Introduction
Several metabolites of abscisic acid (ABA) have been isolated from various plant materials. ABA catabolism proceeds through two major pathways: the ‘oxidative pathway’ involving oxidation at different positions, and the ‘conjugation pathway’. The pathway used depends on the plant species, developmental stage or tissue type. The oxidative pathway, considered the more common in plant catabolism (Nambara and Marion-Poll, Reference Nambara and Marion-Poll2005), is initiated by hydroxylation at C-8′ to produce 8′-hydroxy-ABA (8′-HOABA), which rearranges to phaseic acid (PA) (Cutler and Krochko, Reference Cutler and Krochko1999; Todoroki et al., Reference Todoroki, Hirai and Ohigashi2000). PA is subsequently reduced to dihydrophaseic acid (DPA) and/or its analogue, epi-dihydrophaseic acid (epi-DPA) (Zeevaart et al., Reference Zeevaart, Rock, Fantauzzo, Heath, Gage, Davies and Jones1991).
Recently, Zhou et al. (Reference Zhou, Cutler, Ambrose, Galka, Nelson, Squires, Loewen, Juadhav, Ross, Taylor and Abrams2004) described a new oxidative pathway in which hydroxylation of ABA occurred at the 9′-methyl group of ABA, as well as at the 7′- and 8′-methyl groups. The new metabolite isolated from the plant extract was identified as the closed form of 9 ′-hydroxy-ABA and was named neophaseic acid (neoPA). Levels of neoPA were high in immature tomato seeds, but disappeared at later stages of seed development, suggesting further metabolism (Zaharia et al., Reference Zaharia, Walker-Simmons, Rodríguez and Abrams2005).
ABA and hydroxyl-ABA can be conjugated as glucose esters (ABA-GE) or glycosides. ABA-GE, a major ABA-conjugated metabolite, is formed by an ABA glucosyltransferase (Xu et al., Reference Xu, Nakajima, Suzuki and Yamaguchi2002). The abundance of ABA-GE is low in comparison with ABA, and it may be part of a reversible inactivation reaction used to regenerate free ABA (Sauter et al., Reference Sauter, Abrams and Hartung2002). The fate of ABA-GE is still unknown (Zaharia et al., Reference Zaharia, Walker-Simmons, Rodríguez and Abrams2005), although it has been hypothesized to contribute to ABA homeostasis in plant cells (Chiwocha et al., Reference Chiwocha, Cutler, Abrams, Ambrose, Yang, Ross and Kermode2005).
The hormonal activity reported for some ABA metabolites (Zhou et al., Reference Zhou, Cutler, Ambrose, Galka, Nelson, Squires, Loewen, Juadhav, Ross, Taylor and Abrams2004) has raised the intriguing possibility that these metabolites may mediate hormonal effects hitherto associated only with ABA per se. Thus, further elucidation of the physiological roles of ABA requires quantitative analysis of not only ABA, but also of its metabolites (Setha et al., Reference Setha, Kondo, Hirai and Ohigashi2005).
In angiosperms, the ABA that accumulates during mid-maturation of seeds is synthesized in both zygotic and maternal tissues; thus, the latter is most likely transported from the mother plant. The site of ABA synthesis specifies its physiological action. Maternal ABA promotes reserve accumulation, and embryonic ABA induces seed dormancy and desiccation tolerance (Lefebvre et al., Reference Lefebvre, North, Frey, Sotta, Seo, Okamoto, Nambara and Marion-Poll2006). Many mutations affecting plant responses to a particular hormone have been identified through genetic analysis, including several mutants with altered sensitivity to ABA (Leung and Giraudat, Reference Leung and Giraudat1998; Moller and Chua, Reference Moller and Chua1999). A number of salt (NaCl)-hypersensitive mutants have been isolated in Arabidopsis thaliana (Zhu et al., Reference Zhu, Liu and Xiong1998) and Solanum lycopersicum (Borsani et al., Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2001, Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2002).
In Solanum lycopersicum, these mutants have lost certain salt-tolerance mechanisms, and are therefore more sensitive to salt stress than the wild-type cv. Moneymaker. The tss1 (tomato salt sensitive 1) mutant is hypersensitive to growth inhibition by Na+ but not to osmotic stress induced by mannitol, whereas tss2 (tomato salt sensitive 2) is hypersensitive to both ionic and osmotic stresses (Borsani et al., Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2001). Another mutant, tos1 (tomato osmotic sensitive), has a single recessive nuclear mutation responsible for its hypersensitivity to general osmotic stress (Borsani et al., Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2002). These three mutants are all similar to the wild type in their growth characteristics, with the distinctive phenotypes becoming evident only under stress conditions. Borsani et al. (Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2002) observed that growth inhibition by applied ABA was greater in tss2 (hypersensitive to ABA) than in the wild type, tss1, or tos1. They proposed that the decreased sensitivity of tos1 seedlings and increased sensitivity of tss2 seedlings to ABA could result from either abnormal ABA metabolism or altered ABA signal transduction, and that tss2 and tos1 are not ABA-deficient mutants.
ABA clearly plays a key role in seed germination, but the contribution of ABA catabolism to the regulation of endogenous ABA content in this process is not clear. Likewise, in the above tomato mutants, modifications in ABA metabolism during seed imbibition, and the possible role of ABA metabolism in germination and sensitivity to osmotic and ionic stress, remain to be studied. Thus, we have investigated: (1) the correlation between endogenous ABA content and germination response of non-dormant seeds of cv. Moneymaker and the hypersensitive tomato mutants tss1, tss2, and tos1; (2) ABA, PA, DPA, and ABA-GE accumulation during the imbibition process; and (3) possible relationships between ABA content and ABA metabolic patterns with previously reported differential sensitivity of the mutants.
Materials and methods
Plant materials
Seeds of tomato (Solanum lycopersicum Mill.) cv. Moneymaker (wild type), and its homozygous tss1, tss2, and tos1 mutants, generously provided by Dr Miguel A. Botella (Department of Molecular Biology and Biochemistry, University of Málaga, Spain), were used for the assays. Two alleles of the tss1 mutant were reported by Borsani et al. (Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2001); in our experiments the allele tss1-1 was used. Variability due to different growth and seed storage conditions was avoided by growing wild-type and mutant plants side by side. Seeds were extracted from mature fruits, fermented in the juice for 1 d, rinsed, dried at room temperature and stored at 4°C for 6 months. For each genotype, three biological replicates of equivalent age were processed.
Imbibition and germination assays
A simple random design consisting of ten imbibition times was used. For each experiment, 20 seeds were placed in a 7-ml flask with 300 μl distilled water (three replicate flasks per experiment). The number of germinated seeds was recorded daily at 12, 24, 48, 72, 96, 120, 144, 168, 192 and 216 h, and expressed as a percentage of the total (%G). Germination was defined as the presence of a clearly visible radicle protrusion (Ni and Bradford, Reference Ni and Bradford1993). Experiments were performed under controlled conditions in a Conviron G 30 germination chamber (Winnipeg, Manitoba, Canada) at 25 ± 1°C, relative humidity 80%, in the dark.
Results were analysed using the program Statgraphics Plus, version 3 (Manugistics, Rockville, Maryland, USA). Germination data as percentage of total number of seeds were arcsine transformed. A one-way ANOVA procedure was used to compare the effects of different imbibition times in distilled water. Normality was verified by the Shapiro–Wilk test. Homogeneity of variance was verified by the Bartlett test. P values ≤ 0.05 were considered statistically significant.
Endogenous concentrations of ABA, PA, DPA and ABA-GE were determined in dry and imbibed seeds at 12, 24, 48 and 72 h. The total volume of imbibition water was tested for the presence of free ABA at each imbibition time.
Abscisic acid extraction procedure
ABA was extracted and pre-purified by a modified protocol of Luna et al. (Reference Luna, Soriano, Bottini, Sheng and Pharis1993). The equivalent of 200 mg dry weight of dry or imbibed seeds was ground in a mortar with liquid nitrogen and 20 ml imidazole buffer (pH 7) plus 2,6-di-tert-butyl-p-cresol as antioxidant. Fifty nanograms of [2H6]ABA (OlChemIm Ltd, Olomouc, Czech Republic) were added as internal standard, and the sample was incubated overnight at 4°C to allow extraction and standard equilibration. After centrifugation and isopropanol evaporation, the aqueous fraction was loaded on to a conditioned amino anion exchange minicolumn [BAKERBOND speTM Amino (NH2), Mallinckrodt Baker, Inc., Phillipsburg, New Jersey, USA] and washed sequentially with 6 ml each of hexane, ethyl acetate and acetonitrile. These fractions were discarded, and then ABA was eluted with methanol:acetic acid (95:5, v/v) and evaporated to dryness.
HPLC procedure for abscisic acid purification
Dried extracts were dissolved in 100 μl of elution solvent [methanol:water:acetic acid (70:30:0.1 v/v)], and separated on a preparative high-performance liquid chromatography (HPLC) system (KNK-500, Konic Instruments, Barcelona, Spain) equipped with an RP C18 column (μ-Bondapack, 300 × 3.9 mm internal diameter, 5 μm particle size; Waters Associates, Milford, Massachusetts, USA) coupled to a spectrometry system (UV-Vis) with diode array detector. Samples were subsequently eluted at a flow rate of 1 ml min− 1 with an isocratic mixture of methanol:water:acetic acid (70:30:0.1 v/v). Fractions corresponding to ABA (retention time: 2.77–5.00 min), as determined spectrophotometrically at 262 nm on a previous HPLC run, were pooled and dried.
Phaseic acid, dihydrophaseic acid and ABA-glucose ester extraction and purification
PA, DPA and ABA-GE were extracted and pre-purified as described by Zhou et al. (Reference Zhou, Squires, Ambrose, Abrams, Ross and Cutler2003) with modifications. The equivalent of 200 mg dry weight of dry or imbibed seeds was ground in a mortar with liquid nitrogen and extracted with 3 ml of acetone:water:acetic acid (80:19:1 v/v). One hundred nanograms of [2H3]PA, [2H3]DPA and [2H5]ABA-GE (NRC-Plant Biotechnology Institute, Saskatoon, Canada) were added as internal standards. Extracts were transferred to 50 ml tubes, centrifuged at 5000 rpm for 15 min, and the supernatant was collected and evaporated at 35°C under vacuum in a SpeedVac. Dried extracts were dissolved in 100 μl methanol:acetic acid (99:1 v/v), then mixed with 900 μl of 1% acetic acid. The samples were filtered through a syringe filter tip and purified with 3 ml BondElut-C18 cartridges (Varian, Palo Alto, California, USA) on a vacuum manifold at a flow-rate less than 1 ml min− 1. The cartridges were conditioned with 1.5 ml methanol and equilibrated with 1.5 ml methanol:water:acetic acid (10:89:1 v/v). Samples (each ~1.5 ml volume) were loaded on to the cartridges and washed with 1.5 ml methanol:water:acetic acid (10:89:1 v/v). ABA metabolites were eluted by 1.5 ml methanol:water: acetic acid (80:19:1 v/v) and collected in a 2-ml flat-bottom Eppendorf tube. The eluate was dried under vacuum at 35°C during centrifugation.
GC–MS–SIM
Samples containing ABA or ABA metabolites were dissolved in 100 μl of derivatization solution N, O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and then converted to methylester-trimethylsilylether (MeTMSi) derivatives. The samples were placed in an oven with increasing temperature from 70°C to 90°C for 30 min, and 1 μl was injected split-splitless in a gas chromatography–mass spectrometry with selected ion monitoring (GC–MS–SIM) system (Hewlett Packard 5890 Series II GC, with capillary direct interface to a 5972 Mass Selective Detector) equipped with a 25 m Chrompack CPSil 19 capillary column (internal diameter 0.25 mm; film thickness 0.22 μm). Carrier gas: He at flow rate of 1 ml min− 1; GC injector temperature: 280°C; oven temperature: initially maintained at 100°C for 1 min, then increased from 100 to 195°C at a rate of 15°C min− 1, then at a rate of 4°C min− 1 up to 280°C. For ABA quantification, ions 196 (deutero) and 190 (proto) were monitored at 9–10 min; for PA, ions 299 and 296 were monitored at 9–10 min; for DPA, ions 297 and 294 were monitored at 10–11 min; for ABA-GE, ions 268 and 263 were monitored at 11–12 min. Quantification of ABA-GE was performed as derivatized conjugate. For unequivocal determination, back-up ions (labelled ABA 134, labelled PA 279, labelled DPA 281, labelled ABA-GE 430) were monitored for peak confirmation. The amounts of endogenous ABA, PA, DPA and ABA-GE were calculated by comparison of the peak areas of the ions for the [2H] isotope internal standard versus its proto counterpart, and the analysis was done in triplicate.
Data were expressed as means ± SE of three independent determinations of three samples extracted from experimental treatments (imbibition time) and analysed using the Statgraphics Plus program. ABA and metabolite content in dry and imbibed seeds, as well as effects of different imbibition times on endogenous ABA and metabolite content, were compared by one-way ANOVA and Tukey's HSD test. P values ≤ 0.05 were considered to be statistically significant.
Results
Endogenous content of ABA and its metabolites in dry seeds
Endogenous contents of ABA and its metabolites in dry seeds of cv. Moneymaker (wild type), and its tss1, tss2, and tos1 mutants, are summarized in Fig. 1. ABA content was highest in wild-type seeds followed by tss1 and tos1, and lowest in tss2 [732 pmol (g DW)− 1]. Differences in ABA content were significant between wild type and tss2 and tos1, and among all mutants. PA, DPA and ABA-GE were detected in wild-type and mutant seeds. ABA metabolites were much lower than ABA in all genotypes. Mutants tss2 and tos1 showed similar distribution patterns, with PA being the least abundant metabolite. In the wild type, the total metabolites [PA+DPA+ABA-GE = 1.149 pmol (g DW)− 1] content was 83-fold lower than that of ABA (Fig. 1A). In tss1 and tos1, metabolite contents were, respectively, 84-fold and 37-fold lower than that of ABA (Fig. 1B and D). In contrast, the difference between ABA and total metabolite level [650 pmol (g DW)− 1] in tss2 was only slight (Fig. 1C).
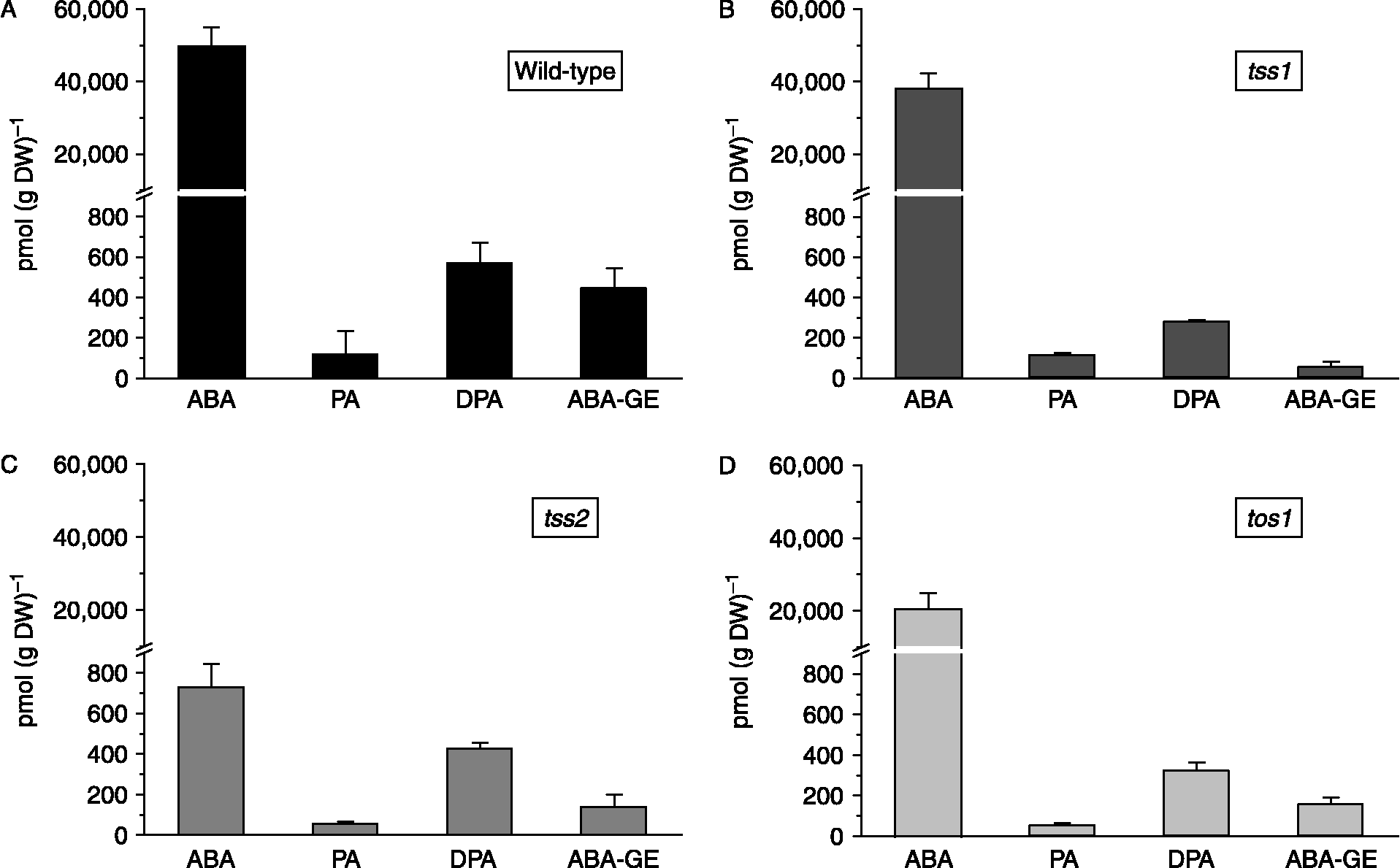
Figure 1 Abscisic acid (ABA) and ABA metabolite content [phaseic acid (PA), dihydrophaseic acid (DPA) and ABA glucose ester (ABA-GE)] of dry tomato seeds. (A) Solanum lycopersicum Mill. cv. Moneymaker (wild type); (B) tss1 mutant; (C) tss2 mutant; (D) tos1 mutant. Data are means of three replicates with SEs (P ≤ 0.05).
DPA was consistently the most abundant ABA metabolite, and was higher in the wild type than in the mutants. Among the mutants, DPA level was highest in tss2. PA was a minor component in all genotypes, and conjugated ABA were twofold higher in the wild type than in the mutants.
Germination of wild-type and mutant seeds
The time course of germination of tomato wild-type and mutant seeds is shown in Fig. 2. None of the genotypes showed germination before 24 h of imbibition. In tss1, germination percentage was 33% at 48 h and 41% at 72 h of imbibition. Tss2 showed 5% germination at 48 h and 62% germination at 72 h. The slowest germination was found for the wild type and tos1 mutant: 23% and 25%, respectively, after 72 h of imbibition. Differences in germination percentage at 72 h were significant between the wild type and tss2, and between tss2 and tos1. At 216 h, germination for the wild type and tss2 mutant was 100%, and for the tss1 and tos1 mutants it was around 70%.

Figure 2 Germination time course of wild-type, tss1, tss2 and tos1 tomato seeds. Data are means of three replicates with SEs. Values with the same letter at 72 h are not significantly different (P ≤ 0.05). Data points ≥ 96 h are from single observations, due to lack of seed material.
Endogenous levels of ABA in imbibed seeds and imbibition water
We studied free ABA content during the imbibition process, and possible secretion of ABA from seeds into the imbibition water. During the imbibition time course, wild-type seeds showed a significant ABA increase from 12 to 48 h, a maximum [749 pmol (g DW)− 1] at 48 h, and decrease to 468 pmol (g DW)− 1 at 72 h. Secretion of ABA into the water was low and sustained during the imbibition process (Fig. 3A).

Figure 3 Abscisic acid content of imbibed tomato seeds and their incubation water during the germination time course: (A) cv. Moneymaker (wild type); (B) tss1 mutant; (C) tss2 mutant; (D) tos1 mutant. Results are expressed in pmol (g DW)− 1 for seeds and in pmol ml− 1 for imbibition water. Data are means of three replicates with SEs (P ≤ 0.05).
During tss1 imbibition, seed ABA increased sharply at 24 h, reached a maximum [754 pmol (g DW)− 1] at 48 h, then decreased sharply at 72 h. There were no significant changes in ABA secretion into the imbibition water (Fig. 3B).
ABA content in tss2 imbibed seeds was considerably lower than that in wild type and tss1, and no significant variations were observed during the course of imbibition. Interestingly, ABA was secreted at much higher levels than found in the imbibed seeds (Fig. 3C).
In tos1 imbibed seeds, ABA content was highest [186 pmol (g DW)− 1] at 12 h, and was slightly lower at 24 h and thereafter. Tos1 seeds also secreted high amounts of ABA into the imbibition water. ABA secreted by tos1 seeds reached a peak of 496 pmol (g DW)− 1 at 24 h, 200% higher than the level of free ABA measured in the seeds at the same time. Similar values were found at 48 and 72 h (Fig. 3D).
Thus, levels of ABA secreted by tos1 and tss2 into imbibition water were high, as compared to endogenous levels in the seeds at various times. In contrast, patterns of secreted and endogenous ABA level for tss1 were similar to those of the wild type.
Endogenous levels of PA, DPA and ABA-GE in imbibed seeds
ABA metabolites from barley are low in liquid medium, making identification difficult (Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002). However, in tomato seeds, ABA metabolites in imbibed seeds were much higher than ABA, and their profiles differed among the genotypes.
In wild-type tomato seeds, DPA was the major metabolite, increasing significantly from 2006 up to 7370 pmol (g DW)− 1 during the time course of imbibition. PA was very low at 12 h and 48 h, and declined to near zero at 72 h. ABA-GE was not detectable at any imbibition time (Fig. 4A).

Figure 4 Abscisic acid (ABA) metabolite content [phaseic acid (PA), dihydrophaseic acid (DPA) and ABA glucose ester (ABA-GE)] of imbibed tomato seeds: (A) cv. Moneymaker (wild type); (B) tss1 mutant; (C) tss2 mutant; (D) tos1 mutant. Data are means of three replicates with SEs (P ≤ 0.05).
Imbibed tss1 seeds had the lowest levels of ABA metabolites among the four genotypes. DPA was again the major metabolite, with a maximal value [1326 pmol (g DW)− 1] at 48 h. PA reached its maximum [837 pmol (g DW)− 1] at 72 h. ABA-GE was detectable only at 48 and 72 h (Fig. 4B).
In tss2, DPA was again the major metabolite, and was unusual in showing its maximal level [8407 pmol (g DW)− 1] at 12 h, after which it gradually decreased. PA and ABA-GE were minor metabolites (Fig. 4C).
In tos1 DPA was also the major metabolite, showing high levels at 12 and 48 h and low levels at 24 and 72 h, coincident with increased PA. The tos1 mutant was the only genotype producing high levels of ABA-GE, which was notable at 24 h and maximal [7850 pmol (g DW)− 1] at 72 h (Fig. 4D).
Discussion
The different basal levels of ABA and metabolites may be correlated with differential germination capacities between the wild type and the several mutant genotypes. ABA content in dry seeds was always higher than for imbibed seeds. For example, ABA in wild-type and tss1 dry seeds were 67- and 50-fold higher, respectively, than after 48 h of imbibition. This suggests that ABA is synthesized or accumulated during seed formation. ABA present in mature seeds is generally considered to be a remnant of the ABA pools that are involved in the regulation of seed development (Hilhorst, Reference Hilhorst1995). Some studies indicated that the osmotic environment experienced by the seeds during development, more than ABA content, determines whether tomato seeds progress into events associated with germination or remain arrested at the completion of development (Berry and Bewley, Reference Berry and Bewley1992; Groot and Karssen, Reference Groot and Karssen1992). Jacobsen et al. (Reference Jacobsen, Pearce, Poole, Pharis and Mander2002) have also failed to demonstrate a consistent relationship between ABA content of mature barley dry grains and germinability.
During imbibition, all tomato genotypes showed an abrupt decrease in ABA compared to dry seeds. Further, the ABA decline was accompanied by a corresponding increase in ABA metabolites, mainly DPA and ABA-GE, and there were qualitative and quantitative differences among the four genotypes. In contrast, for imbibed seeds of Arabidopsis thaliana mutant etr1-2 (mutation that confers ethylene insensitivity and affects the ABA metabolic pathway), the decline in ABA was not accompanied by an increase in any of the ABA catabolites monitored (Chiwocha et al., Reference Chiwocha, Cutler, Abrams, Ambrose, Yang, Ross and Kermode2005).
The delayed germination of wild-type seeds may be due to not only high basal ABA content, but also newly synthesized ABA during imbibition, since ABA levels increased sharply up to 48 h, before declining at 72 h when visible germination was present. These findings are consistent with those of Grappin et al. (Reference Grappin, Bouinot, Sotta, Miginiac and Jullien2000), who showed that some of the endogenous ABA detected in Nicotiana plumbaginifolia seeds resulted from de novo synthesis during early imbibition. In non-dormant and dormant Arabidopsis seeds, there was a decrease of ABA during early imbibition, although the rate of decrease was slower in the dormant seeds. Three days after sowing, the ABA content in dormant seeds increased and then remained high. Therefore, dormant and non-dormant seeds differ in their ability to neosynthesize ABA following imbibition (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004).
Dry and imbibed seeds of the tss1 mutant had ABA contents similar to that of the wild type. Amounts of ABA and its metabolism may have a reduced influence on germination of this mutant, considering its faster germination at 48 h relative to the other genotypes. Valpuesta and Botella (Reference Valpuesta and Botella2007) suggested that TSS1 may encode for a Ca2+ sensor. A member of the Arabidopsis calmodulin-like (CML) gene family, CML24, was recently reported to act downstream from ABA perception, perhaps mediating cellular responses to ABA-induced Ca2+ fluctuations, thereby delaying germination and seedling growth (Delk et al., Reference Delk, Johnson, Chowdhury and Braam2005). In the present study, tss1 exhibited high endogenous ABA during imbibition, but germinated earlier than the wild type. Thus, the low sensitivity of tss1 to endogenous ABA could be due to the mutation affecting a Ca2+ sensor similar to CML24, perhaps resulting in reduced intracellular signalling.
While most varieties of mature tomato seeds are generally non-dormant, the ABA-deficient sitiens (sit w) tomato mutant seeds have been observed to germinate about a day earlier than wild-type seeds (Downie et al., Reference Downie, Gurusinghe and Bradford1999). In this work, the highest germination percentages obtained with tss2 seeds were associated with low ABA content in dry and imbibed seeds, suggesting that this mutant has an active ABA catabolism, and perhaps a low rate of ABA biosynthesis. The tss2 mutant is hypersensitive to exogenous ABA, and the TSS2 locus has been suggested to encode a protein that negatively regulates ABA signalling (Borsani et al., Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2001). A similar pattern of ABA biosynthesis was obtained with tos1 imbibed seeds; supporting the suggestion of Borsani et al. (Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2002) that tos1 is not an ABA-deficient mutant.
While Jacobsen et al. (Reference Jacobsen, Pearce, Poole, Pharis and Mander2002) observed leakage of ABA and PA from barley seeds into the imbibition liquid, major decreases in ABA content in the seeds were not reflected by increased ABA or PA in the liquid. In our experiments, ABA diffused readily from tos1 seeds into the liquid medium, and its level was twofold higher than that in tss1 or tss2. The tos1 mutant (characterized by sensitivity to osmotic stress) could have altered cellular membrane properties that result in an appreciable loss of ABA from the seed. That said, reduced ABA content in the seed of tos1 had no effect on germination at 72 h, i.e. germination percentages for tos1 were similar to those of the wild type, and lower than that of tss1 or tss2. Borsani et al. (Reference Borsani, Cuartero, Fernández, Valpuesta and Botella2002) proposed that the osmotic hypersensitivity of tos1 is due to a defect at the intracellular level of the ABA-dependent signalling pathway, which is necessary for osmotic tolerance, and not to reduced seed ABA. In view of the high ABA leakage from tos1 seeds into the medium, our data suggest that either extracellular ABA perception or signalling could also be altered in this mutant.
Millar et al. (Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006) suggested that ABA-mediated physiological processes are correlated with fluctuating endogenous levels of the hormone, dynamically maintained by continual synthesis, transport and catabolism. The sharp decrease in ABA content following seed imbibition observed in the present study suggests activation of catabolic pathways, in agreement with previous reports (Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002; Kushiro et al., Reference Kushiro, Okamoto, Nakabayashi, Yamagishi, Kitamura, Asami, Hirai, Koshiba, Kamiya and Nambara2004; Zhou et al., Reference Zhou, Cutler, Ambrose, Galka, Nelson, Squires, Loewen, Juadhav, Ross, Taylor and Abrams2004; Nambara and Marion-Poll, Reference Nambara and Marion-Poll2005). In barley and Arabidopsis, the decrease of ABA content during seed imbibition is associated with increases in PA (Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002; Kushiro et al., Reference Kushiro, Okamoto, Nakabayashi, Yamagishi, Kitamura, Asami, Hirai, Koshiba, Kamiya and Nambara2004) or PA metabolites.
Of interest here is the finding that rapid ABA catabolism during Arabidopsis seed imbibition is associated with increased mRNA levels of CYP707A2, a member of the CYP707A gene family that encodes ABA 8′-hydroxylases (Kushiro et al., Reference Kushiro, Okamoto, Nakabayashi, Yamagishi, Kitamura, Asami, Hirai, Koshiba, Kamiya and Nambara2004). This increase in AtCYP707A2 expression was also associated with the rapid decline of ABA in germinating seeds. More recently, Okamoto et al. (Reference Okamoto, Kuwahara, Seo, Kushiro, Asami, Hirai, Kamiya, Koshiba and Nambara2006) reported that CYP707A1 and CYP707A2 are complementary and necessary for seed dormancy and germination control in Arabidopsis.
We identified PA and DPA in dry and imbibed wild-type and mutant seeds by GC–MS–SIM, confirming that 8′-hydroxylation of ABA is the main catabolic route. In imbibed wild-type seeds, all of the PA was metabolized to DPA, with DPA rising throughout the imbibition time course. Reduced ABA and higher DPA were observed in tss2 (sensitive to ionic and osmotic stress) and in tos1 (which is sensitive to osmotic stress), relative to tss1. These results suggest, for tss2 and tos1, that the correlation between sensitivity to osmotic stress and endogenous ABA/DPA differs from that of tss1. In tss1, which is hypersensitive to ionic stress but not to general osmotic stress, the 8′-hydroxylation pathway was almost inactive, yielding the lowest PA and DPA values among the studied genotypes. It should be noted that the earlier germination of tss1 was coincident with its low ABA catabolism, which is in agreement with the findings of Chiwocha et al. (Reference Chiwocha, Abrams, Ambrose, Cutler, Loewen, Ross and Kermode2003), e.g. that 8′-hydroxylation and DPA formation may represent a minor pathway under conditions that promote germination.
DPA was the main ABA metabolite in apple seeds and sweet cherry fruit and seeds, with levels higher than those of ABA, suggesting that DPA, via feedback inhibition, may help regulate ABA in seeds (Setha et al., Reference Setha, Kondo, Hirai and Ohigashi2005). Our observation that PA is a minor compound in imbibed seeds may be explained by its rapid reduction to DPA, as was also seen for germinating seeds of Brassica napus (Zhou et al., Reference Zhou, Squires, Ambrose, Abrams, Ross and Cutler2003). In the present study, only tos1 seeds showed major changes in ABA metabolism during imbibition. The decline in DPA levels at 24 and 72 h may have resulted from further metabolism to its 4′-glucoside, which was not monitored in this study. Such a reaction has been reported in tissues of several species (Walton and Li, Reference Walton, Li and Davies1995).
The wild type was the only genotype in which ABA catabolism by glucosylation was not detected. In contrast, ABA-GE was the predominant ABA catabolite in lettuce seeds during germination (Chiwocha et al., Reference Chiwocha, Abrams, Ambrose, Cutler, Loewen, Ross and Kermode2003), and the high ABA-GE in germinating wild-type Arabidopsis seeds suggests that this is the major reason for decreased ABA accumulation during germination (Chiwocha et al., Reference Chiwocha, Cutler, Abrams, Ambrose, Yang, Ross and Kermode2005). Finally, for germinating maize kernels, Wang et al. (Reference Wang, Mambelli and Setter2002) found that conjugation to ABA-GE was not a major pathway for endogenous ABA catabolism. Thus, ABA catabolic pathways vary considerably among different plant species, or even in different tissues of the same species. While ABA-GE has been considered to be biologically inert, and thus not constitute a stored form (Dietz et al., Reference Dietz, Sauter, Wichert, Messdaghi and Hartung2000), Sauter et al. (Reference Sauter, Abrams and Hartung2002) provided evidence that ABA-GE contributes to ABA homeostasis. Adding further support for a role of ABA-GE in homeostasis is the finding that the β-glucosidase homologue AtBG1 generates ABA from ABA-GE (Lee et al., Reference Lee, Piao, Kim, Choi, Jiang, Hartung, Hwang, Kwak, Lee and Hwang2006).
In our study increased ABA-GE was measured in tos1 seeds from 24 to 72 h of imbibition. This indicates that the ABA glucosylation pathway is active in tos1 and, together with an active 8′-hydroxylation pathway, likely accounts for the very low level of free ABA during germination. Interestingly, at 72 h tos1 showed the same germination response as the wild type, even though their pattern of ABA metabolism was very different. An active glucosylation pathway in tos1 seeds may be a direct response to the mutation for this osmotic stress-sensitive mutant.
In conclusion, wild-type and mutant seeds exhibited differing ABA contents and different ABA metabolism patterns that may be related to their differential sensitivity to abiotic stress. ABA metabolism differs in dry and imbibed seeds of wild-type tomato cv. Moneymaker and the tss1, tss2 and tos1 mutants, and metabolism to DPA is the most important pathway for ABA catabolism.
Acknowledgements
We thank Dr Miguel A. Botella (University of Málaga, Spain) for kindly providing the tomato seeds used in this study. This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), SECYT-UNRC and UNRC-ANPCYT grants awarded to G.A. and fellowships from CONICET to A.A.






