Introduction
Verbascum is one of the largest genera of the Scrophulariaceae family, with about 360 species worldwide (Judd et al., Reference Judd, Campbell, Kellogg, Stevens and Donoghue2008; Benedí, Reference Benedí, Castroviejo, Aedo, Laínz, Muñoz Garmendia, Nieto Feliner, Paiva and Benedí2009). It is distributed in the temperate northern hemisphere (Europe and Asia) and shows its highest species diversity in the Mediterranean region. The main centre of speciation and diversification for this genus is located in the Balkan and Irano-Turanian biogeographic regions, where nine-tenths of all the species can be found (Benedí, Reference Benedí, Castroviejo, Aedo, Laínz, Muñoz Garmendia, Nieto Feliner, Paiva and Benedí2009). Ferguson (Reference Ferguson, Tutin, Burges, Chater, Edmondson, Heywood, Moore, Valentine, Walters and Webb1972) recognized about 100 species of Verbascum in Europe (including the genus Celsia). Twenty-nine species of Verbascum can be found in Italy (Conti et al., Reference Conti, Abbate, Alessandrini and Blasi2005) and nine taxa can be found in Sicily (Pignatti, Reference Pignatti1982; Conti et al., Reference Conti, Abbate, Alessandrini and Blasi2005), the largest island in the centre of the Mediterranean basin, i.e. Verbascum blattaria L., V. creticum (L.) Kuntze, V. macrurum Ten., V. phlomoides L., V. pulverulentum Vill., V. rotundifolium Ten. subsp. rotundifolium, V. siculum Tod., V. sinuatum L. and V. thapsus L.
The genus Verbascum includes species that grow in different habitats, such as sandy places, rocks, open and semi-open natural habitats. Many species of Verbascum usually colonize disturbed environments, such as abandoned agricultural fields, margins of cultivated fields, burnt areas and roadsides. The success of these species in colonizing disturbed habitats is very often the result of some type of disturbance that reduces the density of the existing plant species (Reinartz, Reference Reinartz1984). The establishment is driven by the very persistent soil seed bank (Darlington and Steinbauer, Reference Darlington and Steinbauer1961; Kivilaan and Bandurski, Reference Kivilaan and Bandurski1981), which may last several decades in V. thapsus (Hoshovsky, Reference Hoshovsky1986; Remaley, Reference Remaley1998) and even up to 90 years in V. blattaria (Kivilaan and Bandurski, Reference Kivilaan and Bandurski1981). After their establishment, these species produce ephemeral populations that very rarely persist for several years, unless the sites are very severely disturbed, for example by intensive grazing.
Several species of Verbascum have been used traditionally as medicinal plants. From a commercial point of view, the importance of this genus has been increasing for the past few years and nowadays it has also become important for gardening and landscaping, thanks to the ability of several species to flower over a long period of time (V. arcturus, V. blattaria, V. creticum), even in dry soils. However, as the result of their intentional introduction as medicinal herbs, V. thapsus and V. blattaria have recently become invasive weed species in North America, where they are very difficult to eradicate (Remaley, Reference Remaley1998), due to their extreme plasticity (Parker et al., Reference Parker, Rodriguez and Michael2003), optional self-pollination, high seed production and the above-mentioned persistence in soil seed banks.
It is clear that the ecophysiology of seed germination is essential to understanding the establishment of these ruderal species, their succession and natural regeneration (Vázquez-Yanes and Orozco-Segovia, Reference Vázquez-Yanes, Orozco-Segovia, Caldwell and Pearcy1994). In particular, it is necessary to evaluate the combined role of temperature and light, which have usually shown a very high level of interaction (Demel, Reference Demel2005), particularly when seeds are exposed to regimes with ample daily fluctuations, which may typically happen when they are brought near to the soil surface in disturbed habitats.
When studying the effect of temperature, it is meaningful to consider the thermal-time (TT) modelling approach, which assumes that the germination behaviour (capacity and velocity) relates to three cardinal (threshold) temperatures (Gardarin et al., Reference Gardarin, Guillemin, Munier-Jolain and Colbach2010; Parmoon et al., Reference Parmoon, Moosavi, Akbari and Ebadi2015), i.e. minimum or base temperature (Tb), optimum temperature (To) and cut-off temperature (Tc). At temperatures lower than Tb or higher than Tc germination does not occur, between T b and T o (sub-optimal temperatures) germination rates increase with temperature, while between T o and T c (supra-optimal temperatures) germination rates decrease with temperature, mainly as an effect of increased base osmotic potential level, i.e. the capacity of seeds to use environmental water to trigger germination (Rowse and Finch-Savage, Reference Rowse and Finch-Savage2003; Wang et al., Reference Wang, Cheng and Song2013).
To our knowledge, only a few studies are available in literature relating to the ecophysiology of seed germination for Verbascum spp. and the information available concerns only V. bithynicum, V. blattaria, V. phlomoides, V. sinuatum, V. thapsus and V. wiedemannianum (Gross and Werner, Reference Gross and Werner1978; Baskin and Baskin, Reference Baskin and Baskin1981; Gross, Reference Gross1985; Puech et al., Reference Puech, Ribannier and Martin1997; Senel et al., Reference Senel, Ozdener and Incedere2007). Furthermore, threshold temperatures have never been determined for any of the species of this genus.
The present study attempted to fill the above gaps of knowledge and investigated the seed germination behaviour in nine species of Verbascum, which can easily be found in ruderal habitats, coastal sand dunes and rocky areas of the Mediterranean region. The aims were as follows: (1) to evaluate germination capacity and velocity as affected by light regime, temperature level and regime (constant or fluctuating temperatures), storage time and their interactions; (2) to obtain estimates of threshold temperatures for seed germination; and (3) to discuss the role of these threshold temperatures for modelling purposes.
Materials and methods
Plant species
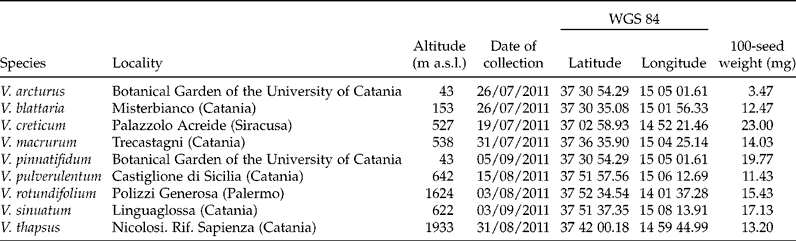
This research was carried out with nine species belonging to the genus Verbascum: Verbascum arcturus L., V. blattaria L., V. creticum (L.) Kuntze, V. macrurum Ten., V. pinnatifidum Vahl., V. pulverulentum Vill., V. rotundifolium Ten. subsp. rotundifolium, V. sinuatum L. and V. thapsus L. The selection of these species was based on a preliminary survey, which suggested that, at present, no other species can be found in Sicily. It should be noted that V. arcturus and V. pinnatifidum were not found in natural conditions (see later) but they were included in this study in order to increase the range of geographical distributions, life forms and growing habitats.
Seven of these species are biennial, while V. arcturus and V. pinnatifidum are semi-perennial (chasmophyte) and perennial, respectively. V. arcturus is usually found on calcareous cliffs and rocks, while V. pinnatifidum prefers the sandy coasts of the Aegean Sea (Greece and Western Turkey) or the northern coasts of the Black Sea (Ukraine, Crimea). V. rotundifolium subsp. rotundifolium is endemic to Italy (Sicily and Campania) while V. arcturus is an endemic species of Western Crete (Greece). These three species (V. arcturus, V. pinnatifidum and V. rotundifolium) are characterized by a narrow natural distribution area and thus they should be considered as very vulnerable, while all the other species commonly grow in a variety of ruderal habitats and uncropped fields and have a much broader geographical distribution (i.e. chorological types): palaeotemperate (V. blattaria), south-western Mediterranean (V. creticum), Mediterranean-montane (V. macrurum), central–south European (V. pulverulentum), Euro-Mediterranean (V. sinuatum) and European–Caucasian (V. thapsus) (Pignatti, Reference Pignatti1982).
Seed collection
For all species except V. arcturus and V. pinnatifidum, seeds were collected between July and September 2011. Collection sites were selected considering the geographical distribution of these species in Sicily and the fitness of the populations, e.g. high number of plants per population and high plant densities. A GPS (iFINDER® HUNTTM, Lowrance Electronics Inc., Oklahoma, USA) was used to record positioning and elevation for each collection site (Table 1).
Table 1 Characteristics of the sampled populations of the nine Verbascum species

From each plant, ripe capsules (dry and brownish) were harvested from the lowest part of the infructescence in order to avoid the selection of immature seeds that are normally located in the uppermost part of the infructescence.
For each population, at least 30 adult plants were randomly selected, except for V. arcturus and V. pinnatifidum, for which five adult plants located in the botanical garden of Catania (Hortus Botanicus Catinensis) were selected.
Germination assays
Following harvest, fruits were kept for a 4-week period in our laboratory to ensure uniform seed drying. Afterwards, the seeds were removed from the fruits, manually cleaned and stored in paper bags at room temperature (22 ± 2°C) until the initiation of the germination assays. The difference between two different storage periods was studied, which is also indicated by the terms ‘short storage’ and ‘long storage’. Before the assays, mean seed weight was determined for each species, on five replicates of 100 seeds, by using an analytical balance Mettler Toledo AE-50 (Greifensee, Zurich, Switzerland).
Germination assays were performed at seven constant temperature regimes (10, 15, 20, 25, 30, 35, 40°C) and seven fluctuating temperature regimes (5/30, 10/25, 10/30, 10/35, 15/25, 15/30, 20/30°C – 12/12 h thermoperiod). Each of the above thermal conditions was tried both in continuous darkness (D) and in an alternating light/dark regime (L/D), with a 12/12 h photoperiod. In the experiments with alternating temperature, the exposure to light coincided with the highest temperature. Fixed temperature regimes were selected in order to be able to explore the whole range of germination responses, reaching both below T b and above T c. Fluctuating temperature regimes (T 1/T 2) were selected with the aims of achieving: (1) T b < T 1 < T o and T o < T 2 < T c; (2) a large number of T 1 and T 2 averages (i.e. 17.5, 20, 22.5, 25°C); and (3) a large number of daily thermal excursions (10, 15, 20, 25°C), considering also the values that are normally experienced in natural conditions during the germination season (spring or autumn), near the soil surface and within canopy gaps.
Germination assays were performed in 9-cm Petri dishes on top of three filter papers, previously moistened with 5 ml of distilled water. Four replicated batches of 25 seeds were used for each treatment level and combination. The Petri dishes were incubated in a thermostatically controlled growth chamber (Sanyo - model MLR-351H, Tokyo, Japan), equipped with cool white fluorescent tubes (Osram FL 40 SS W/37). For treatments in darkness, the Petri dishes were immediately wrapped in two layers of aluminium foil and incubated together with those exposed to the alternating light regime. All Petri dishes were wrapped with Parafilm M® to prevent moisture loss, and water was added to the dishes as needed to keep an adequate moisture level. To ensure no systematic effects due to the positioning within the chamber, Petri dishes were re-randomized every 2 d (Yang et al., Reference Yang, Lovett-Doust and Lovett-Doust1999). The dishes were monitored daily and seeds with an emerged radicle were counted and removed from the Petri dishes.
For the dark-treated seeds, counts were made in a darkroom, under a green safe light, to eliminate red–far-red light effects on seed germination. It has been shown that green safe light may have a stimulating effect on germination (Doussi and Thanos, Reference Doussi, Thanos, Ellis, Black, Murdoch and Hong1997; Walck et al., Reference Walck, Baskin and Baskin2000; Luna et al., Reference Luna, Pérez, Fernández-González and Moreno2004; Goggin and Steadman, Reference Goggin and Steadman2012), although, in this case, preliminary assays did not show such effects for the populations under study.
A seed was considered to have germinated when the radicle protrusion was more than 1 mm. The final germination percentage was scored after an incubation period of 15 d. At the end of each germination test, ungerminated seeds were cut-tested to confirm that they were either empty or not viable.
Data analysis
Final germinated proportions (FGPs) obtained at constant temperature regimes were regarded as measures of germination capacity and were submitted to analysis of variance (ANOVA), following arcsin-square root transformation (Sileshi, Reference Sileshi2012). This same analysis was also performed separately on data obtained at fluctuating temperatures. Back-transformed means, together with back-transformed standard errors (by using the Delta method; Bolker, Reference Bolker2008), were obtained and reported in figures.
With respect to germination velocity, the germination times for the 10th, 30th and 50th percentiles in each Petri dish were obtained (t 10, t 30 and t 50; percentiles refer to the whole seed lot, as shown in Bradford, Reference Bradford2005). To this aim, Kaplan–Meyer estimates of germination functions were used (Onofri et al., Reference Onofri, Gresta and Tei2010a; McNair et al., Reference McNair, Sunkara and Frobish2012) together with mid-point imputation to comply with interval censoring (Law and Brookmeyer, Reference Law and Brookmeyer1992), considering that the consequences of this type of censoring for germination assays with daily monitoring have been found to be very small (Onofri et al., Reference Onofri, Mesgaran, Neve and Cousens2014). Germination rates for each Petri dish and for each percentile g (GR g ) were obtained as the inverse of germination times and they were set to 0 when germination was not achieved for the corresponding percentile (Bradford, Reference Bradford2005); means across replicates and standard errors were calculated and reported in the figures.
Thermal-time parameters
The observed GR g s at constant temperatures and L/D (germination in D at constant temperatures was almost always negligible) were used to parameterize the following threshold thermal-time model:
 $$\left \{{\begin{array}{ccc} GR _{ g } = a _{ g }( T - T _{ b ( g )})\{1 - \,exp\,[ b _{ g }( T - T _{ c ( g )})]\} \\ GR _{ g } = 0\quad if \, T < T _{ b ( g )}\, or \, T > T _{ c ( g )} \\ \end{array} }\right. $$
$$\left \{{\begin{array}{ccc} GR _{ g } = a _{ g }( T - T _{ b ( g )})\{1 - \,exp\,[ b _{ g }( T - T _{ c ( g )})]\} \\ GR _{ g } = 0\quad if \, T < T _{ b ( g )}\, or \, T > T _{ c ( g )} \\ \end{array} }\right. $$
where GR g is the germination rate for the percentile g, T is the temperature, T b is the base temperature, T c is the cut-off temperature, a and b are regression parameters, respectively relating to the speed of increase in GR above T b and to the speed of decrease in GR above T o. When parameters are allowed to vary with germination percentiles, a wide range of germination trends can be flexibly described.
This model was fit by using non-linear least squares and, to compensate for heteroscedasticity, robust standard errors for estimated parameters were obtained by using a ‘sandwich’ estimator (Onofri et al., Reference Onofri, Carbonell, Piepho, Mortimer and Cousens2010b). For each species, storage time and germination percentile, T o was derived as the abscissa corresponding to the maximum GR value.
By considering that GR g is the inverse of germination time for a given percentile (t g ), we can see that, for temperatures between T b and T c, it is:
where θ g = 1/a g represents the thermal-time constant for the germination of percentile g (in °C d), that is independent from temperature, while the quantity at the denominator represents the daily requirement of temperature (°C).
The above equation can be used to predict the germination time or germination rate for percentile g with fluctuating temperatures, as shown by Garcia-Huidobro et al. (Reference Garcia-Huidobro, Monteith and Squire1982) and Finch-Savage et al. (Reference Finch-Savage, Steckel and Phelps1998). These predictions assume that germination depends only on the prevailing temperature and not on thermal sequence. In particular, we used equation (1) to simulate the germination rate for the 10th, 30th and 50th percentiles (GR10, GR30 and GR50 as d− 1), for all fluctuating temperature regimes in the presence of alternating light. These predictions were compared with the observed GR g at fluctuating temperatures in order to understand whether thermal sequence played a role in seed germination.
Classification of species
In order to group the species based on their germination characteristics, it was decided to consider the following observed variables: (1) FGPs in D and L/D, as observed with short storage time and averaged over constant temperatures; (2) absolute increase of FGP in D and L/D when passing from short to long storage time; (3) germination rates for the 30th percentile in D and L/D, as observed with short storage time and averaged over constant temperatures; (4) for alternating temperatures, absolute difference between observed and predicted (by using equation 1) GR30s, averaged over fluctuating temperatures and storage times, both in D and L/D; (5) threshold temperatures (T b, T o and T c for the 30th percentile) in L/D, averaged over storage times; (6) absolute difference between the above T b and T c.
The standardized matrix with the nine species on the rows and the 12 observed variables on the columns (see later) was submitted to principal components analyses (PCA). The first four columns of PC scores along with the first four columns of the rotation matrix were displayed on a ‘distance’ biplot, wherein distances among species approximate their original Euclidean distance on the space defined by the original variables (Legendre and Legendre, Reference Legendre and Legendre1998).
Results
Germination capacity at constant temperature regimes
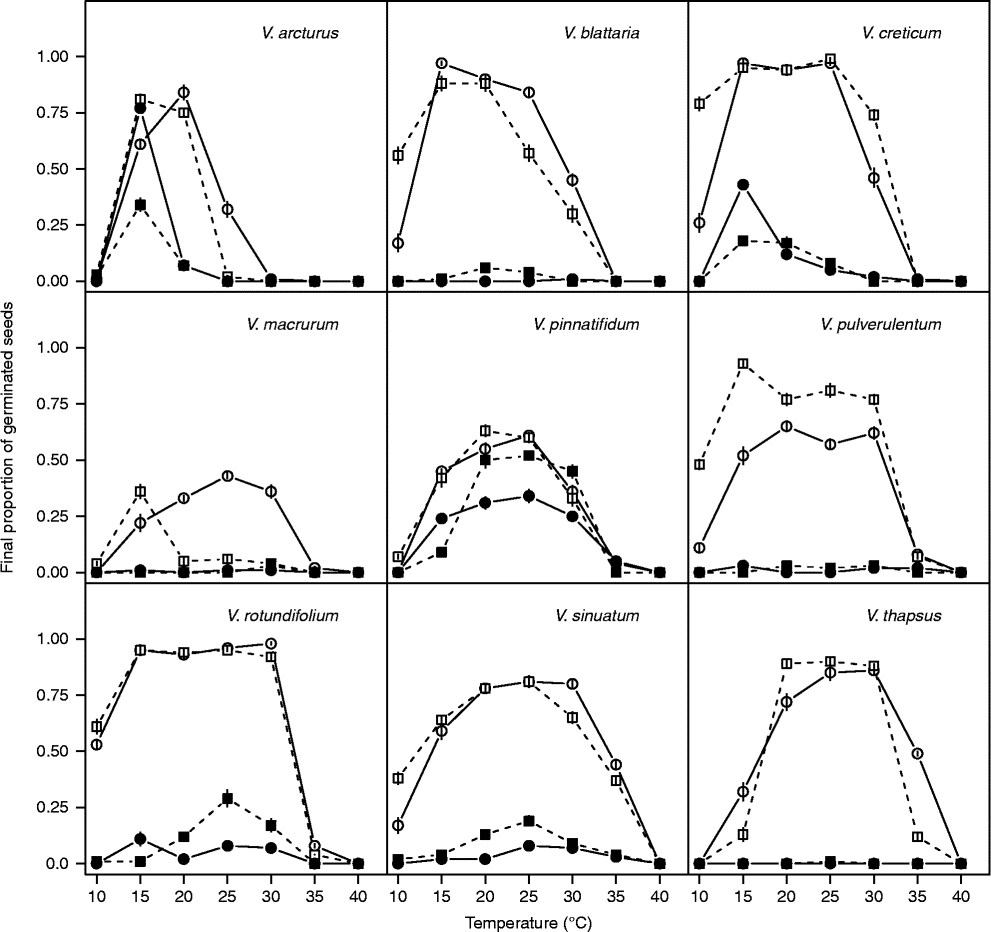
Considering germination capacity, as measured by the FGPs, there was a significant ‘temperature × light × storage’ interaction (P < 10− 16). Light preference was detected in all Verbascum species; indeed, seed germination was strongly suppressed under continuous darkness at all constant temperature treatments, though such a suppression was less dramatic with V. arcturus and V. pinnatifidum (Fig. 1). In the case of the L/D (12/12 h light/dark), the effect of storage was very small with V. creticum, V. pinnatifidum, V. rotundifolium, V. sinuatum and V. thapsus, while in the case of all other species germination increased slightly after long storage, with very low or very high temperature regimes, except in the case of V. pulverulentum which showed decreased germination capacity with long storage at temperatures from 10°C to 30°C. Also, in the case of the alternating light regime, a main part of seeds of all species were able to germinate 2 months after collection, with the highest germination capacity from 15 to 30°C. FGPs dropped suddenly at high temperatures and became negligible in all cases at 30°C (V. arcturus), 35°C (V. blattaria, V. creticum and V. macrurum) or 40°C (all other species). On average, FGPs at 20–25°C ranged from 0.75 to 1 in all species except V. macrurum, V. pinnatifidum and V. pulverulentum (this latter species only with long storage), in which they were between 0.40 and 0.60.

Figure 1 Final germinated proportion (at 15 d after the beginning of assays) for nine species of Verbascum at two storage periods, two light conditions and seven constant temperatures. Open symbols: alternating light/darkness; closed symbols: continuous darkness; squares: short storage; circles: long storage; vertical bars: standard errors.
Germination rates at constant temperature regimes
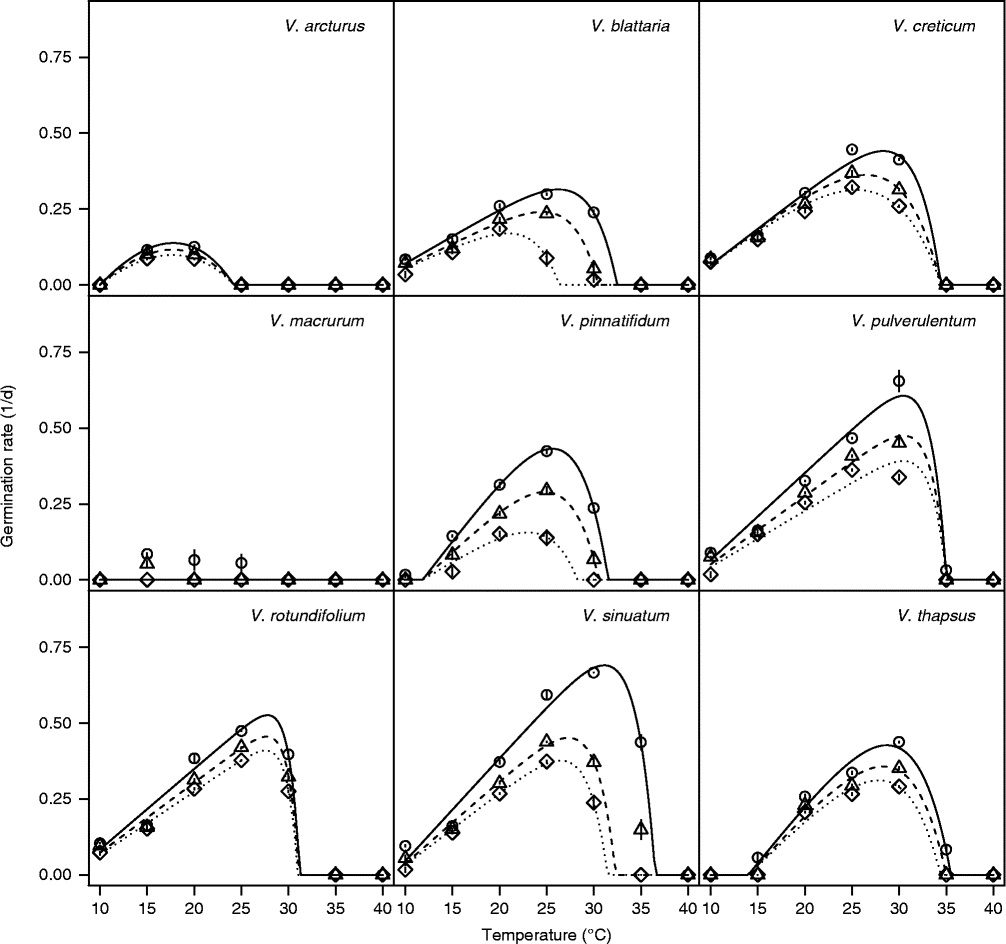
The threshold model in equation (1) always provided a good description of the effect of constant temperatures on GRs (Figs 2 and 3). The list of estimated parameters together with robust standard errors is reported as supplementary material (Table S1). In most cases, only a and T c (and consequently T o) varied with the germination percentile, while in a few cases (V. arcturus, V. creticum and V. pulverulentum with short storage and V. pinnatifidum with long storage) there was no evidence to support the dependency of threshold temperatures on germination percentile. In one case, there was evidence that T b (and not T c) varied with germination percentile (V. macrurum with long storage).

Figure 2 Germination rates (inverse of germination times) at seven constant temperatures for the 10th, 30th and 50th percentiles, as derived by interpolation from the cumulative germination curves (Covell et al., Reference Covell, Ellis, Roberts and Summerfield1986). This dataset refers to the alternating light regime and short storage time. Symbols show observed data (circle: GR10; triangle: GR30; diamond: GR50), while lines show fitted equations.

Figure 3 Germination rates (inverse of germination times) at seven constant temperatures for the 10th, 30th and 50th percentiles, as derived by interpolation from the cumulative germination curves (Covell et al., Reference Covell, Ellis, Roberts and Summerfield1986). This dataset refers to the alternating light regime and long storage time. Symbols show observed data (circle: GR10; triangle: GR30; diamond: GR50), while lines show fitted equations.
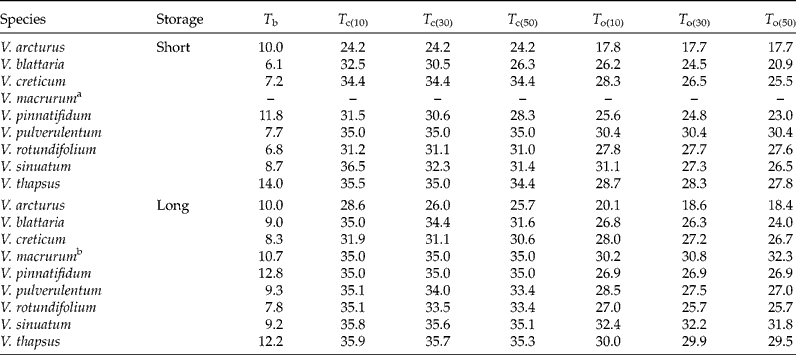
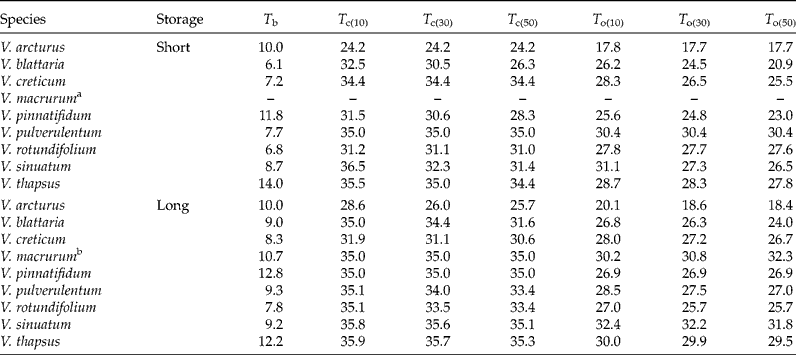
With short storage time (Fig. 2), V. macrurum showed very low GR values and it hardly reached 30% germination capacity, so that equation (1) could not be fitted. Apart from this species, in all other cases seed germination was relatively fast at near-to-optimal temperatures, with observed t 50 values (days ± SEs) of 11.7 ± 0.72 (V. arcturus), 5.5 ± 0.46 (V. blattaria), 3.1 ± 0.24 (V. creticum), 5.8 ± 1.04 (V. pinnatifidum), 2.8 ± 0.06 (V. pulverulentum), 2.7 ± 0.03 (V. rotundifolium), 2.7 ± 0.06 (V. sinuatum) and 3.4 ± 0.14 (V. thapsus). The estimated T b levels ranged from below 7 (V. blattaria and V. rotundifolium) to above 10°C (V. thapsus and V. pinnatifidum) while T c levels were often above 30°C. Considering T o for the 30th percentile, the ranking among species was: V. arcturus < V. blattaria < V. pinnatifidum < V. rotundifolium < V. sinuatum < V. creticum < V. thapsus < V. pulverulentum.
With long storage time (Fig. 3), the situation was very similar and the observed t 50s ( ± SEs) were 11.3 ± 0.44 (V. arcturus), 4.3 ± 0.22 (V. blattaria), 3.3 ± 0.14 (V. creticum), 3.3 ± 0.24 (V. pinnatifidum), 4.8 ± 0.38 (V. pulverulentum), 2.7 ± 0.01 (V. rotundifolium), 2.7 ± 0.01 (V. sinuatum) and 3.6 ± 0.06 (V. thapsus). As shown previously (Fig. 1), V. macrurum hardly reached 50% germination at any temperature. It may be interesting to note that all these germination times are similar to those observed with short storage time, except for V. blattaria and V. pinnatifidum, which were quicker with long storage time, while V. pulverulentum was quicker with short storage time. Almost all threshold temperatures proved to be slightly higher than with short storage time (Table 2). The ranking in optimal temperature for the 30th percentile was V. arcturus < V. rotundifolium < V. blattaria < V. pinnatifidum < V. creticum < V. pulverulentum < V. thapsus < V. macrurum < V. sinuatum, which is fairly consistent with that observed with short storage time, apart from V. pulverulentum and V. rotundifolium which became less demanding in terms of temperatures with increased duration of storage.
Table 2 Threshold germination temperatures for nine species of Verbascum, as estimated from the threshold model in equation (1). Apart from base temperature, all the thresholds were different for the different germination percentiles (10, 30 and 50%). The complete set of estimated parameters together with standard errors are reported in supplementary Table S1
a In the short storage time V. macrurum showed very low GR values and it hardly reached 30% germination capacity, so that equation (1) could not be fitted.
b In the case of V. macrurum, base temperatures were different for the 10th, 30th and 50th germination percentiles and equal to 10.7, 16.5 and 26.9°C, respectively.
Germination responses at fluctuating temperatures
In some cases, germination was higher when seeds were exposed to fluctuating temperature regimes, with respect to constant regimes. With the L/D regime, germination capacity of V. macrurum was always better with fluctuating temperatures than the maximum level observed at constant temperatures (Fig. 4). V. macrurum, with little or no germination at low and high constant temperatures (Fig. 1), responded positively to temperature fluctuation with a large amplitude (5/30 and 10/35°C). In the case of V. pulverulentum, such an effect was only observed with long storage, at all fluctuating temperatures except 10/35°C. Likewise, V. pinnatifidium did not germinate when the daytime temperature was 35°C (i.e. 10/35°C) which is higher than the corresponding T c. V. arcturus responded negatively to temperature fluctuation with an amplitude of 20 and 25 (i.e. 5/30, 10/35°C) (Fig. 4). The increase in germination capacity was much more evident in continuous darkness, where most species (except V. arcturus and, only with short storage, V. pinnatifidum) reached FGPs almost similar to those observed in the L/D regime (Fig. 5). Other species, such as V. creticum, V. rotundifolium and V. sinuatum, showed a full germination response to all the combinations of alternating temperature regimes with both a large and a small amplitude, as well as to all average temperatures.

Figure 4 Final germinated proportion (FGP at 15 d after the beginning of assays) for nine species of Verbascum at two storage periods, seven fluctuating temperatures and an alternating light regime (photoperiod of 12/12 h, with the highest temperature during daytime). At the extreme right of each graph, maximum observed FGPs (MAX) at constant temperatures are added for the sake of comparison. White bars: short storage; grey bars: long storage; vertical lines: standard errors.

Figure 5 Final germinated proportion (FGP at 15 d after the beginning of assays) for nine species of Verbascum at two storage periods, seven fluctuating temperatures and continuous darkness regime (thermoperiod of 12/12 h). Maximum observed FGPs (MAX) at constant temperatures are added for the sake of comparison. White bars: short storage; grey bars: long storage; vertical lines: standard errors.
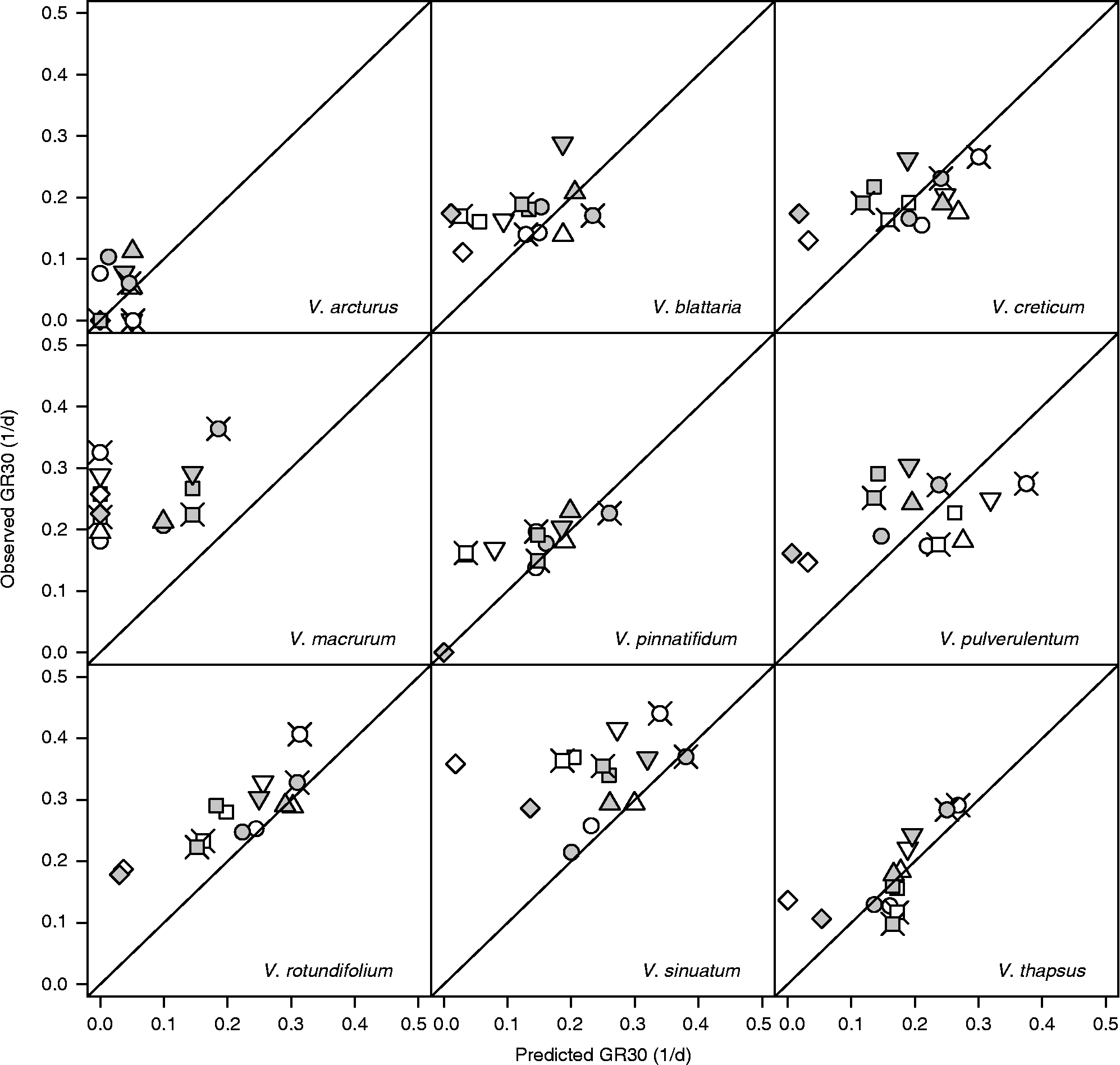
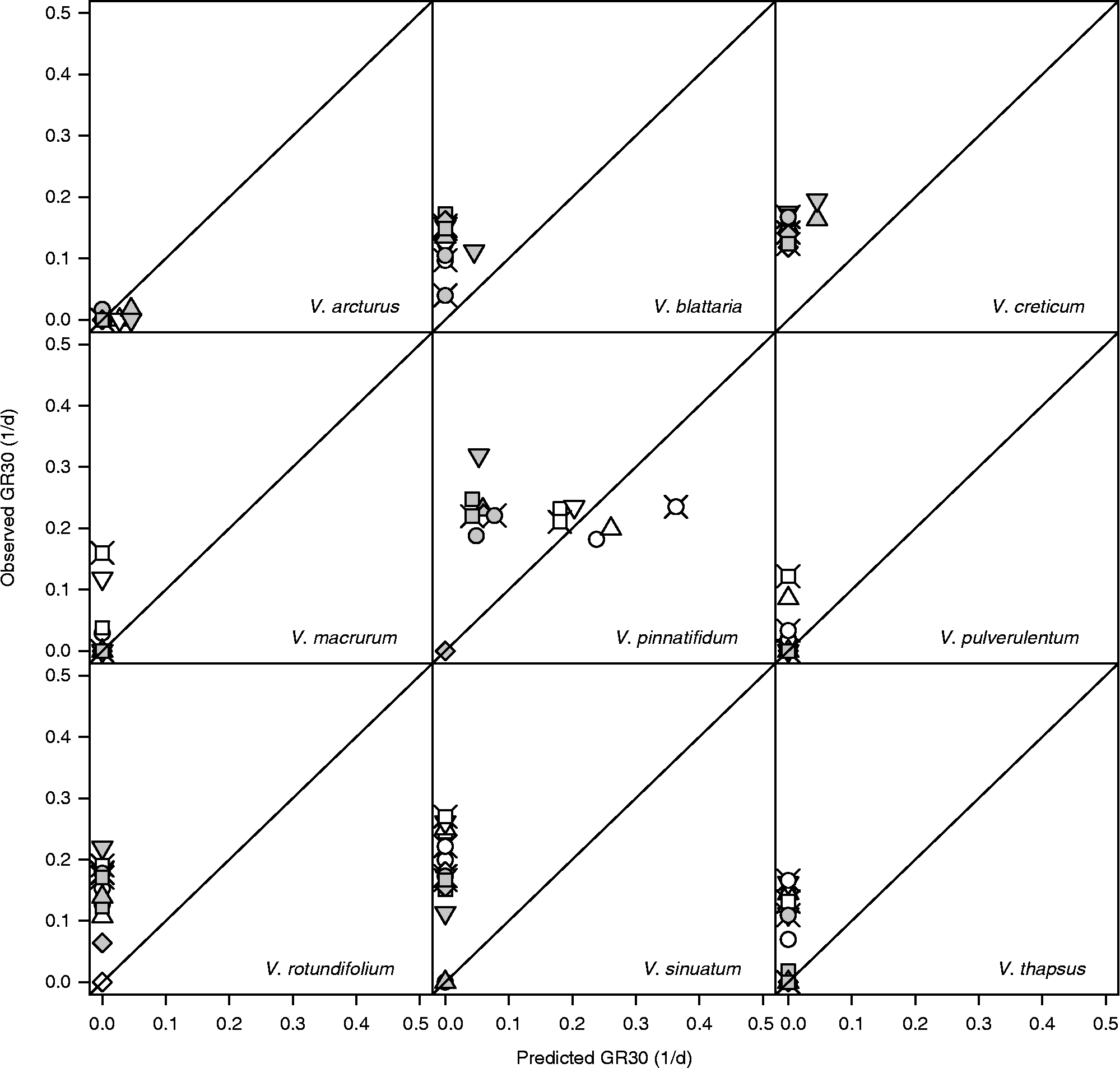
The above comparison with FGPs observed at near-to-optimal constant temperatures may be misleading because it is performed against sub-optimal alternating temperature conditions. A more appropriate comparison should be performed by considering the thermal-time concept and comparing the observed germination rates for the 10th, 30th and 50th percentiles (GR10, GR30 and GR50 as d− 1) with those predicted by using equation (1). These latter predictions assume that germination rates are only determined by the prevailing temperatures and therefore they should be sensibly underestimated/overestimated if the thermal history plays a relevant role. Figure 6 displays the results relating to GR30 at the alternating light regime (results relating to GR10 and GR50 are substantially similar): data lying above the diagonal show a ‘stimulating’ effect of alternating temperatures with respect to the predictions of the thermal-time model.
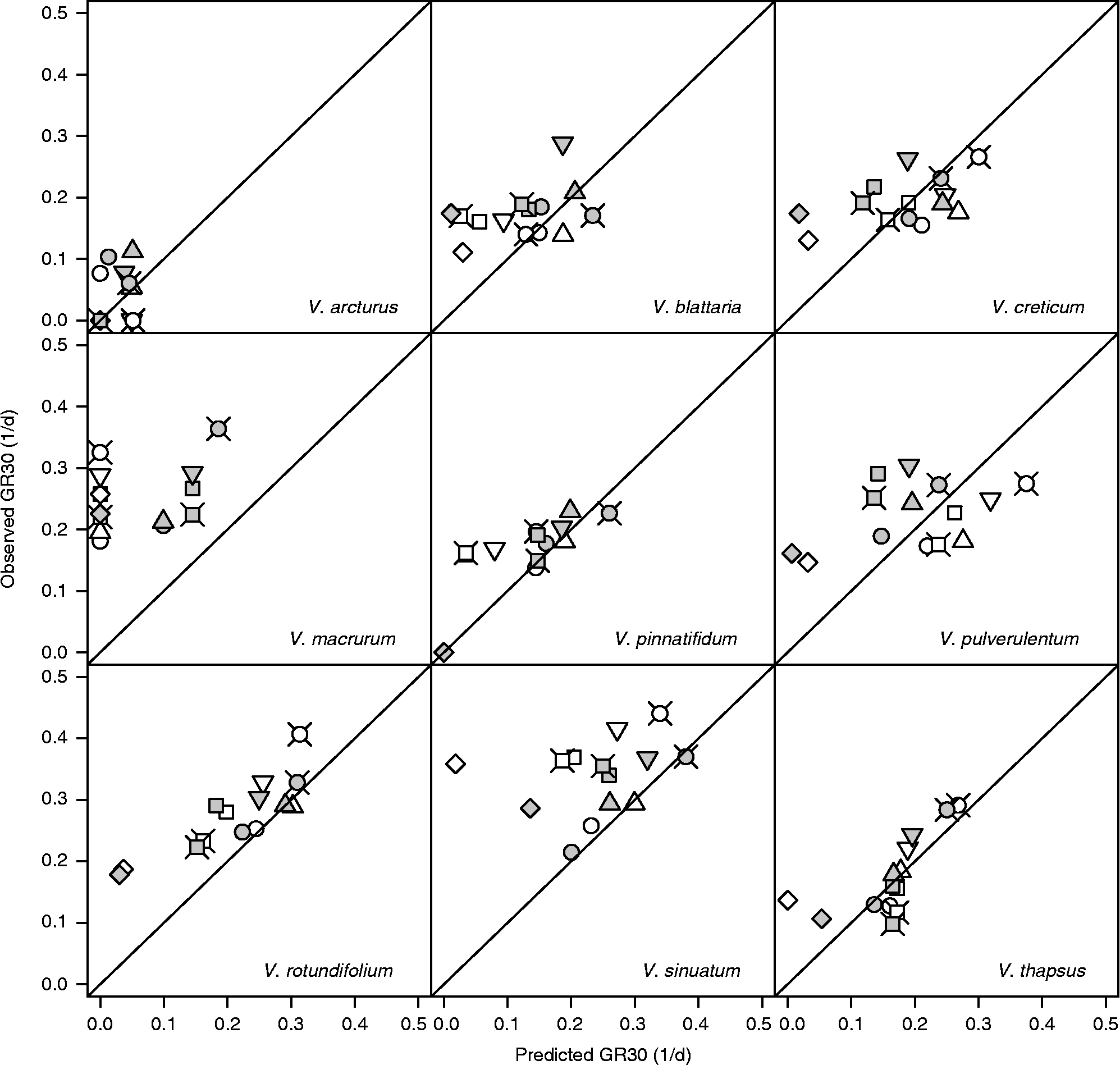
Figure 6 Observed and predicted GR30s [germination rates for the 30th percentile, as obtained from equation (1)] for nine species of Verbascum at fluctuating temperatures, alternating light and after short (white symbols) and long (grey symbols) storage. Circles: 10–25°C; squares: 10–30°C; diamonds: 10–35°C; triangles: 15–25°C; inverted triangles: 15–30°C; crossed circles: 20–30°C; crossed squares: 5–30°C.
In the presence of light, the germination of V. arcturus was always rather slow and at all alternating temperature regimes it did not deviate significantly from model predictions. V. macrurum always germinated faster with respect to model predictions. As for the other species, the situation is less clear and it may be useful to consider the level of maximum daily temperature. When this level was 25°C (10/25 and 15/25, i.e. when both temperatures were sub-optimal) none of the species appeared to deviate from model predictions. On the other hand, when the maximum temperature was 35°C (10/35, i.e. when temperatures were respectively slightly above T b and slightly below T c(30)), all the species germinated faster than expected on the basis of the thermal-time model. When the maximum temperature was 30°C, the germination of V. thapsus was never stimulated, while the germination of the other species was faster than expected only at some temperature regimes with long storage time. In other words, it would appear that, apart from V. arcturus, which was rather insensitive to thermal history, and V. macrurum, which always proved very sensitive, the germination speed of all the other species was increased with fluctuating temperatures, but conditional to a high temperature range, including both the sub-optimal and the supra-optimal range. Considering continuous darkness (Fig. 7), while the model did not predict any germination, observed rates were comparable to those observed in light, except in the case of V. pinnatifidum. Indeed, this latter species showed good germination rates in darkness at constant temperatures (compare with Fig. 1) and these rates were enhanced at fluctuating temperatures only in the case of long storage.
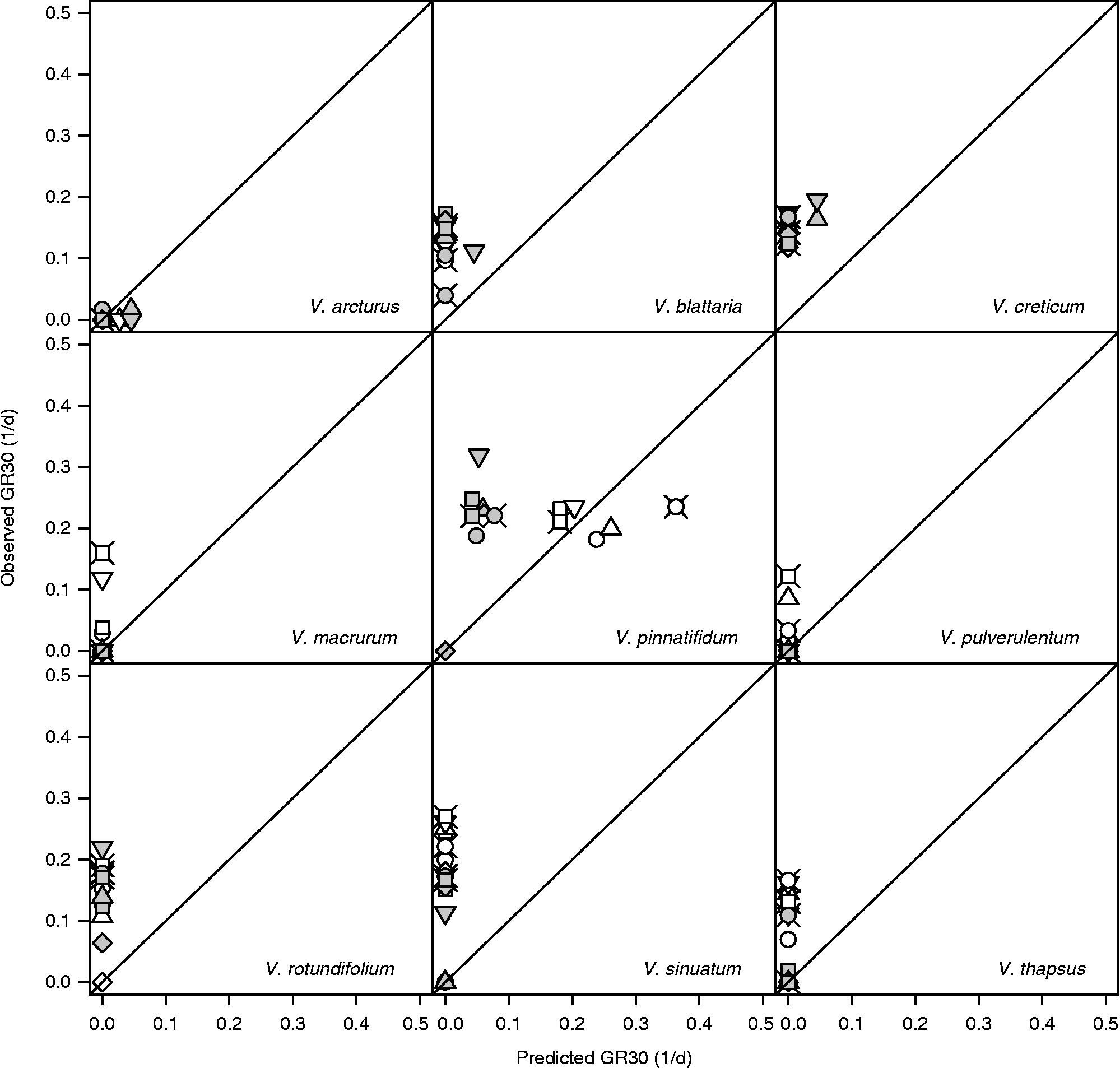
Figure 7 Observed and predicted GR30s [germination rates for the 30th percentile, as obtained from equation (1)] for nine species of Verbascum at fluctuating temperatures, continuous darkness and after short (white symbols) and long (grey symbols) storage. Circles: 10–25°C; squares: 10–30°C; diamonds: 10–35°C; triangles: 15–25°C; inverted triangles: 15–30°C; crossed circles: 20–30°C; crossed squares: 5–30°C.
Classification of species
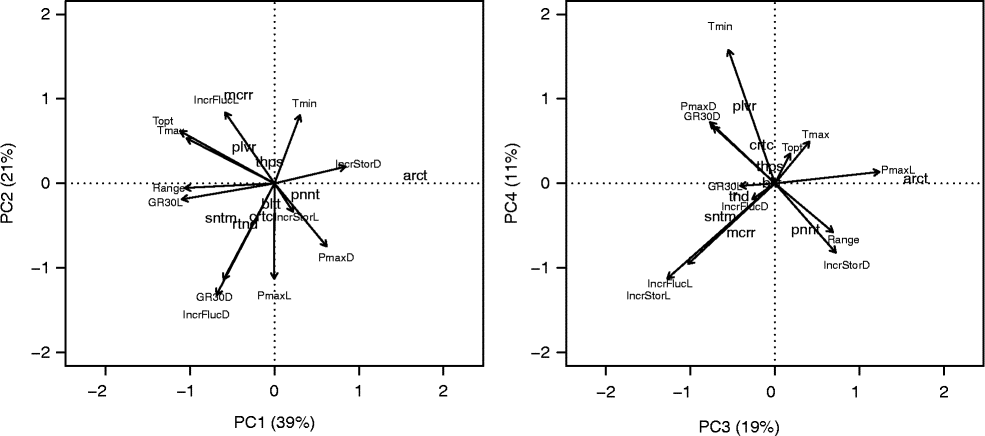
The 12 variables submitted to PCA were not highly correlated among each other, and thus four PCs were necessary to represent 90% of the variability of the original data. On the first two PCs (Fig. 8, left), V. arcturus is represented as an isolated observation due to its high germination capacity in darkness and high positive effect of storage time on PmaxD (average maximum germination capacity with continuous darkness, constant temperature and short storage time). On the other hand, this species showed very low optimal temperature, T c and range. Also on the first two PCs, V. macrurum appears to form a group by itself due to its very high sensitivity to fluctuating temperatures, high T o and T c and very high light requirement. V. sinuatum, V. rotundifolium, V. creticum and V. blattaria were mainly characterized by high germination capacity and speed in light, low capacity and speed in darkness, but with a sensible improvement with fluctuating temperatures. All the other species are far less characterized. V. pinnatifidum lies on the same part of the biplot as V. arcturus because of its high germination capacity in the dark, which is, however, counterbalanced by higher range and storage effect in the dark (see the third and fourth components in Fig. 8, right) and high germination speed. On the other hand, V. pulverulentum and V. thapsus are more similar to V. macrurum as regards their high thermal requirements, but with higher germination capacity.
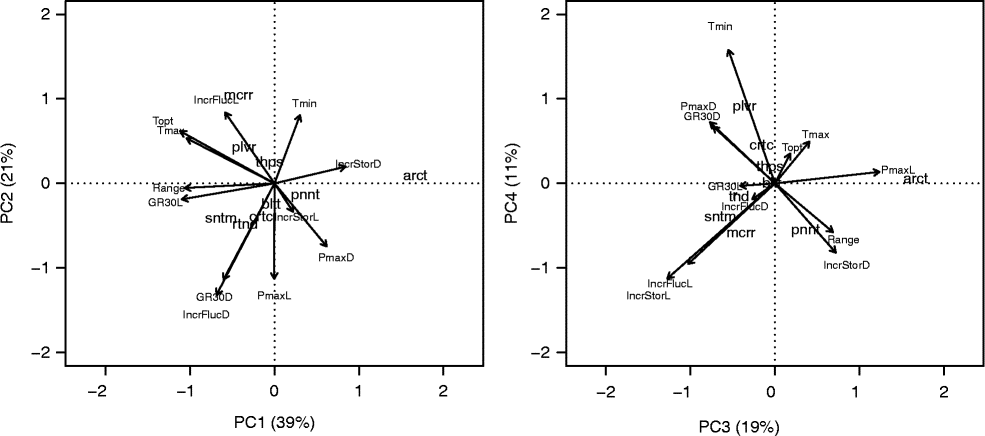
Figure 8 ‘Distance’ biplot from the principal component analysis for germination characteristics of nine species of the genus Verbascum. Species scores are represented by codes (arct: V. arcturus; bltt: V. blattaria; crtc: V. creticum; mcrr: V. macrurum; pnnt: V. pinnatifidum; plvr: V. pulverulentum; rtnd: V. rotundifolium; sntm: V. sinuatum; thps: V. thapsus), while variable scores are represented by arrows. PmaxL: average maximum germination capacity with alternating light, constant temperature and short storage time; PmaxD: same as PmaxL, but in continuous darkness; IncrStorL: absolute increase in average PmaxL with long storage; IncrStorD absolute increase in average PmaxD with long storage; GR30L: average observed GR30 with alternating light, fluctuating temperature and short storage; GR30D: average observed GR30 with darkness, fluctuating temperature and short storage time; IncrFlucL: absolute increase in predicted GR30 with respect to the observed GR30L, with fluctuating temperatures in alternating light; IncrFlucD: absolute increase in predicted GR30 with respect to the observed GR30D, with fluctuating temperatures in darkness; Tmin: base temperature (Tb(30)); Tmax: cut-off temperature (Tcut(30)); Topt: optimal temperature (To(30)); Range: range between base temperature and cut-off temperature. All threshold temperatures are estimated with alternating light and short storage. See text for more details.
Discussion
This paper presents new data on the germination behaviour of nine species belonging to the genus Verbascum. Seven of these species were selected after preliminary surveys, as they should represent the whole list of species in our region. Two other species could be found at the Botanical Garden of the University of Catania and, although these species are not found in natural conditions in our region, they were included in this study to enlarge the range of geographical distributions, life forms and growing habitats. As we worked with only one population per species, we cannot draw comprehensive conclusions relating to the ecophysiology of seed germination for the whole genus Verbascum under Mediterranean conditions, but, nonetheless, we are confident that the wide list of our selected taxa can provide a good overview about the variability of behaviour between species.
Light appears to play a key ecological role in the germination of Verbascum, particularly for V. blattaria, V. pulverulentum and V. thapsus. The sensitivity of seeds to light is common within species that colonize open areas (Ballaré, Reference Ballaré, Caldwell and Pearcy1994) and may give an adaptive advantage to pioneer ruderal species, colonizing roadsides, building sites and rubbish dumps, where the existing vegetation canopy is of low density. Several authors have also shown that high light requirements for seed germination are typical of species that produce numerous small seeds, with long persistence in the soil (Grime et al., Reference Grime, Mason, Curtis, Rodman, Band, Mowforth, Neal and Shaw1981; Vázquez-Yanes and Orozco-Segovia, Reference Vázquez-Yanes, Orozco-Segovia, Caldwell and Pearcy1994; Milberg et al., Reference Milberg, Andersson and Thompson2000; Baskin and Baskin, Reference Baskin and Baskin2014), which should be the case for Verbascum (Thompson and Grime, Reference Thompson and Grime1979; Thompson, Reference Thompson, Hendry and Grime1993). In this study we did not consider soil persistence, although we noted that, 10 months after harvest, the final germinated proportions of all species, except V. pulverulentum, were equal to or higher than those observed immediately after harvest.
As shown by Gardner (Reference Gardner1921) with V. thapsus, light sensitivity can persist for long periods of time during seed storage, which is confirmed by our results, as we did not notice any increase in germination capacity in dark conditions 10 months after seed collection, apart from the cases of V. arcturus (40% increase) and, to a lesser extent, V. creticum (24% increase). V. pinnatifidum was positively affected by light, though it also proved able to germinate in the dark, similarly to other psammophilous plants (Thanos et al., Reference Thanos, Georghiou, Douma and Marangaki1991). In general, V. arcturus and V. pinnatifidum proved less sensitive to light with respect to the other species, which might give them the ability of germinating in rocky areas or sandy dunes, respectively (where these species grow), also when they are not directly exposed to sunlight, e.g. because they are buried in cliff crevices (V. arcturus) or under a layer of sand (V. pinnatifidum).
In the presence of light, with the exception of V. arcturus, all the other species included in this experiment showed a good germinating ability in a wide range of temperatures. This confirms and extends the validity of the results obtained by other authors with V. thapsus (Semenza et al., Reference Semenza, Young and Evans1978) and explains why, in temperate climates, these species can germinate at any time from spring to early autumn and in a wide range of locations, altitudes and environments (Baskin and Baskin, Reference Baskin and Baskin1981). On the other hand, in Mediterranean climates, germination in summer under field conditions is usually prevented by the dry condition of soils. In the case of V. thapsus, T b was higher than that of other species, which might prevent germination in autumn, especially at high altitudes (1800–2000 m) (Baskin and Baskin, Reference Baskin and Baskin1981; Gross and Werner, Reference Gross and Werner1982). This has been regarded as an adaptive process, by which plants can ‘compensate’ for their incapacity to achieve sufficient biomass to survive the winter (see, for example, Maruta, Reference Maruta1983, Reference Maruta1994).
The very high Tc values (>30°C) observed for most of these species are not common for Mediterranean plants, where germination mainly takes place at low temperatures (Thanos and Doussi, Reference Thanos and Doussi1995). In this respect, V. arcturus seems to be more ‘normal’, with a T c lower than 20°C. High T c values make the germination range very wide for these species, particularly for V. rotundifolium, which supports the idea that these species might show a good ability to respond to climate changes or anthropogenic management (Cochrane et al., Reference Cochrane, Yates, Hoyle and Nicotra2014), thanks to their ability to survive in several environmental niches.
There was a strong interaction between light and temperature, and the need for light during germination was partly released in the case of fluctuating temperatures, particularly when the range of fluctuation was very wide. Literature data relating to laboratory experiments with other species have demonstrated that germination of light-requiring seeds can be modulated by temperature conditions, especially diurnal temperature fluctuations (Thompson and Grime, Reference Thompson and Grime1983; Vázquez-Yanes and Orozco-Segovia, Reference Vázquez-Yanes, Orozco-Segovia, Caldwell and Pearcy1994; Koutsovoulou et al., Reference Koutsovoulou, Daws and Thanos2014). The results of Demel (Reference Demel1996) and Demel and Granström (Reference Demel and Granström1997), working with seeds of Achyrospermum, Conyza, Laggera, Phytolacca and Urera, confirm that the effect of alternating temperatures is the highest when the range of fluctuation is wide. The capacity of seeds of certain species to germinate in response to diurnal temperature fluctuations has been regarded as a mechanism of depth-sensing and gap detection by buried seeds (Demel, Reference Demel2005; Koutsovoulou et al., Reference Koutsovoulou, Daws and Thanos2014). Indeed, for seeds that are buried near the soil surface, and thus not directly exposed to light, the diurnal fluctuations of temperature are lower below a thick vegetation cover than on bare soils (Balisky and Burton, Reference Balisky and Burton1993). This might give buried seeds the ability to germinate when they are close to the soil surface and in the presence of vegetation gaps (Thompson et al., Reference Thompson, Grime and Mason1977; Pons and Schröder, Reference Pons and Schröder1986), which may frequently happen in disturbed ground. In this respect, seeds of V. macrurum showed a remarkably increased germination capability with fluctuating temperatures, especially with very large amplitudes, which might explain its widespread occurrence in disturbed ground.
The threshold model in equation (1) proved to flexibly describe several types of patterns by allowing parameters to change with germination percentiles. In most cases, this threshold model closely resembled the hydrothermal-time model proposed by Rowse and Finch-Savage (Reference Rowse and Finch-Savage2003) and modified by Mesgaran et al. (Reference Mesgaran, Mashhadi, Alizadeh, Hunt, Young and Cousens2013). Other similar types of threshold models have recently been proposed by Wang et al. (Reference Wang, Cheng and Song2013) and Parmoon et al. (Reference Parmoon, Moosavi, Akbari and Ebadi2015). Indeed, when only a and T c vary within a seed lot, equation (1) depicts a relationship where T b is common to all the seeds within the lot, while the increase in GR g in the suboptimal temperature range is higher for the lowest percentiles. Above T o, the decrease in GR g is similar for all seeds, which implies that cut-off temperatures will be higher for the lowest germination percentiles.
With the exception of V. macrurum, which showed high intra-population variability in T b, our results are in agreement with the most widely accepted assumption of a common T b value for the entire seed population (Bradford, Reference Bradford, Kigel and Galili1995). At the same time, T c and consequently T o varied among different seed fractions, in almost all species (with the exception of V. arcturus), especially in long storage. From an ecological perspective, this situation could be associated with a distinct genetic variability within the seed population and might be considered as an adaptive strategy, where the different fractions of the population accumulate thermal-time at distinct rates, spreading germination over time and thus increasing the probability of seedling establishment (Chantre et al., Reference Chantre, Batlla, Sabbatini and Orioli2009; Cristaudo et al., Reference Cristaudo, Gresta, Catara and Mingo2014a, b).
However, equation (1) cannot describe a germination time-course, unless some of the parameters are given a frequency distribution across percentiles. So far, this result has been attained by assuming that the base osmotic potential within the seed lot follows a normal (Bradford, Reference Bradford2005), log-logistic (Mesgaran et al., Reference Mesgaran, Mashhadi, Alizadeh, Hunt, Young and Cousens2013) or other types of distribution. None of the modelling approaches proposed so far appeared to work in practice with this dataset. We postulate that the current approach taken in the literature to describe the effect of sub- and supra-optimal temperatures on the time course of germination might be overly simplistic, but more research is needed to clear this up.
Fluctuating temperatures, light regime and storage time are other factors of concern in terms of modelling and, in this case, sensibly different threshold parameters were established. These aspects should not be neglected and we argue that parameters estimated from germination assays at constant conditions are not very likely to work well in natural environments. Considering the wide range of variability in the fluctuation of temperature and light conditions, building models that predict seed germination reliably in natural environments might be a difficult task.
In general, this study confirms the difficulties that are inherent to germination studies, in relation both to (1) the selection of an efficient experimental plan and (2) the interpretation of results. With reference to the first issue, it is not always easy to sample appropriately from the possible range of temperatures and daily fluctuation regimes that are found in natural conditions, while the complex pattern of interaction between environmental factors makes the interpretation of results an often daunting task. In any case, knowledge about the ecophysiology of seed germination seems to be fundamental, either to develop meaningful germination models or to support the rational use of these species of Verbascum in gardening and landscaping plans, or for the conservation of biodiversity in natural populations.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0960258515000343
Acknowledgements
The authors are grateful to three anonymous reviewers and the associate editor for useful suggestions.
Financial support
This research was supported by the Project P.O.N. 2007-2013 (grant number PON01_01 611) ‘Sustainable production of pot plant in Mediterranean environment (SO.PRO.ME.)’.
Conflicts of interest
None.