Introduction
Temperature is a major environmental factor responsible for the timing of seed germination and for changes in dormancy states (Baskin and Baskin, Reference Baskin and Baskin2014). Under a Mediterranean climate, characterized by a high seasonality with hot dry summers and cold wet winters (Joffre et al., Reference Joffre, Rambal, Damesin, Pugnaire and Valladares1999; Valladares and Sánchez-Gómez, Reference Valladares and Sánchez-Gómez2006), plant reproduction must occur in a window of favourable conditions that may vary in length, particularly with altitude, and in which environmental cues and constraints play a central role (Thanos et al., Reference Thanos, Costas and Skarou1995; Debussche et al., Reference Debussche, Garnier and Thompson2004; Gresta et al., Reference Gresta, Cristaudo, Onofri, Restuccia and Avola2010). A ‘Mediterranean germination syndrome’ has been identified for typical Mediterranean coastal species (Thanos et al., Reference Thanos, Georghiou, Dimitra and Marangaki1991; Skordilis and Thanos, Reference Skordilis and Thanos1995; Thanos et al., Reference Thanos, Costas and Skarou1995; Doussi and Thanos, Reference Doussi and Thanos2002). This seed germination pattern is characterized by low optimal germination temperatures (≤15°C; Thanos et al., Reference Thanos, Georghiou and Skarou1989) and slow germination rate (Kadis, Reference Kadis1995; Doussi and Thanos, Reference Doussi and Thanos2002). It represents an advantageous ecological adaptation of species, as it ensures that germination occurs well into the wet season, in late autumn, and maximizes the length of the growing season before the onset of summer drought (Doussi and Thanos, Reference Doussi and Thanos2002). Within this ecological context, dry after-ripening, i.e. a period of warm temperatures under dry conditions, may be considered a favourable treatment for seeds of Mediterranean coastal species, for which high temperature pre-treatment might increase the germination response (Schütz, Reference Schütz, Breckle, Schweizer and Arndt1999; Pérez-Fernández et al., Reference Pérez-Fernández, Lamont, Marwick and Lamont2000; Baskin and Baskin, Reference Baskin and Baskin2014). On the contrary, cold stratification may have a detrimental effect on seed germination of these species, as reported by Skordilis and Thanos (Reference Skordilis and Thanos1995) for seeds of Pinus halepensis.
However, Mediterranean mountain species are exposed to a different set of environmental conditions and are likely to show different seed germination traits than the typical lowland species. For example, avoiding germination during or immediately before severe winters is important for successful regeneration (Baskin and Baskin, Reference Baskin and Baskin2003; Fenner and Thompson, Reference Fenner and Thompson2005; Baskin and Baskin, Reference Baskin and Baskin2014; Rosbakh and Poschlod, Reference Rosbakh and Poschlod2015) and most alpine species are ‘programmed’ to germinate after snowmelt in spring/early summer, preferring high temperatures for germination (Mooney and Billings, Reference Mooney and Billings1961; Billings and Mooney, Reference Billings and Mooney1968; Körner, Reference Körner1999; Niederfriniger Schlag and Erschbamer, Reference Niederfriniger Schlag and Erschbamer2000; Mondoni et al., Reference Mondoni, Rossi, Orsenigo and Probert2012). Giménez-Benavides et al. (Reference Giménez-Benavides, Escudero and Pérez-García2005) detected that most of the species from high altitude Mediterranean climates readily germinate without treatment, reaching an optimum at relatively high temperatures (20°C). Furthermore, cold-wet stratification increased germination capacity and broke seed dormancy in several species (e.g. García-Fernández et al., Reference García-Fernández, Escudero, Lara-Romero and Iriondo2015; Cuena-Lombraña et al., Reference Cuena-Lombraña, Porceddu, Dettori and Bacchetta2017), as widely reported also for many arctic, boreal and alpine species (Baskin and Baskin, Reference Baskin and Baskin2014).
Theoretical cardinal temperatures for germination have been used to model responses to temperature in crops (e.g. Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982; Covell et al., Reference Covell, Ellis, Roberts and Summerfield1986; Ellis et al., Reference Ellis, Covell, Roberts and Summerfield1986) and wild species (e.g. Pritchard and Manger, Reference Pritchard and Manger1990; Seal et al., Reference Seal, Daws, Flores, Ortega-Baes, Galíndez, León-Lobos, Sandoval, Ceroni Stuva, Ramírez Bullón, Dávila-Aranda, Ordoñez-Salanueva, Yáñez-Espinosa, Ulian, Amosso, Zubani, Torres Bilbao and Pritchard2017; Tudela-Isanta et al., Reference Tudela-Isanta, Ladouceur, Wijayasinghe, Pritchard and Mondoni2018). In these models, seeds accumulate units of thermal time (degree days: °Cd) to germinate above a base temperature for germination (T b), with germination rate generally increasing linearly with temperature to an optimum temperature (T o), above which germination rate decreases (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982). Pritchard et al. (Reference Pritchard, Steadman, Nash and Jones1999) reported that physiological dormancy release in the seeds of a temperate tree, Aesculus hippocastanum, can be described simply in terms of T b reduction gradually allowing germination to occur at progressively lower temperatures, and eventually at the temperature for dormancy alleviation. In the same species, local environment across Europe during seed development had an impact on features of the seeds’ thermal response (Daws et al., Reference Daws, Lydall, Chmielarz, Leprince, Matthews, Thanos and Pritchard2004). Similarly, seed dormancy in Centaurium somedanum varied with local climatic gradient and the seed maturation environment, suggesting that dormancy and germination are influenced by both long- and short-term climatic variation (Fernández-Pascual et al., Reference Fernández-Pascual, Jiménez-Alfaro, Caujapé-Castells, Jaén-Molina and Díaz2013, Reference Fernández-Pascual, Mattana and Pritchard2018). Likewise, Rosbakh and Poschlod (Reference Rosbakh and Poschlod2015), studying species with different distributional ranges in a temperate climate zone, found that the initial temperature of seed germination (i.e. a temperature enabling the first 5% of all seeds to germinate) is negatively correlated with habitat temperature.
The application of threshold models to predict seed germination and dormancy release has been reported in several studies (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982; Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2004; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008; Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015). Moreover, thermal time modelling is a valuable tool to assess and predict seed germination in native species (e.g. Galíndez et al., Reference Galíndez, Seal, Daws, Lindow, Ortega-Baes and Pritchard2017; Seal et al., Reference Seal, Daws, Flores, Ortega-Baes, Galíndez, León-Lobos, Sandoval, Ceroni Stuva, Ramírez Bullón, Dávila-Aranda, Ordoñez-Salanueva, Yáñez-Espinosa, Ulian, Amosso, Zubani, Torres Bilbao and Pritchard2017), including in the field (e.g. Chantre et al., Reference Chantre, Batlla, Sabbatini and Orioli2009) and in relation to the environment (Trudgill et al., Reference Trudgill, Honek, Li and Van Straalen2005). Some general principles have emerged of higher T b values and shorter thermal time requirements for seeds of tropical and temperate species (Tompsett and Kemp, Reference Tompsett and Kemp1996; Trudgill et al., Reference Trudgill, Squire and Thompson2000; Finch-Savage, Reference Finch-Savage, Benech-Arnold and Sánchez2004; Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015). However, little is known about seed thermal thresholds of native Mediterranean species (e.g. Orrù et al., Reference Orrù, Mattana, Pritchard and Bacchetta2012; Porceddu et al., Reference Porceddu, Mattana, Pritchard and Bacchetta2013; Mattana et al., Reference Mattana, Picciau, Puddu, Cuena Lombraña and Bacchetta2016). Although the low optimal germination temperatures and slow germination rate which characterize the ‘Mediterranean germination syndrome’ suggest low values of T b and longer thermal times for typical Mediterranean lowland species, this has not been quantitatively assessed yet. Therefore, the aim of this study was to evaluate the effect of temperature and various pre-treatments (cold and warm wet stratification, dry after-ripening, gibberellic acid) on the thermal threshold for seed germination of Mediterranean lowland and mountain species in the context of their varying natural environment.
Materials and methods
Study areas and species
This study was carried out in Sardinia, the second largest island in the Mediterranean Sea (ca 24,090 km2), from sea level up to 1834 m above sea level (masl) in the highest mountain region (Gennargentu Massif, CE-Sardinia) (Table 1). The experimental sites were chosen to represent a diversity in bioclimatic conditions (Bacchetta et al., Reference Bacchetta, Bagella, Biondi, Farris, Filigheddu and Mossa2009) and geological substrata (Carmignani et al., Reference Carmignani, Oggiano, Barca, Conti, Salvadori, Eltrudis, Funedda and Pasci2001). Species belonging to the most representative families in the Mediterranean area were chosen, with priority given to: (1) exclusive Sardinian endemics; (2) the Sardinian, the Corsican and the Tuscan Archipelago endemics; and (3) Tyrrhenian insular endemics (Table 1).
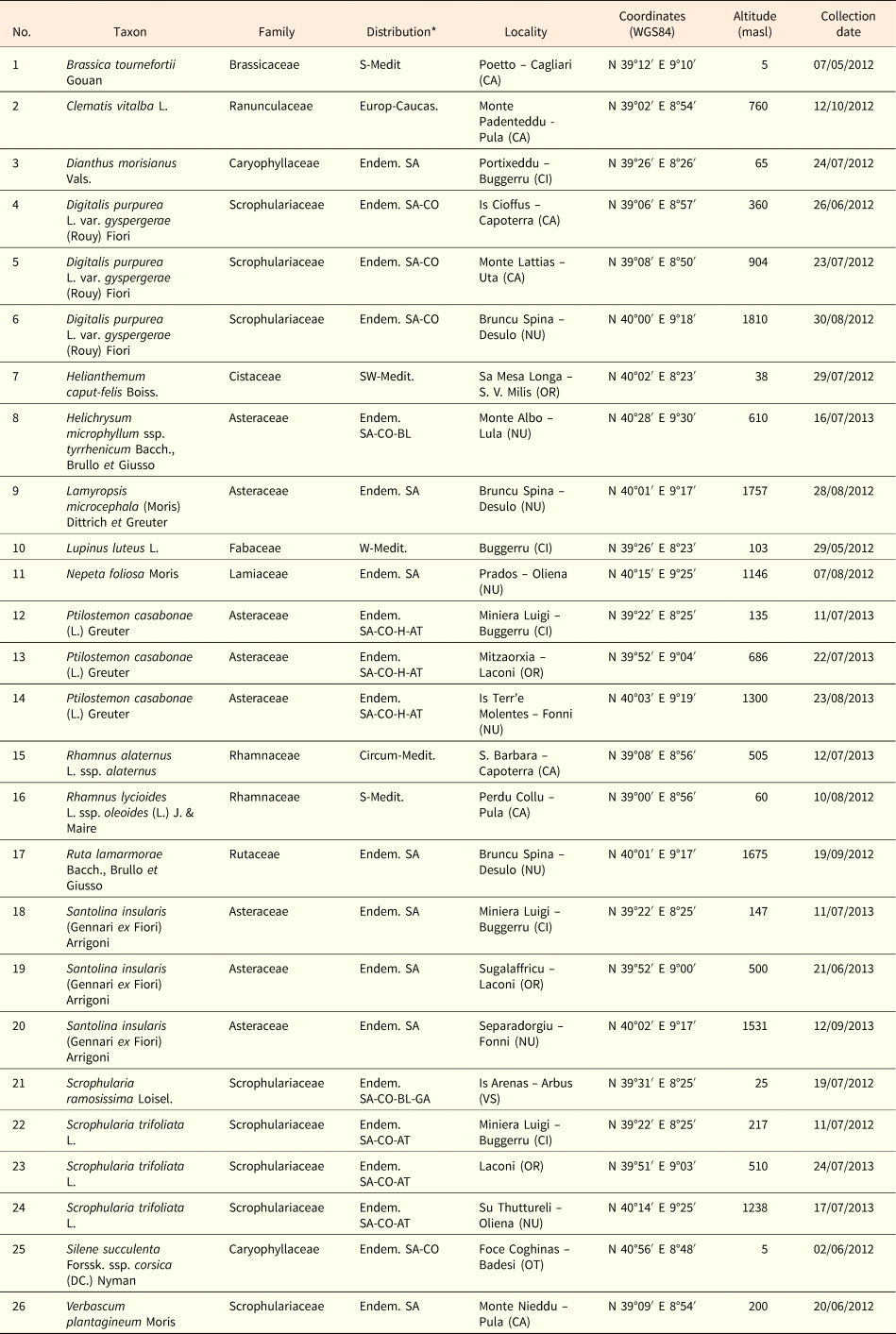
Table 1. Seed lot details and information on the species collected. *Sources: Bacchetta, Reference Bacchetta2006; Bacchetta et al., Reference Bacchetta, Fenu and Mattana2012; Fenu et al., Reference Fenu, Fois, Cañadas and Bacchetta2014. Abbreviations on the endemic species distribution: SA, Sardinia; CO, Corsica; BL, Balearic Islands; GA, France; H, Hyères Islands; AT, Tuscan Archipelago. Species were collected close to the data loggers (see Table 2).

Seed collecting and soil temperature recording
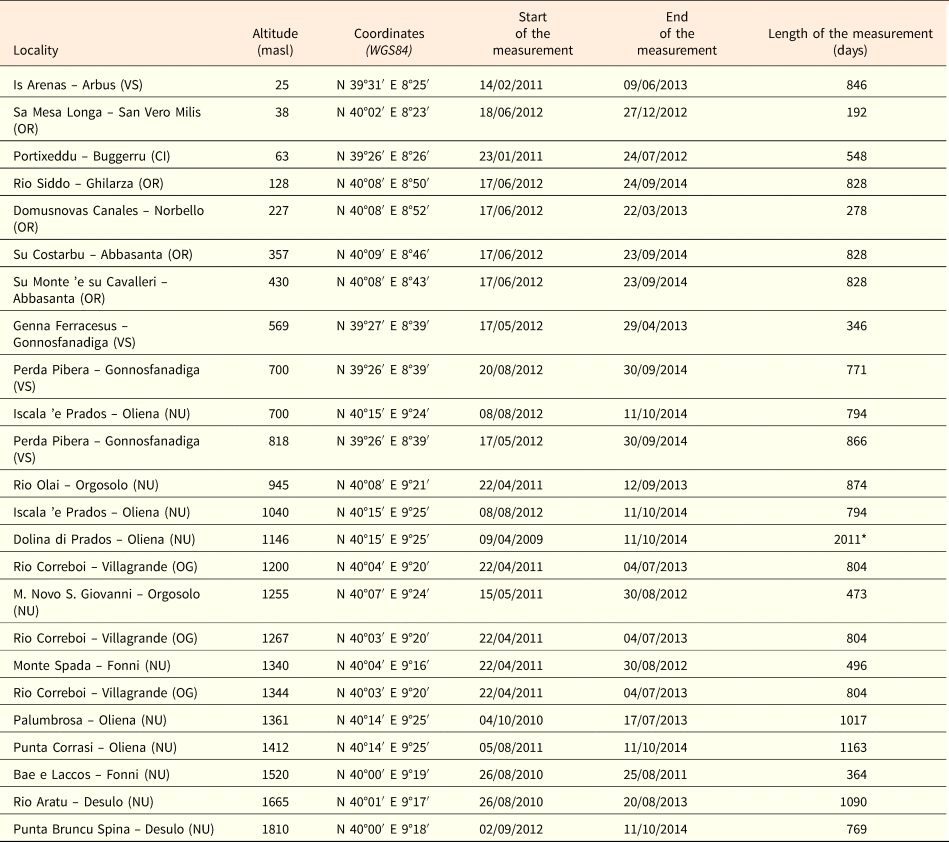
Seeds of 18 species were collected from 26 provenance localities, considering also different populations of the same species (Table 1). Several collecting trips were carried out each year from late spring (May) to autumn (October), during the period 2012–2013 and seeds were collected directly from the plants at the time of natural dispersal (see Table 1). For the species listed in the Habitat Directive annexes, as required by the European and national laws (articles 9 and 10 of DPR 357/97, modified by DPR 120/03), seeds were collected after obtaining permits from the ‘Ministero dell'Ambiente e della Tutela del Territorio e del Mare’. To study and monitor the annual trend of soil temperature, 24 data-loggers (TidbiT v2 temperature logger, Onset Computer Corporation, Cape Cod, MA, USA) were buried at ca 2–3 cm depth, at sites at different altitudes (from ca 25 to ca 1800 masl) and time periods (from April 2009 to September 2012; Table 2). The loggers recorded the soil temperature at 90-min intervals and most of them covered at least two winter and summer seasons (Table 2).
Table 2. Site information and data loggers for soil temperature measurement details.
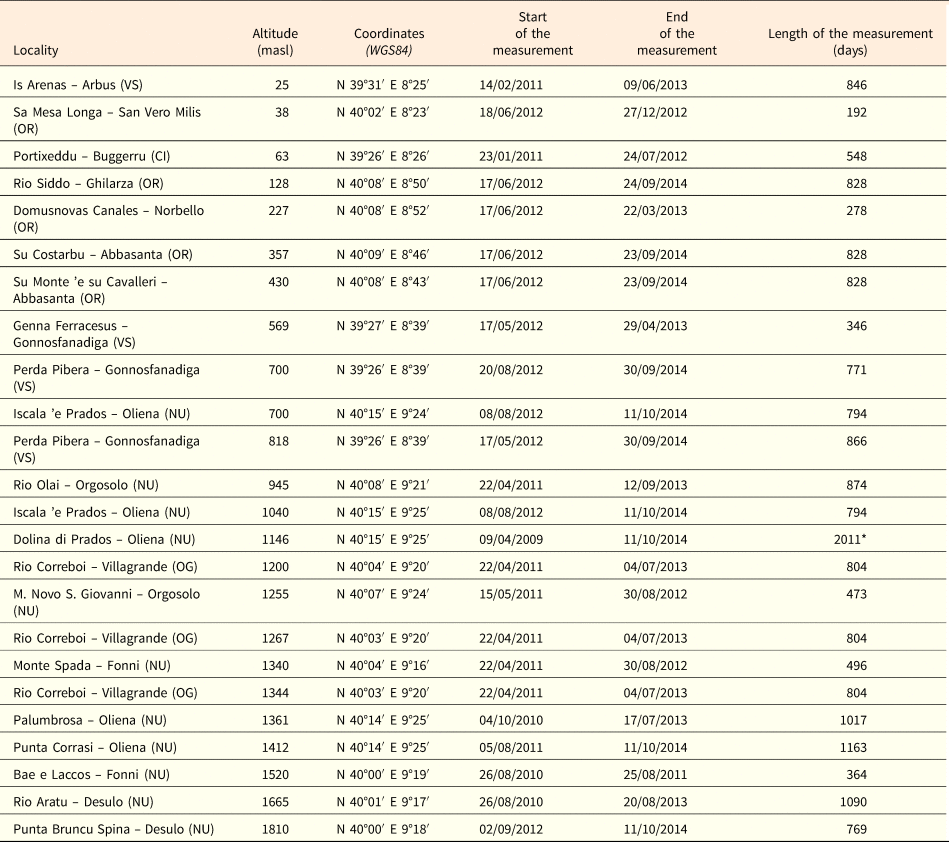
Abbreviations on the province: VS, Medio Campidano; OR, Oristano; CI, Carbonia-Iglesias; UN, Nuoro; OG, Ogliastra. *Data deficient from 19/08/2011 to 08/08/2012.
Germination experiments
According to seed availability, four replicates of 30 seeds (n = 120) or three replicates of 20 seeds (n = 60) were sown on the surface of 1% agar water in 60 or 90 mm diameter plastic Petri dishes for small or large seeds, respectively.
Seeds were incubated for a maximum of 4 months in the light (12 h light/12 h dark) at a range of constant germination temperatures (5, 10, 15, 20 and 25°C) and under an alternating temperature regime (25/10°C), with light applied during the warm phase. In addition, depending on the seed availability and according to prior knowledge of the study species, different pre-treatments were also applied: (1) pre-chilling (‘C’, seeds were incubated for 3 months at 5°C in 1% agar water); (2) pre-warming (‘W’, seeds were incubated for 3 months at 25°C in 1% agar water); and (3) dry after-ripening (‘DAR’, seeds were stored for 3 months at 25°C inside a sealed glass container with silica gel in a ratio seed:silica gel of 1:1). At the end of each pre-treatment, seeds were incubated at the above listed temperatures. During stratifications (i.e. C and W) seeds were incubated in continuous dark achieved by wrapping dishes in aluminium foil and then incubated to the light condition at the germination temperatures specified above. Furthermore, GA3 treatment was applied in the investigated species, with the exception of six taxa (Dianthus morisianus, Helianthemum caput-felis, Lamyropsis microcephala, Lupinus luteus, Rhamnus alaternus ssp. alaternus, Silene succulenta ssp. corsica; Appendix 1), by sowing seeds on the surface of 1% agar water with 250 mg l–1 of GA3 and incubating them in the light (12 h light/12 h dark), at the previously cited temperatures.
Seeds of Helianthemum caput-felis and Lupinus luteus, for which physical dormancy (PY) was reported (Royal Botanic Gardens Kew, 2014), were scarified by chipping with a scalpel, before starting the germination experiments.
Germination, defined as visible radicle emergence (>1 mm), was recorded three times a week. During stratification in the dark, seeds were scored only at the end of the pre-treatment to avoid any exposure to light. At the end of the germination tests, when no additional germination had occurred for 2 weeks, and no less than 1 month from sowing, a cut-test was carried out to determine the viability of the remaining seeds. Firm seeds were considered to be viable.
Data and statistical analyses
Soil temperatures were analysed for winter and summer seasons. The winter season was considered to be from 21 December to 20 March, while summer ran from 21 June to 21 September. The minimum daily temperature, the mean daily temperature and the duration of the cold period (i.e. number of days with mean daily temperatures ≤5°C) were calculated for the winter season. The maximum daily temperature, the mean daily temperature and the duration of the warm period (i.e. days with mean daily temperatures ≥25°C) were calculated for the summer season.
Final germination percentages were calculated on the basis of the total number of filled seeds as the mean of the four or three replicates ± standard deviation (SD) for each tested condition. Seed germination during pre-treatments (i.e. C and W stratifications) was also recorded and when seeds germinated during pre-treatments before moving to the incubation temperatures, these were not considered in the final germination percentages.
Theoretical base temperature for germination (T b) at which the germination rate is equal to zero (Ellis et al., Reference Ellis, Covell, Roberts and Summerfield1986) was evaluated for each seed lot. T b was calculated by determining the seed germination rate, defined as the reciprocal of time to reach 50% of actual germination for the tests carried out at constant temperatures (5–25°C). Subsequently, data were regressed using a linear model, by averaging the x-intercept for the sub-optimal temperature range. However, when 50% of final germination was not reached, the T b value was not established.
The thermal constant (S) expressed in degree days (°Cd), i.e. the thermal requirement to achieve 50% of germination, given by the reciprocal of the slope of the linear regression (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982; Trudgill et al., Reference Trudgill, Squire and Thompson2000), was also calculated for seed lots that reached 50% of final germination after each pre-treatment. Regression analyses were carried out using SigmaPlot version 11.0 (Systat Software, Inc., San Jose, CA, USA).
In addition, the dataset of Trudgill et al. (Reference Trudgill, Squire and Thompson2000), composed of 31 temperate species, was employed to compare their findings with the results of this study.
Effect of pre-treatments, temperatures and their combinations on germination percentages were determined by generalized linear models (GLMs), with a logit link function and a quasi-binomial error structure, using F-tests with an empirical scale parameter to overcome residual overdispersion (Crawley, Reference Crawley2007). GLMs were also used for analysing the effect of base temperatures and the thermal constant for 50% of germination (as detailed above) of the three groups identified (i.e. Mediterranean lowland, Mediterranean high mountain and temperate species), followed by a post-hoc pairwise comparisons t-test (with Bonferroni adjustment). Two-sample Student's t-tests were used to test whether the means of two normally distributed populations were equal (McDonald, Reference McDonald2008). Furthermore, the Wilcoxon rank sum test, which is based solely on the order in which the observations from the two samples fall, was applied. All the analyses were carried out using R v. 2.14.1 (R Core Team, 2011).
Results
Climatic data
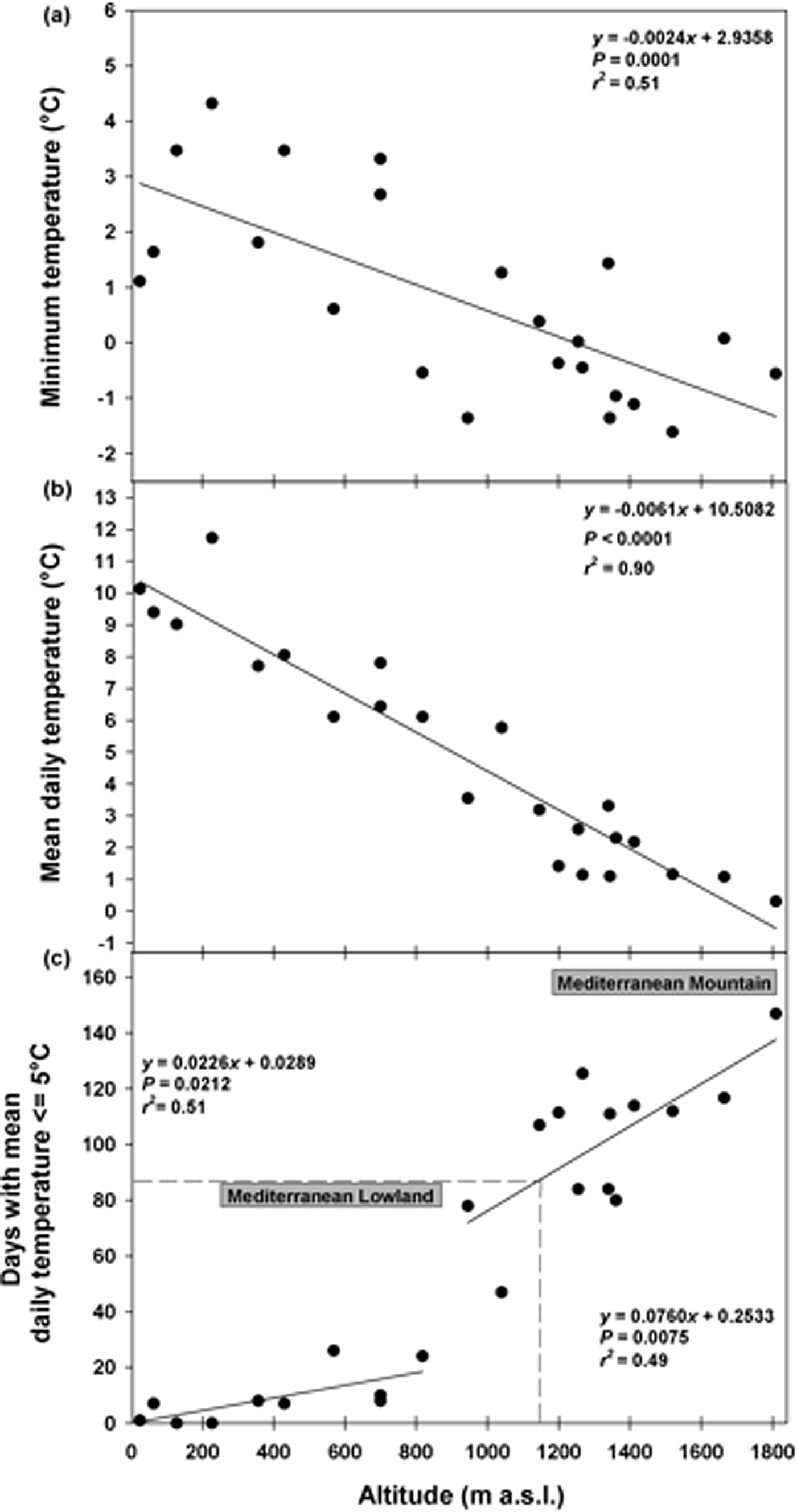
Soil temperature data highlighted that in winter the minimum daily temperatures decreased with altitude from ca 4°C (ca 200 masl) to ca –1°C (ca 1800 masl) and this pattern could be modelled by a linear correlation (Fig. 1a). The mean daily temperatures followed the same pattern decreasing from ca 12°C (ca 200 masl) to 0°C (ca 1800 masl; Fig. 1b). The cold period increased linearly with altitude from 0 to 25 days at 800 masl (Fig. 1c). At altitudes >800 masl, this period increased considerably, reaching a duration of 147 days at 1810 masl (Fig. 1c). The correlation resulting from fitting data with two straight lines represented a more suitable model, showing a higher proportion of the variance (higher r 2 values) than the continuous line. A cold period of 90 days, corresponding to the duration of the cold stratification at 5°C (C) in the laboratory, was reached at altitudes ≥1180 masl (Fig. 1c). According to this threshold, it was possible to distinguish between ‘Mediterranean lowland’ (ML) up to altitudes of 1180 masl, and ‘Mediterranean mountain’ (MM) at altitudes ≥1180 masl (Fig. 1c).
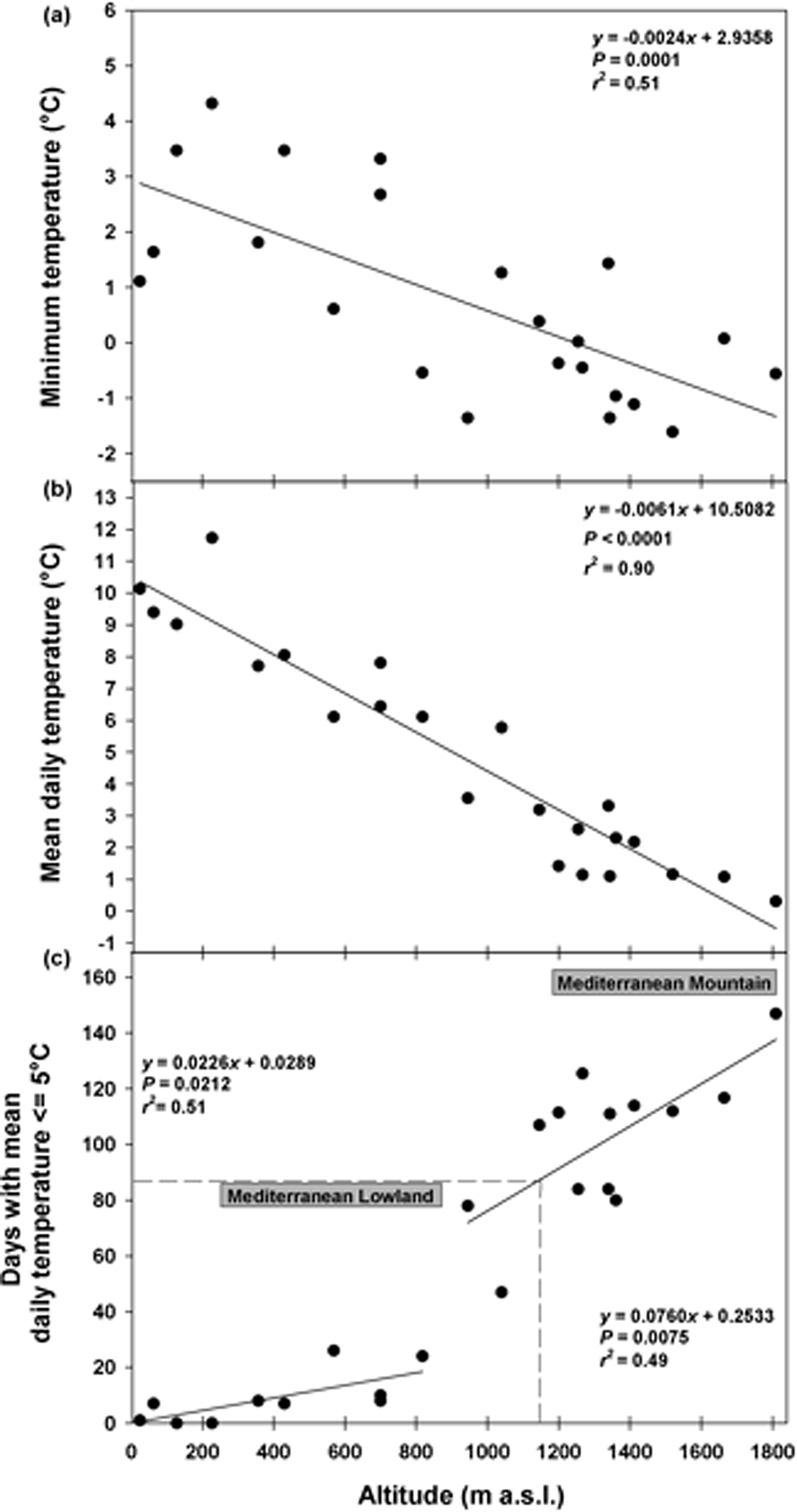
Fig. 1. Soil temperatures recorded in winter at different altitudes: minimum temperature (a), mean daily temperature (b), and days with mean daily temperature ≤5°C (c). Fitted line parameters are shown in each plot. In (c) the dashed lines highlight the value of 90 days which corresponds to the cold stratification (C; 5°C for 3 months) applied in the laboratory. According to this threshold, which was reached at altitudes ≥1180 masl, it was possible to distinguish between Mediterranean lowland and Mediterranean mountain species.
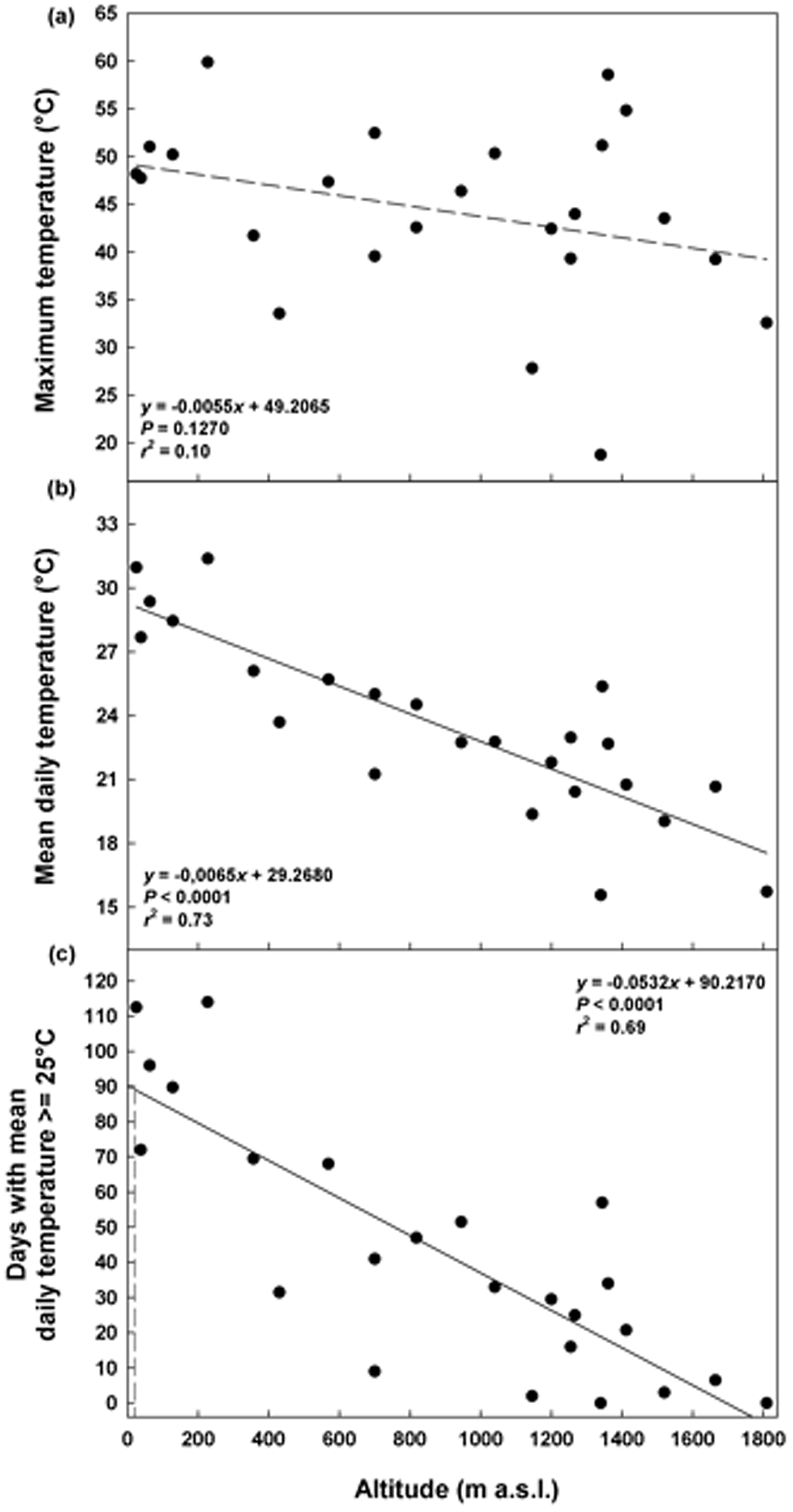
The maximum daily temperatures in summer ranged from 19°C (ca 1350 masl) to 60°C (ca 200 masl), without a significant correlation with altitude (P = 0.1270; Fig. 2a). The mean daily temperatures decreased with altitude from 31°C (25 masl) to 16°C (ca 1350 masl; Fig. 2b) as well as the duration of the summer period from 113 (25 masl) to 0 days (1810 masl; Fig. 2c). A summer period of 90 days, corresponding to the duration of the warm stratification (W) and dry after ripening (DAR) at 25°C in the laboratory, was estimated to be achieved only at altitudes ≤4 masl (Fig. 2c).
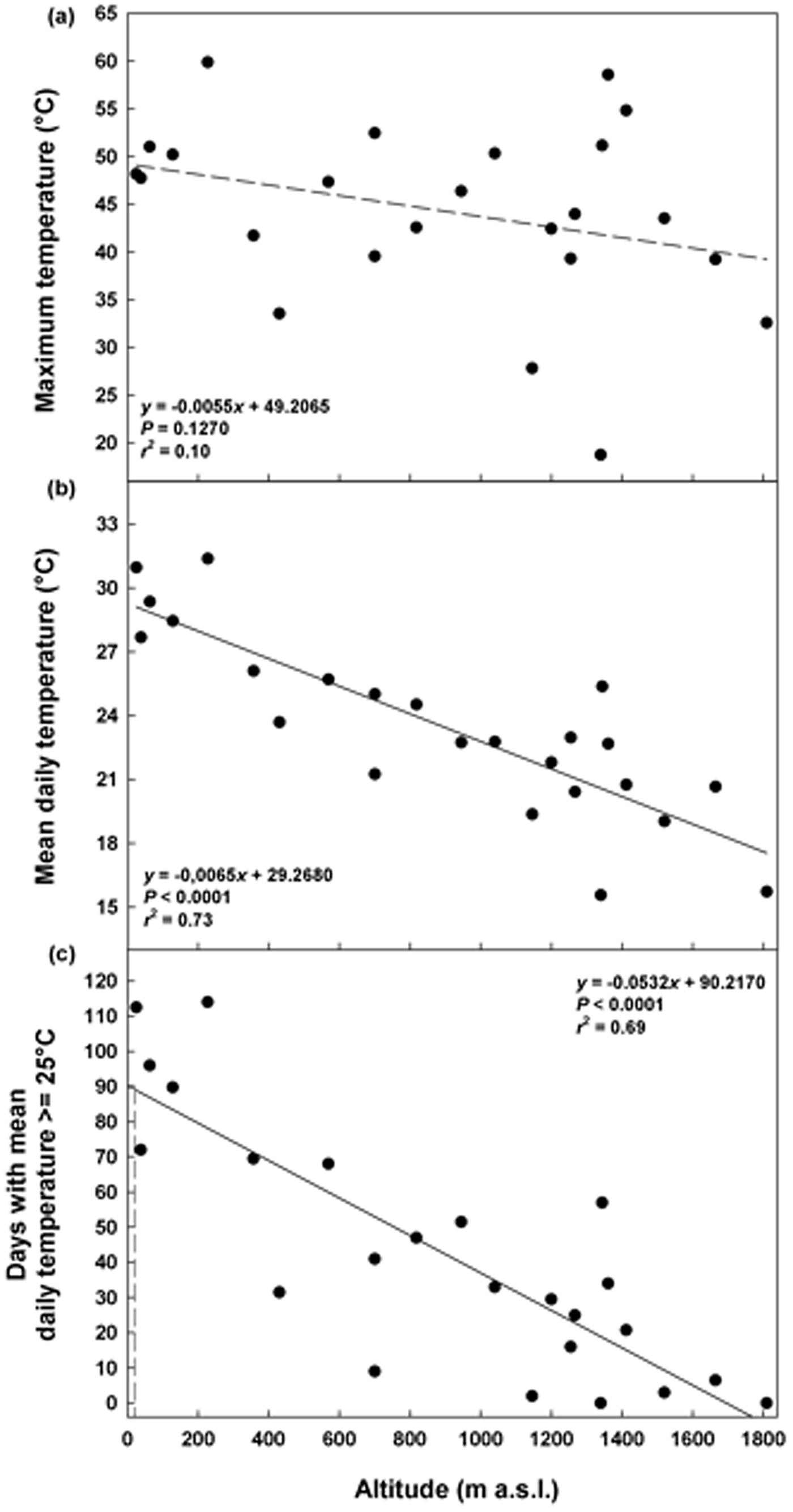
Fig. 2. Soil temperatures recorded in summer at different altitudes: maximum temperature (a), mean daily temperature (b) and days with mean daily temperature ≥25°C (c). Fitted line parameters are shown in each plot. In (c) the dashed lines highlight the value of 90 days which corresponds to the warm stratification (W; 25°C for 3 months) and the dry after-ripening (DAR; 25°C for 3 months on silica gel) applied in the laboratory.
Seed germination
Seed germination results of each taxon, including different populations of the same species, are reported in Appendix 1. In the control test, the highest germination percentage was reached by Lupinus luteus (100% at all tested temperatures). However, high mean germination percentages >75% were also recorded for eight taxa: Helianthemum caput-felis; all three populations of Ptilostemon casabonae; the lowest altitude population of Digitalis purpurea var. gyspergerae; Helichrysum microphyllum ssp. tyrrhenicum; and populations of Santolina insularis at middle and high altitude. Seeds of six other species germinated to maxima between 50 and 75%: Silene succulenta ssp. corsica; the low altitude population of Santolina insularis; Rhamnus alaternus ssp. alaternus; Nepeta foliosa; Lamyropsis microcephala; and the highest altitude population of Digitalis purpurea var. gyspergerae. Finally, only two species, Brassica tournefortii and Ruta lamarmorae, failed to achieve 25% seed germination; the cut-test on the non-germinated seeds revealed that they were viable (Appendix 1).
A positive effect of GA3 treatment on seed germination was observed, reaching final germination >50% compared with seeds under control conditions. Overall in relation to the control, GA3 increased germination significantly in eight taxa (Appendix 1). For example, Brassica tournefortii seeds reached final germination close to 100% at all tested temperatures.
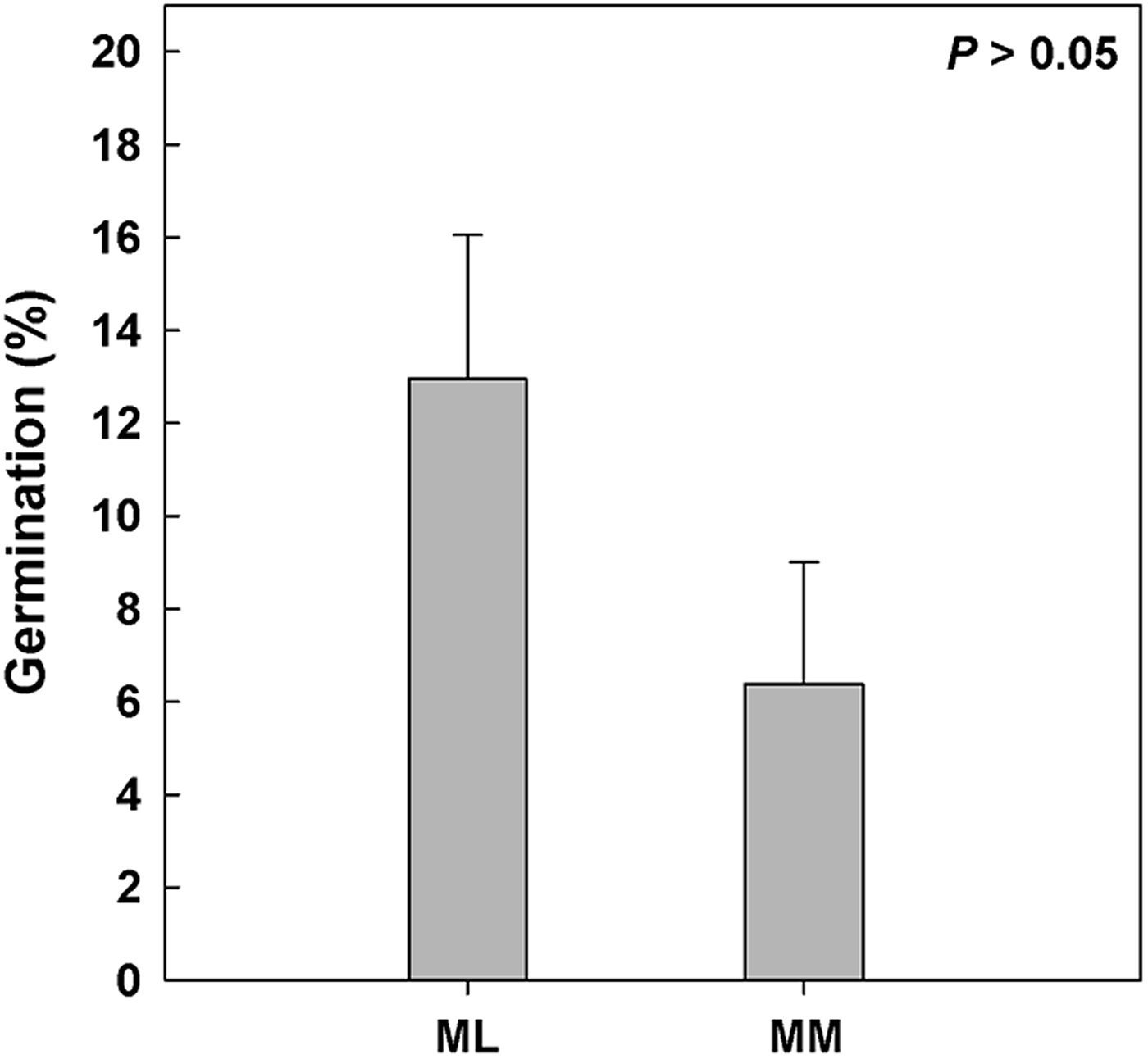
Seeds of 14 taxa and 21 seed lots (i.e. Clematis vitalba, Dianthus morisianus, all three populations of Digitalis purpurea, Helichrysum microphyllum ssp. tyrrhenicum, Lamyropsis microcephala, Nepeta foliosa, all three populations of Ptilostemon casabonae, Rhamnus alaternus ssp. alaternus, Rhamnus lycioides ssp. oleoides, Ruta lamarmorae, Santolina insularis population at low and high altitude, Scrophularia ramosissima, all three populations of Scrophularia trifoliata and Verbascum plantagineum) germinated during C stratification, before moving to the range of incubation temperatures. Differences in seed germination were observed according to altitude, in particular 15 seed lots from lowlands germinated during C, with higher values (ca 13%) than those of high altitudes (six seed lots with ca 6%; Fig. 3).

Fig. 3. Seed germination during pre-chilling (C, 5°C for 3 months). Referring to climatic data achieved by data loggers (Fig. 1c), the study species were divided depending on altitude: Mediterranean lowland ‘ML’ species (0–1180 masl) and Mediterranean mountain ‘MM’ species (1180–1810 masl). P > 0.05 by post hoc pairwise comparisons t-test.
After C treatment the highest germination was observed for Clematis vitalba seeds (ca 95%). Nevertheless, high germinations (i.e. >75%) were also recorded for ten species, while seeds of six other taxa reached maxima between 50 and 75%, whereas those of four species only reached 25–50%. In contrast, seed germination was low (<25%) for three species (see details in Appendix 1). C increased germination significantly in only two species (i.e. Clematis vitalba and Nepeta foliosa) in comparison with the control. C decreased significantly the mean germination of nine species, mostly from lowlands (Appendix 1).
After W treatment, seeds of four species (all three populations of Ptilostemon casabonae, the middle altitude population of Scrophularia trifoliata, Helichrysum microphyllum ssp. tyrrhenicum, and the highest altitude population of Santolina insularis) achieved mean germination >75%. Seeds of two species germinated to maxima of 50–75%, while only one species reached 25–50% (Appendix 1). W treatment did not significantly increase germination of all the tested species in comparison with the control. In contrast, W decreased significantly the mean seed germination of five lowland species (Appendix 1).
The highest seed germination percentages following DAR were observed in two species (Helianthemum caput-felis and Lupinus luteus). However, high mean germination percentages >75% were also recorded for five taxa. Furthermore, seeds of seven species reached maxima between 56 and 75%, including Ruta lamarmorae, with 56% (Appendix 1). DAR only increased germination significantly for the study species compared with the control in Brassica tournefortii, Helianthemum caput-felis and Ruta lamarmorae, while it decreased germination in two taxa.
Amongst the investigated species, generalized linear models highlighted a statistically significant effect (P < 0.001) on germination of treatment and temperature factors as well as of their interactions (P < 0.001, P < 0.01 and P < 0.05); with the exception of four taxa (see details in Appendix 2).
Thermal thresholds for seed germination
Thermal threshold estimates of each seed lot are reported in Appendix 3. T b of seeds under control conditions (0) varied by 18°C, from –9 to ca 9°C; while GA3-treated seeds had T b values varying by 11°C, from –5 to 6°C. C treatment reduced the inter-species range of values to 6°C, from ca 1 to ca 7°C. In DAR-treated seeds, T b varied by 14°C, from –5 to 9°C. Finally, T b of seeds treated with W varied from 0 to 9°C (see details in Appendix 3).
Control seeds had S values that varied more than ca 15-fold, between 22 to 357°Cd. With GA3, the S value ranged less than fourfold, from 53 to 196°Cd. Cold (C) had a similar effect on S to GA3 with values recorded from 33 to 204°Cd. In contrast, in DAR treatment, S values of 41 to 313°Cd were similar to the control. Finally, for seeds treated with W, S varied from 56 to 238°Cd (Appendix 3).
Amongst species and all treatments there was a general tendency for T b and S to be negatively correlated, but this was statistically significant for seeds under control conditions only (P = 0.0190, r 2 = 0.88).
Correlation between base temperature and altitude
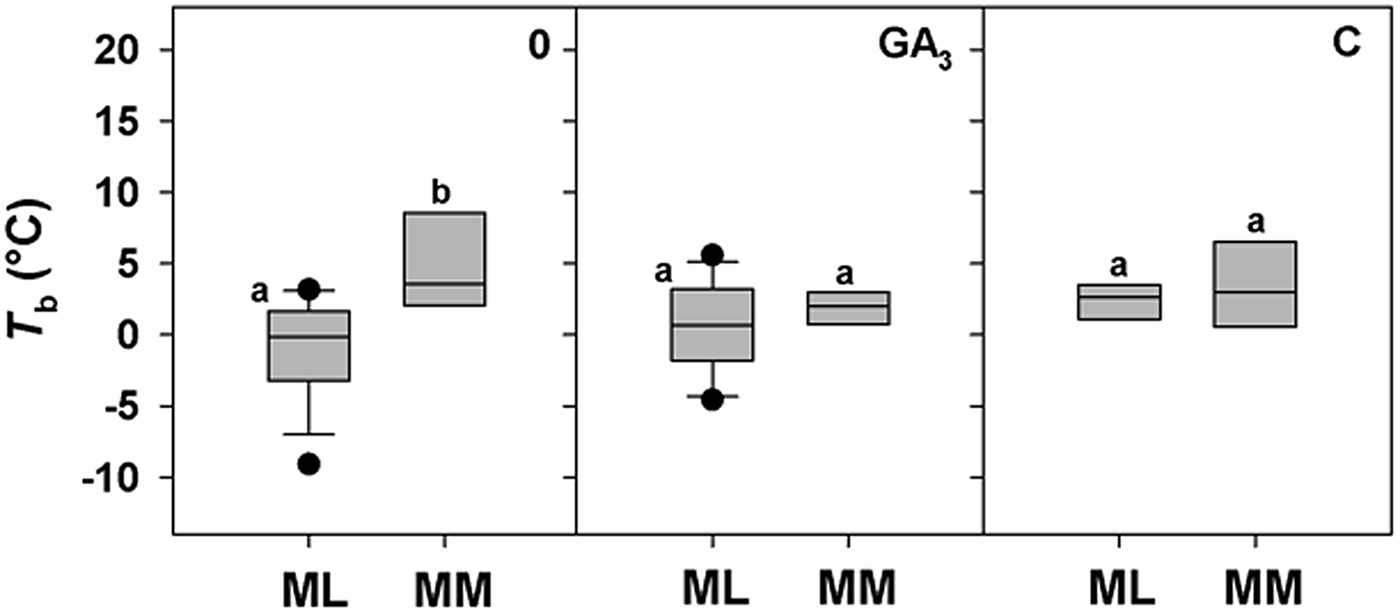
A positive correlation between base temperature (T b) and altitude was highlighted only for DAR-treated seeds (Fig. 4d), while a positive trend between T b and altitude for control seeds (0) just failed statistical significance (P = 0.0651; Fig. 4a). No correlation was found for seeds treated with GA3, C and W, with T b estimated to be around 2°C for all species, regardless of altitude (Fig. 4b, c and e). To explore further these apparent differences in T b with altitude, species were divided between ML and MM based on the climate data from the data-loggers (Fig. 1c). For control seeds post hoc pairwise t-test comparison highlighted significant differences among ML and MM. Both GA3 and C treatment caused a reduction in T b values of MM species, nullifying the effect of altitude (Fig. 5). GLMs highlighted a statistically significant effect of the treatment and altitude factors (P < 0.001 and P < 0.01) on T b of the study species. Also, their interactions were statistically significant (P < 0.01; Table 3).

Fig. 4. Relationships between base temperatures for germination (T b) of the investigated species, for which it was possible to calculate T b, and altitude after each pre-treatment: 0; control (a); GA3, 250 mg l–1 of GA3 in the germination substrate (b); C, 5°C for 3 months (c); DAR, 25°C for 3 months on silica gel (d); W, 25°C for 3 months (e).
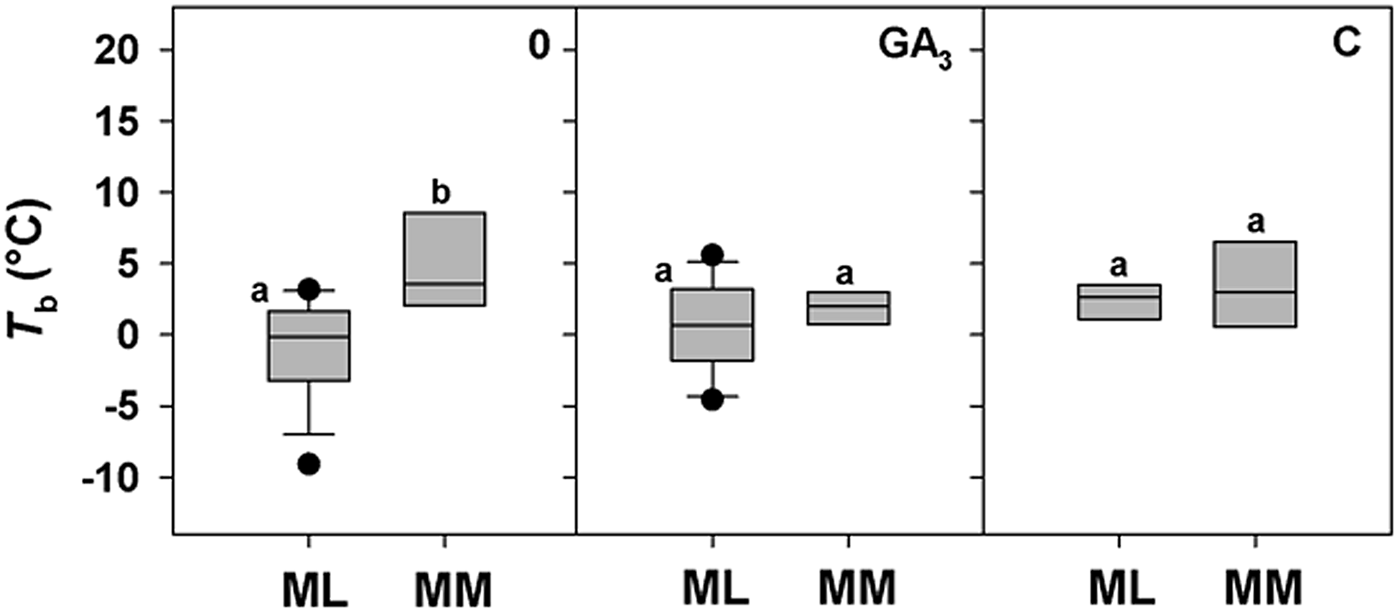
Fig. 5. Relationships between base temperatures for germination (T b) and the Mediterranean lowland ‘ML’ species (0–1180 masl) and Mediterranean mountain ‘MM’ species (1180–1810 masl) for 0 (control), GA3 (250 mg l–1 of GA3 in the germination substrate) and C (5°C for 3 months) treatments. Box plots with the same letter are not different at P > 0.05 by post hoc pairwise comparisons t-test.
Table 3. GLMs results for base temperature of germination (T b) of the following factors: ‘Treatment’ (0, control; GA3, 250 mg l–1 in the germination substrate; C, 5°C for 3 months) and ‘Altitude’ (Mediterranean lowland 0–1180 masl; Mediterranean mountain 1180–1810 masl), and their interaction.

Thermal thresholds of Mediterranean vs temperate species
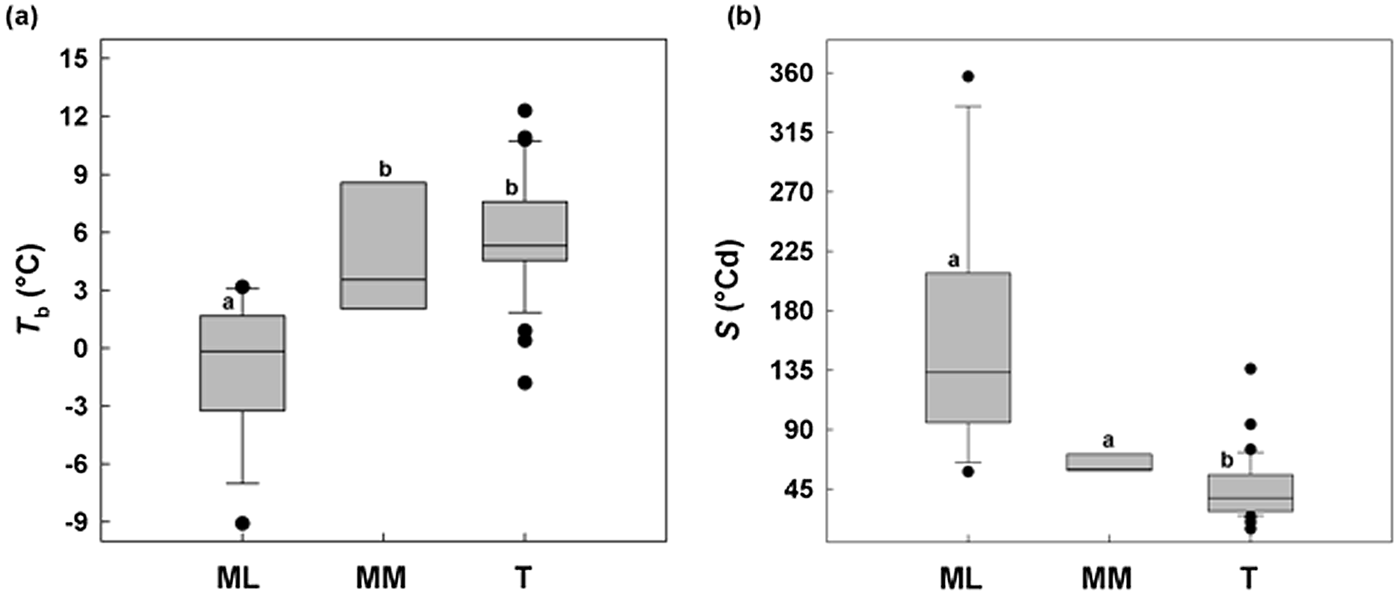
ML showed a minimum T b of –9°C and MM a minimum of 2°C, while in temperate species (T) Tb values were not below –2°C (Fig. 6a). Concerning S, Mediterranean species (sensu lato) were different from temperate species, with a relatively slower germination (longer thermal times), while among MM and ML there was no difference (Fig. 6b).
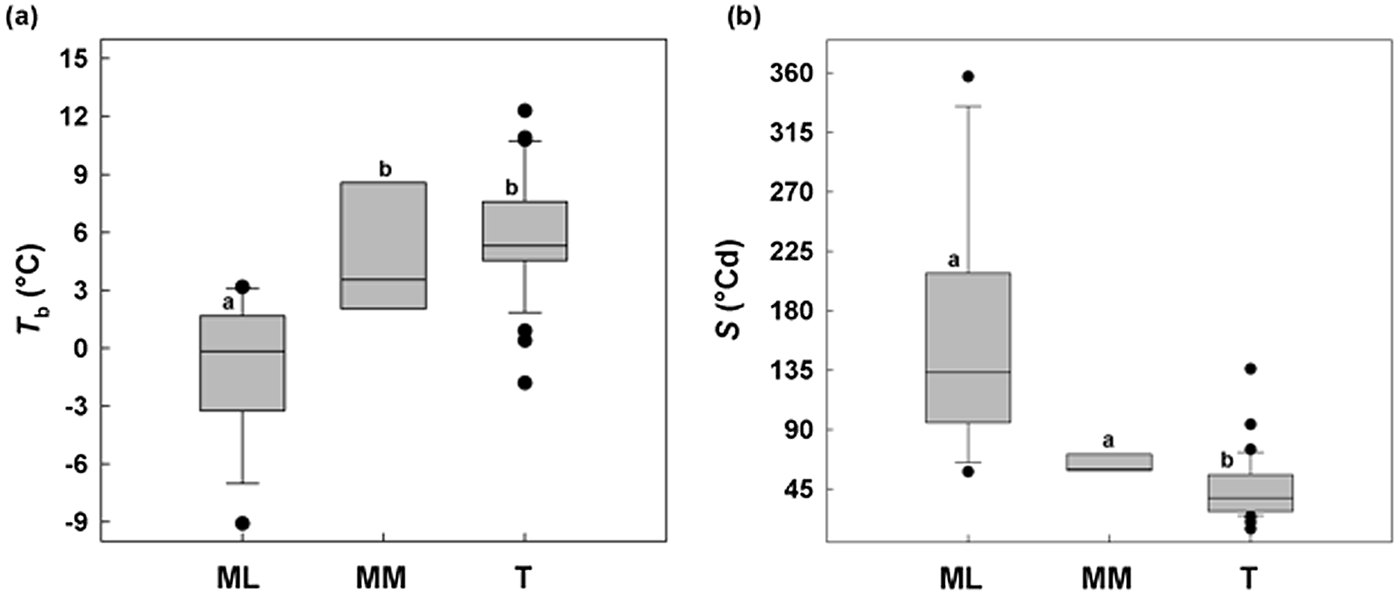
Fig. 6. Relationships between base temperatures for germination (T b) and thermal time constant S (°Cd) without any pre-treatment for 50% of germination between the study species divided on the basis of altitude: Mediterranean lowland ‘ML’ species (0–1180 masl) and Mediterranean mountain ‘MM’ species (1180–1810 masl). A comparable dataset for temperate species (Trudgill et al., Reference Trudgill, Squire and Thompson2000) is also presented. Box plots with the same letter are not different at P > 0.05 by post hoc pairwise comparisons t-test.
Discussion
The soil temperature data recorded in Sardinia during this study confirmed a typical pattern of the Mediterranean climate with high temperatures in summer and cool winters (Joffre et al., Reference Joffre, Rambal, Damesin, Pugnaire and Valladares1999; Medrano et al., Reference Medrano, Flexas and Galmés2009; Kadis and Georghiou, Reference Kadis and Georghiou2010). In particular, colder winters occur at high altitudes than in the lowlands (until 800 masl). Conversely, much warmer summers are detected at lower elevations (up to 200 masl). However, the high temperatures recorded in summer along the whole altitudinal gradient, highlight the fact that Mediterranean mountains have the potential for high evaporation and imposition of water stress, which can have important effects on the timing of seed germination and on plant growth (Mooney et al., Reference Mooney, Hillier and Billings1965; Rundel et al., Reference Rundel, Gibson, Midgley, Wand, Palma, Kleier and Lambrinos2003; Giménez-Benavides et al., Reference Giménez-Benavides, Escudero and Pérez-García2005). Indeed, it has recently been shown that the base water potential for germination in alpine species can vary with niche preference for siliceous and calcareous soils (Tudela-Isanta et al., Reference Tudela-Isanta, Ladouceur, Wijayasinghe, Pritchard and Mondoni2018). However, such determinations were beyond the scope of the study reported here.
An inter-specific variation in the sensitivity of seed germination to the applied treatments was identified among species and altitudes. GA3-treated species responded positively to the treatment. Furthermore, GA3 widened the temperature range for germination in eight species, triggering germination at 5°C compared with the control test. This indicates that a certain degree of dormancy is found in the study species’ seed lots. Indeed, it is well known that GA plays a key role in dormancy release and in the promotion of seed germination in species exhibiting physiological dormancy (PD) or morphophysiological (MPD) dormancy (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Baskin and Baskin, Reference Baskin and Baskin2014). Seeds collected from low altitude sites can germinate during cold stratification before moving to higher temperatures, confirming the general benefits of cold temperature to seed germination in coastal Mediterranean species (‘Mediterranean germination syndrome’; Thanos et al., Reference Thanos, Georghiou, Dimitra and Marangaki1991; Skordilis and Thanos, Reference Skordilis and Thanos1995; Thanos et al., Reference Thanos, Costas and Skarou1995; Doussi and Thanos, Reference Doussi and Thanos2002). This temperature sensitivity provides a considerable ecological advantage to seeds by ensuring that germination is completed at the most appropriate season (mid to late autumn), thus allowing seedlings to avoid arid conditions during summer (Luna et al., Reference Luna, Pérez, Céspedes and Moreno2008; Kadis and Georghiou, Reference Kadis and Georghiou2010). Furthermore, with the exception of three species, cold stratification either inhibits or has a neutral effect on seed germination compared with the control. This is especially the case for lowland species, which, on the basis of logged data, are not naturally exposed to 90 days continually at 5°C. Nonetheless, this behaviour is in agreement with previous studies on Mediterranean species, where relatively long-term chilling may not enhance seed germination and may even be detrimental to seed germination, e.g. Pinus halepensis (Skordilis and Thanos, Reference Skordilis and Thanos1995).
The investigated species did not benefit from the W treatment, especially at constant temperatures. This is not surprising as the climatic data detected in this study showed that 90 days continually at 25°C does not naturally occur at middle–high altitudes in Sardinia. Such W treatment is known to overcome dormancy in endospermic seeds with an under-developed embryo, e.g. Paeonia corsica (Porceddu et al., Reference Porceddu, Mattana, Pritchard and Bacchetta2016) or with larger embryos, such as in Arum maculatum (Pritchard et al., Reference Pritchard, Wood and Manger1993). Consequently, we expected a similar response in Clematis vitalba, as seeds of Ranunculaceae species have been reported to exhibit MD and MPD (e.g. Baskin and Baskin, Reference Baskin and Baskin1994, Reference Baskin and Baskin2014; Porceddu et al., Reference Porceddu, Mattana, Pritchard and Bacchetta2017). However, W treatment did not enhance seed germination of C. vitalba. Interestingly, the alternating temperature regime triggered seed germination in C. vitalba under control conditions, while cold stratification and GA3 treatments promoted seed germination at all tested temperatures (Picciau et al., Reference Picciau, Porceddu and Bacchetta2017).
DAR is an important treatment to break seed dormancy in species growing in dry environments (Bewley, Reference Bewley1997; Probert, Reference Probert and Fenner2000; Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005). In our study, the application of DAR improved the fit of the linear regression in the relationship between T b and altitude compared with the other pre-treatments and had a positive effect only in few cases; in particular, in seeds of species from coastal environments that faced a long dry summer and dispersed their seeds before the wet season, as for example Brassica tournefortii and Helianthemum caput-felis. Similarly, storage <18 months at high temperatures improved germination in seeds of three Australian Asteraceae (Peishi et al., Reference Peishi, Plummer, Turner, Choengsaat and Bell1999). Moreover, dryland seeds of Arabidopsis thaliana, i.e. Cape Verde Island accessions, respond to DAR dormancy release (Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007).
Thermal thresholds can vary and change significantly among species as well as populations of the same species (e.g. Ellis et al., Reference Ellis, Simon and Covell1987; Daws et al., Reference Daws, Lydall, Chmielarz, Leprince, Matthews, Thanos and Pritchard2004). To explore how much this is the case in Mediterranean species we chose to harvest seed from species along an altitudinal gradient of 1800 m, as the seeds are likely to receive widely differing environmental cues during development and post-dispersal, during autumn to spring. Control seeds of species from higher altitudes were found to have higher T b estimates, suggesting an avoidance of premature germination under cool conditions when the risk of freezing may still persist. Interestingly, the dormancy breaking treatments of GA3 and C are effective at lowering T b of Mediterranean mountain species, indicating the widening of the temperature range over which germination can occur post-treatment (e.g. Pritchard et al., Reference Pritchard, Steadman, Nash and Jones1999; Daws et al., Reference Daws, Lydall, Chmielarz, Leprince, Matthews, Thanos and Pritchard2004; Steadman and Pritchard, Reference Steadman and Pritchard2004). In contrast, C increased T b values in seeds of Mediterranean lowland species, similarly to W, to ca 2°C, suggesting a narrowing of the temperature range for germination through an uplift in the low temperature end of the response. Overall, the differential responsiveness to pre-treatments, specifically C in the lowland species, DAR in the mountain species and W in most of the species, reflect a climate-adapted strategy for the timing of seedling emergence (Skordilis and Thanos, Reference Skordilis and Thanos1995) that is modulated through thermal thresholds and times. Indeed, T b and S values provide insight into the adaptation and the ecological strategies of plants in relation to their thermal environment (Trudgill et al., Reference Trudgill, Honek, Li and Van Straalen2005; Fernández-Pascual et al., Reference Fernández-Pascual, Mattana and Pritchard2018), in our study between Mediterranean lowland and mountain species. Mountain species show quantifiable thermal characteristics between Mediterranean lowland (coastal) species and temperate species. This distinctive germination response enables relatively slow germination typical of Mediterranean species (e.g. Doussi and Thanos, Reference Doussi and Thanos2002), which reduces seed cohort loss as a result of erratic rainfall, and a relatively high T b, typical of the temperate species, so as to reduce the risk of a seed cohort germinating in winter. These results are consistent with previous studies investigating the seed germination of three mountain species of Sardinia, Lamyropsis microcephala (Mattana et al., Reference Mattana, Daws and Bacchetta2009a), Rhamnus persicifolia (Mattana et al., Reference Mattana, Daws and Bacchetta2009b; Porceddu et al., Reference Porceddu, Mattana, Pritchard and Bacchetta2013) and Gentiana lutea ssp. lutea (Cuena-Lombraña et al., Reference Cuena-Lombraña, Porceddu, Dettori and Bacchetta2017) for which an early spring germination prevails due to a requirement for cold stratification over winter.
The existence of adaptively significant variation in seed germination responses (thresholds and thermal time) amongst groups of species or populations has recently been highlighted in relation to the timing of germination in the field and thus niche competitiveness (Meyer et al., Reference Meyer, Allen and Beckstead1997; Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002; Honek et al., Reference Honek, Martinkova, Lukas and Dixon2014; Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015; Picciau et al., Reference Picciau, Serra, Porceddu and Bacchetta2018; Tudela-Isanta et al., Reference Tudela-Isanta, Ladouceur, Wijayasinghe, Pritchard and Mondoni2018). Here we have extended such an approach to model the characteristics of Mediterranean species and, in so doing, revealed a shift in germination syndromes between lowland and upland species. We propose that similar studies are undertaken on species that are representative of the world's floras, thereby underpinning studies in plant ecology and vegetation science.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258518000399.
Author ORCIDs
Rosangela Picciau 0000-0002-7128-830X.
Acknowledgements
The authors thank Luca Frigau for his valuable help with the statistical analysis. Special thanks to Aixa Zunino Luna and Marta Orgaz Diaz for assistance in the laboratory, and to Marco Porceddu, Valerio Lazzeri and Martino Orrù for helping with fieldwork. The ‘Centre for the Biodiversity Conservation (CCB)’ is supported by the ‘Provincia di Cagliari – Assessorato Tutela Ambiente’. The Royal Botanic Gardens, Kew, receives a grant-in-aid from Defra, UK.
Conflicts of interest
None