Introduction
The cultivation of the Andean seed crop quinoa (Chenopodium quinoa) out of its region of origin increases the risk of pre-harvest sprouting. This risk is low in its traditional area of cultivation, where it matures after the end of the rainy season (Geerts et al., Reference Geerts, Raes, García, Del Castillo and Buytaert2006), but coincidence with rains around the maturity date is known to affect seed quality (Jacobsen and Bach, Reference Jacobsen and Bach1998). The occurrence of dormancy after physiological maturity was confirmed for two C. quinoa accessions, as part of a research project aimed at the identification of resistance sources to pre-harvest sprouting. It was found that environmental conditions experienced by quinoa seeds during their development regulate the level of dormancy present at harvest and their release rate afterwards: high temperatures and long photoperiods produced more dormant seeds than low temperatures and short photoperiods (Ceccato et al., Reference Ceccato, Bertero and Batlla2011).
Seed dormancy is usually classified depending on the tissues that determine it – as an embryo- or coat-imposed dormancy – and that imposed by the seed coats is perhaps the most commonly studied (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Nonogaki, Reference Nonogaki2006; Hilhorst, Reference Hilhorst, Bradford and Nonogaki2007; Linkies and Leubner-Metzger, Reference Linkies and Leubner-Metzger2012). Coats impose dormancy through several mechanisms. They can: (1) interfere with water absorption or gas exchange with the environment; (2) impose a mechanical restraint on radicle emergence; (3) prevent the release of inhibitors from the embryo; (4) release inhibitors to the embryo; and/or (5) filter light (Debeaujon et al., Reference Debeaujon, Lepiniec, Pourcel, Routaboul, Bradford and Nonogaki2007). Environmental effects on seed dormancy level can be exerted through changes in seed coat properties. In some cases, these effects are associated with changes in structural or chemical characteristics, such as seed coat thickness or their polyphenolic content. These kinds of association have been reported in some members of the genus Chenopodium. In C. polyspermum and C. album, seed coat thickness and germination are affected by the photoperiod experienced during development (Jacques, Reference Jacques1968; Karssen, Reference Karssen1970; Pourrat and Jacques, Reference Pourrat and Jacques1975). In particular for C. album, darker seed coats (showing higher dormancy) are correlated with the occurrence of longer days (Karssen, Reference Karssen1970). In C. bonus-henricus, Dorne (Reference Dorne1981) found a negative association between altitude of origin and germination percentage. The latter decreased as the altitude at which the mother plant was grown increased. This decrease in germination was related to an increase of seed coat thickness and polyphenol content. The effect of altitude was associated with average temperature 30 d before harvest and this temperature was positively correlated with germination. Dorne suggested that radiation, which increases in association with altitude, may cause a higher content of oxidizable polyphenols, which determines a lower amount of oxygen reaching the embryo.
The possibility of identifying morphological and physiological processes controlling dormancy in quinoa, and how these traits are affected by the environment, is of paramount importance for the selection of sprouting-resistant genotypes in this species. Based on these considerations, the aims of this work were to establish the importance of seed coats in the regulation of dormancy in quinoa, to reveal possible mechanisms of action and to assess associations with changes in dormancy level caused by the environment.
Materials and methods
Seed production and germination
The seed coat in C. quinoa is composed of two cell layers, the endotegmen (the inner layer) and the exotesta (the external one) (Prego et al., Reference Prego, Maldonado and Otegui1998; Sukhorukov and Zhang, Reference Sukhorukov and Zhang2013). Surrounding the seed coat is the pericarp, usually as a discontinuous layer at maturity. Experimental details are as described by Ceccato et al. (Reference Ceccato, Bertero and Batlla2011). Briefly, two quinoa accessions, Chadmo and 2-Want, both presenting dormancy at harvest, were cultivated in the field (34°60′S, 58°65′W, Hurlingham, Buenos Aires, Argentina) on three sowing dates during the first experimental year (2 November, 27 December and 13 March) and on two sowing dates during the second experimental year (7 November and 8 February) during 2005–2006 and 2006–2007. Both accessions were obtained from the Germplasm Bank at the US Department of Agriculture, National Plant Germplasm System at Beltsville, Maryland, where they are identified as PI 614850 and AMES 13 737, respectively. Environmental data were obtained from a conventional meteorological station located about 300 m from the experimental site [Instituto de Clima y Agua, Instituto Nacional de Tecnología Agropecuaria (INTA)]. Flowering date was determined as the date of first anthesis in at least 50% of the plants. Seed dry weight and moisture content were determined during seed filling by weighing 50 seeds per replicate before and after drying at 105°C for 24 h in an oven (Ministério da Agricultura e Reforma Agrária, 1992). Physiological maturity (PM) was considered to be complete when dry weight levelled off. Harvest time (H) was determined based on a 20% seed moisture content, which is common practice in the traditional production areas. Four panicles of each accession were sampled starting 15–20 d after flowering (DAF) and at intervals of 5 d until crop harvest. At each harvest time, 35 seeds were placed on filter paper (Schleicher & Schuell 0859, Dassel, Germany) and moistened with 4 ml of distilled water in plastic boxes of 85 mm diameter, with four replicates. Then they were incubated under fluorescent light, with a photoperiod of 8/16 h (light/dark), at 5, 10 or 25°C in an incubator with a temperature variation of ± 1°C. Seed germination was recorded daily for 15 d, and visible radicle protrusion was used as germination criterion. Seeds used for germination tests were extracted from the middle third of each panicle, and each replicate represents a single plant.
Bois et al. (Reference Bois, Winkel, Lhommee, Rafaillac and Rocheteau2006) indicated that maximum germination in quinoa occurs between 18 and 23°C. Accessions studied in this work reached 100% of germination in just 2 d of incubation at 25°C when dormancy was not present (data not shown), and therefore 25°C was considered an optimal temperature for germination in subsequent experiments. The following treatments were applied to assess the coat's role in seed germination behaviour: control – whole seeds with pericarp; perforated – seeds punctured through the pericarp + seed coat in the perisperm area (to avoid embryo damage). This treatment was chosen in replacement of embryo isolation for operational reasons and based on previous observations by Jacques (Reference Jacques1968) that a perforation treatment led to the complete release of dormancy in C. polyspermum. Ungerminated seeds were tested by the cutting test to rule out dead seeds, and the remaining were considered dormant. Furthermore, random samples of the same seed lot, taken months later, had 100% germination (Ceccato et al., Reference Ceccato, Bertero and Batlla2011).
Seed coat thickness measurement
Seed coat thickness was measured in seeds of both accessions that were sampled at harvest for each sowing date in both experimental years. For this purpose seeds were hydrated in warm water with a few drops of detergent, pre-fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.2) for 2 h and post-fixed in 1.5% osmium tetroxide in the same buffer at 2°C for 3 h, then dehydrated in acetone at increasing concentrations and embedded in Spurr resin. Afterwards, seeds were cut longitudinally with respect to the embryo, with a Reichert–Jung ultramicrotome (Reichert Inc., New York, USA), and thin sections (1 μm thickness) were stained with cresyl violet and mounted in synthetic resin (O'Brien and McCully, Reference O'Brien and McCully1981). Seed coat and pericarp thickness were measured in a section lateral to the radicle, where endosperm that sheaths the radicle is absent (Prego et al., Reference Prego, Maldonado and Otegui1998), under a Wild M20 microscope (Wild Heerbrugg AG, Gais, Switzerland) with a graduated ocular lens. Photomicrographs were taken in a Motic DMWB1-223ASC microscope (Motic Instruments Co., Hong Kong, China) with incorporated digital camera. Ten replicate measurements were performed for each sowing date.
Measurement of abscisic acid leaching and abscisic acid content of seeds
Differences in the amount of abscisic acid (ABA) leaching into the incubation medium between perforated and control seeds (intact seeds) of both accessions were tested for seeds from the first sowing date of the second experimental year. Four replicates of 25 seeds were incubated at 25°C for 8 h in 20-mm plastic boxes containing 300 μl of distilled water. After incubation, the remaining distilled water contained in the boxes of each treatment was stored in a freezer at − 20°C until ABA determination. ABA content in dry seeds from the same harvest was measured. For this purpose, whole seeds in four replicates of 25 were ground and water was added [24 ml (mg dry weight)− 1] for extraction. The amount of ABA was determined by radioimmunoassay, as described by Steinbach et al. (Reference Steinbach, Benech-Arnold, Kristof, Sánchez and Marcucci-Poltri1995), using the monoclonal antibody AFR MAC 252 (Quarrie et al., Reference Quarrie, Whitford, Appleford, Wang, Cook, Henson and Loveys1988) and tritiated ABA (Amersham Biosciences, Little Chalfont, Bucks, UK) (Rodríguez et al., Reference Rodríguez, Mendiondo, Maskin, Gudesblat, Iusem and Benech-Arnold2009; Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010; Di Mauro et al., Reference Di Mauro, Iglesias, Arce, Valle, Benech-Arnold, Tsuda, Yamazaki, Casalongué and Godoy2012). Each sample was assessed twice. The results presented are the mean value of four (for Chadmo) or three (for 2-Want) biological replicates ± standard error (SE). Values are expressed either as picograms of ABA per milligram of seed dry weight [pg ABA (mg dw)− 1] or, as picograms of ABA per microlitre of incubation medium (pg ABA μl− 1).
Data analysis
The data were analysed using the InfoStat software (InfoStat Group, University of Córdoba, Argentina). T 50 was calculated by fitting time-course curves to observed germination data (germination percentage vs. time in days) using a Gómpertz function (Notivol et al., Reference Notivol, García-Gil, Alía and Savolainen2007). Curves were fitted with GraphPad Prism 4 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com) and the fitting value (r 2) was in every case greater than 0.95. Differences in coat thickness between accessions for each sowing date were evaluated by t-test for independent samples. The correlation between coat thickness and environmental conditions was analysed using the complete data set, the mean ± 1 SE is presented for clarity. Differences in amounts of ABA were evaluated by unpaired t-test with Welch's correction (which does not assume equal variances) using GraphPad Prism version 5.00 for Windows (GraphPad Software, www.graphpad.com).
Results
Role of the seed coat in dormancy and sowing date effects
The effect of seed coat perforation on seed germination depended on sowing date and incubation temperature. Coat perforation produced a significant increase in the rate of dormancy loss during development for seeds in both accessions belonging to the first sowing date and incubated at 25°C (Fig. 1A, B). This difference was particularly contrasting in Chadmo, in which perforated seeds showed 84% germination at harvest while the control seeds showed no germination (Fig. 1B). For seeds belonging to the late sowing date (March), the effect of seed perforation was not significant in both accessions when seeds were incubated at 25°C. The seeds still expressed a level of dormancy that was not overcome by coat perforation (Fig. 1C, D).
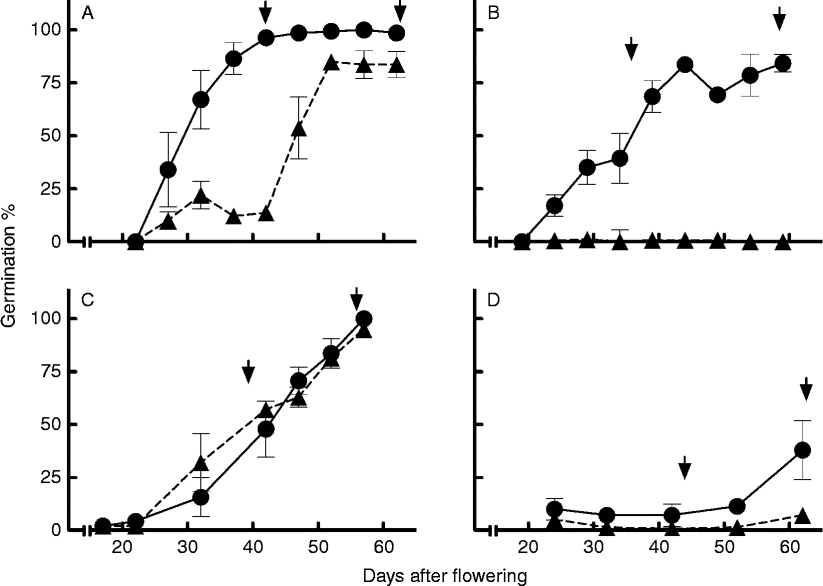
Figure 1 Changes in the maximum germination after incubation at 25°C during seed development and maturation in (▲) control (intact seeds with pericarp) and (●) perforated seeds (seeds punctured through the pericarp + seed coat), in accession 2-Want (A and C) and Chadmo (B and D), from the first (A and B) and third sowing dates (C and D) in the first experimental year. In each panel, the left arrow indicates physiological maturity and the right arrow the harvest date. Symbols represent the mean ± 1SE.
At lower incubation temperatures (10 and 5°C), at the first sowing date, the effect of perforation was still significant in both accessions (Fig. 2A, B). In seeds belonging to the late sowing date a significant level of coat-imposed dormancy was still expressed in 2-Want at 10 and 5°C, overcome by perforation (Fig. 2C). Chadmo seeds expressed strongly reduced germination under low incubation temperatures, which was not overcome by coat perforation (Fig. 2D). The fact that dry afterripened seeds of both accessions achieve maximum germination at 10 and 5°C (Ceccato et al., Reference Ceccato, Bertero and Batlla2011), indicates that the observed reduction in germination was due to dormancy expression, and not a consequence of non-optimal temperatures for germination.
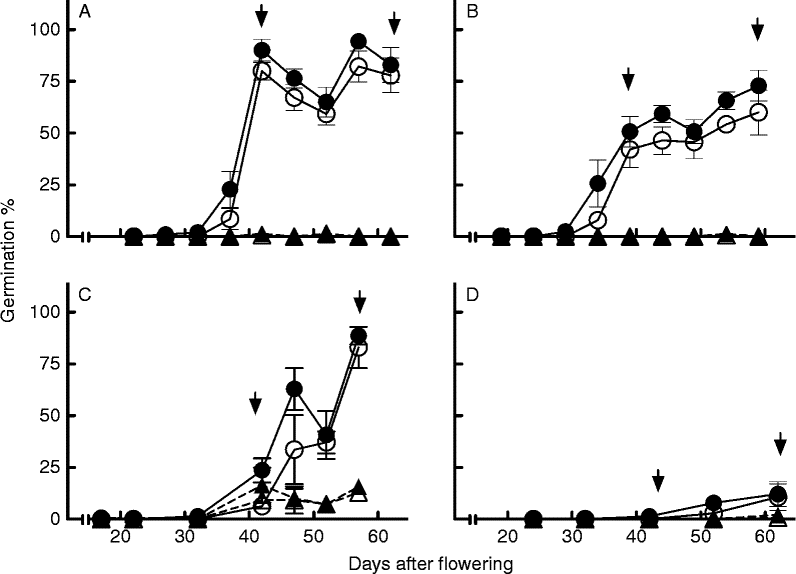
Figure 2 Changes in the maximum germination after incubation at 10 (closed symbols) and 5°C (open symbols) during seed development and maturation in (▲) control (intact seeds with pericarp), and (●) perforated seeds (seeds punctured through the pericarp + seed coat), in accession 2-Want (A and C) and Chadmo (B and D), from the first (A and B) and third sowing dates (C and D) in the first experimental year. In each panel, the left arrow indicates physiological maturity and the right arrow the harvest date. Symbols represent the mean ± 1SE.
Seed coat thickness, sowing date and seed dormancy level
In accession 2-Want, seed coat thickness was reduced in late sowings as compared to the earlier ones, in both experimental years (P≤ 0.05, Table 1, Fig. 3); however, no differences were detected for Chadmo. Additionally, independent samples t-test showed that the seed coat was significantly thicker in Chadmo as compared to 2-Want for each sowing date (P≤ 0.01, Table 1). No consistent variation in pericarp thickness was detected between sowing dates or between accessions (data not shown).
Table 1 Seed coat thickness (μm) of 2-Want and Chadmo seeds, from different sowing dates.
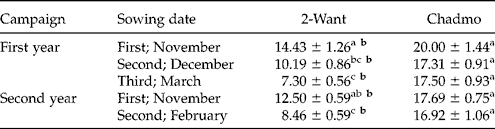
The data are expressed as the mean ± standard error; different letters indicate significant differences between sowing dates for each accession (normal letters, P≤ 0.05) and between accessions for each sowing date (bold letters, P≤ 0.01).

Figure 3 Cross-section of fruit and seed of accession 2-Want (A) and detail of the tissues that composed the seed coat (B). The rectangle indicates the area where measurements of seed coat thickness were performed. emb, embryo; p, perisperm; en, endosperm; per, pericarp; sc, seed coat; tes, testa; teg, tegmen. The scale bars indicate (A) 250 μm and (B) 50 μm.
In the first experimental year, seed filling occurred during the months of January and February, March and April, and June for the first, second and third sowing date, respectively. In the second year seed filling occurred between January and February, and April and May for the first and second sowing date, respectively. Differences in seed coat thickness were associated with different environmental conditions (temperature and photoperiod) experienced by the seeds during their development for each sowing date (Fig. 4). The association of seed coat thickness with T 50 as a dormancy parameter was also significant, for accession 2-Want (P= 0.0009; r 2= 0.21; data not shown). T 50 is the time in days from anthesis for germination at 10°C of harvested seeds to reach 50% (see Materials and methods).

Figure 4 Correlation between the seed coat thickness (μm) at harvest and the mean temperature (A) or photoperiod (B) during seed development for each sowing date. P< 0.001. Accession 2-Want. Data are the mean ± 1SE.
A measurement of whole-seed polyphenol content, based on the assumption that higher polyphenol could lead to higher dormancy and explain variation between sowing dates, showed an opposite trend to that of seed dormancy (data not shown). So, this hypothesis was provisionally discarded, although more specific and localized measurements are needed.
Interference of seed coat properties with leaching of abscisic acid
In accession Chadmo, ABA content was 2.5 times higher in the incubation medium of perforated seeds compared to that of intact seeds (Fig. 5A). For 2-Want, no significant differences were detected (Fig. 5B). Detectable ABA levels in the incubation medium were observed even in control seeds. The amount of ABA leaching from intact (control) seeds was higher in 2-Want than in Chadmo (Fig. 5A, B), and this was consistent with differences in ABA content of dry seeds between accessions (Fig. 5C).
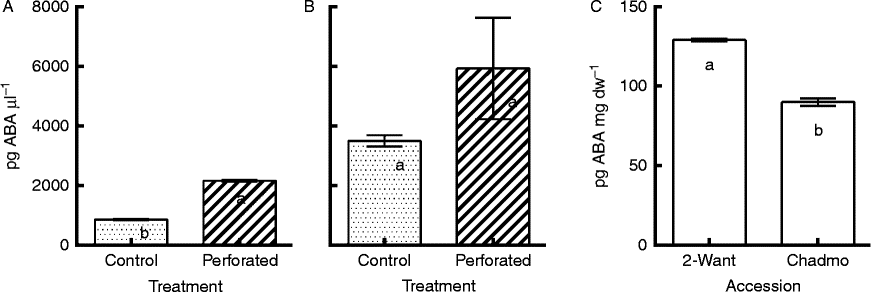
Figure 5 ABA measured in the incubation medium of intact seeds (control) and seeds with perforated coats (perforated), in accessions Chadmo (A) and 2-Want (B); and ABA content in dry seeds at harvest (C). The data are expressed as picograms of ABA per microlitre of incubation medium (pg ABA μl− 1; A and B), or as picograms of ABA per milligram of seed dry weight [pg ABA (mg dw)− 1; C]. Different letters indicate significant differences (P< 0.01) and the bars indicate the mean ± 1SE.
Discussion
In agreement with previous results in other Chenopodium species (Jacques, Reference Jacques1968), the results of perforation treatment indicate that a significant part of dormancy is explained by the action of the seed coat and pericarp tissues, since a discontinuity in these layers resulted in the acquisition of a high germination capability (Fig. 1A, B). On the other hand, since the pericarp is partially separated from the seeds at harvest time, it can be speculated that coat-imposed dormancy at harvest and afterwards is mainly imposed by the seed coat.
The lack of response to perforation in seeds from the late sowing (March) incubated at 25°C may be explained by an increase in embryo dormancy under this maturation condition (Fig. 1C, D). Generally, high temperatures during seed development on the mother plant decrease dormancy (Fenner, Reference Fenner1991; Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sánchez2004; Gualano and Benech-Arnold, Reference Gualano and Benech-Arnold2009). Results obtained in this work suggest that embryo dormancy in quinoa follows this general pattern, therefore in seeds exposed to higher temperatures during development (early sowing date) embryo dormancy was maintained at relatively low levels, while seeds exposed to lower temperatures during development (late sowing date) showed a higher level of embryo dormancy. However, considering dormancy imposed by the seed coats, our results suggest that it responds to environmental conditions in the opposite way: higher in early sowing and lower in late sowings. The reduced effect of perforation treatments in late sowing dates is explained by the combination of low coat dormancy and high embryo dormancy (compare Fig. 1A with C for 2-Want, and Fig. 1B with D for Chadmo). This response is similar to that of sunflower, where high temperatures during seed development increase coat (pericarp and seed membranes) imposed dormancy and decrease embryo dormancy (Bodrone, Reference Bodrone2014).
Under low incubation temperatures (5 and 10°C) the expression of dormancy is stronger in quinoa seeds (Ceccato et al., Reference Ceccato, Bertero and Batlla2011). That could explain the fact that coat-imposed dormancy (and perforation effects) was expressed at 5 and 10°C in 2-Want seeds from the late sowing date (even though embryo dormancy was higher than in early sowing dates). Chadmo seeds showed a very low response to perforation, this might be a consequence of a deeper embryo dormancy expressed under low incubation temperatures in late sowing dates that limits the expression of coat-imposed dormancy in Chadmo seeds. Embryo dormancy was suggested (but not proved) before for C. album, as a possible explanation for the lack of response to seed scarification (Williams, Reference Williams1963). This paper presents firm arguments in support of the existence of embryo dormancy in the genus Chenopodium.
The seed coat thickness of 2-Want was reduced due to late sowings in both years, in accordance with the decrease in dormancy level (Table 1). Similar results were reported previously in the genus Chenopodium for C. polyspermum, C. album and C. bonus-henricus (Jacques, Reference Jacques1968; Karssen, Reference Karssen1970; Pourrat and Jacques, Reference Pourrat and Jacques1975, Dorne, Reference Dorne1981). In those three species the increased coat thickness has been associated with a higher level of dormancy. However, the fact that Chadmo seeds did not respond in that way to sowing date is a warning against generalizations of these patterns to the whole species.
Nevertheless, seed dormancy was associated with exposure of seeds to different environmental conditions during their development. Temperatures and photoperiods experienced during this period are associated with seed dormancy level in quinoa (Ceccato et al., Reference Ceccato, Bertero and Batlla2011). Seed coat thickness in 2-Want seeds is also correlated with the mean temperature and photoperiod during their development (Fig. 4). Due to the covariance of these factors, it became impossible under field conditions to know which factor (temperature and/or photoperiod) is responsible for the observed variation in seed dormancy and seed coat thickness, and this matter deserves further analysis.
Like quinoa, C. polyspermum and C. album seeds formed under a short photoperiod had thinner seed coats and lower dormancy (Jacques, Reference Jacques1968; Karssen, Reference Karssen1970; Pourrat and Jacques, Reference Pourrat and Jacques1975). The relative changes in seed coat thickness, and the germination percentage linked to that, were similar to the ones observed in accession 2-Want, but were obtained by exposing plants to a higher difference between photoperiods (10–16 h). In 2-Want, the difference was of ~3.5 h. Any temperature effect during seed development on seed dormancy has been tested extensively in other species (e.g. Fenner, Reference Fenner1991; Fonseca and Sanchez, 2000; Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sánchez2004). In the case of 2-Want, the difference between average temperatures in the first and last sowing date was 11°C in the first and 7°C in the second year for the effective seed-filling period (between the onset of filling and PM). Thus, possible effects of variation in environmental temperature should not be ruled out.
Archaeological studies in eastern North America related differences in coat thickness to the level of domestication in C. berlandieri ssp. jonesianum. Thicker coats (mean 34 μm) were associated with wild or weedy forms, and thinner coats (mean 15.85 μm) with cultivated forms (Gremillion, Reference Gremillion1993a, b). A similar reduction in seed coat thickness (12.8 to 2.4 μm average) was linked to domestication of C. quinoa, as observed in Chiripa, Bolivia (Bruno, Reference Bruno2005, Reference Bruno, Zeder, Bradley, Emshwiller and Smith2006). These changes were assumed to be the consequence of selection, with the objective of reducing dormancy. This hypothesis, however, was not assessed with viable seeds. According to results of the present work, an association of seed coat thickness with germination per cent between sowing dates was established in one of the two accessions studied. Moreover, Chadmo seeds consistently exhibited a thicker seed coat (Table 1) and were consistently more dormant than 2-Want seeds (Figs 1 and 2). Besides, seed coats measured in accession 2-Want (Table 1) were two times thicker than those reported by Bruno (Reference Bruno2005, Reference Bruno, Zeder, Bradley, Emshwiller and Smith2006) in modern seeds from Chiripa, Bolivia. For BO25, an accession native to central Chile, López Fernández (Reference López Fernández2008) reported an exotesta thickness nearly twice that of another eight cultivars tested (~ 25 μm vs. 12–15 μm), including Chilean and Bolivian accessions. Together, these observations indicate an important variability in coat thickness between accessions of C. quinoa. A comparison of dormancy level between accessions with contrasting coat thickness in a range of environments is needed to provide more information about the association between coat thickness and dormancy level in the species.
Less ABA leached from Chadmo seeds upon imbibition than from 2-Want seeds (compare the two controls in Fig. 5A and B), which suggests that deeper dormancy in Chadmo seeds can be explained partially by less ABA leaching.
More ABA leached from Chadmo seeds upon perforation; therefore, the seed coat plays a stronger role in ABA leaching, and the greater thickness may also play a role. The role of the seed coat as a barrier for ABA diffusion has been reported for several species (Wang et al., Reference Wang, Heimovaara-Dijkstra and Van Duijn1995; Bianco et al., Reference Bianco, Garello and Le Page Degivry1997; Ren and Kermode, Reference Ren and Kermode1999; Feurtado et al., Reference Feurtado, Ren, Ambrose, Cutler, Ross, Abrams and Kermode2008). Interestingly, the dry 2-Want seeds have weaker dormancy, yet a higher ABA content. Apparently, ABA content in dry seeds is not necessarily directly associated with dormancy level. On the other hand, the possibility of differences in the ability of seeds to synthesize ABA de novo, or to catabolize ABA during incubation, should not be neglected.
Among other probable action mechanisms of coats in the regulation of dormancy, interference with the absorption of water was considered unlikely, since a very fast imbibition was found when seeds were incubated in water and 100% germination was reached post-maturation (Ceccato et al., Reference Ceccato, Bertero and Batlla2011). Besides, any mechanical restraint to radicle emergence would be unlikely since a small hole in the middle of the seed (far from the micropyle) was sufficient to remove dormancy, although the possibility of an indirect mechanism cannot be dismissed.
Conclusions
It was suggested that embryo dormancy is present in the genus Chenopodium. Coats affect dormancy in quinoa, and the seed coat tissue is largely responsible for this effect. The contribution of embryo dormancy to whole-seed dormancy is expressed under conditions in which coat-imposed dormancy is reduced.
Environmental conditions during seed development affect the level of dormancy and relative contribution of the seed coats and the embryo to seed dormancy level. Those experienced at early sowing dates (higher temperatures and longer photoperiods) lead to an increase in coat and a decrease in embryo dormancy, while those experienced at late sowing dates (lower temperatures and shorter photoperiods) lead to a decrease in coat and an increase in embryo dormancy.
The obstruction of ABA release to the medium by the seed coats appears to have a central role in dormancy maintenance. This was revealed by germination responses to perforation treatments in both accessions, and the concomitant increase in ABA levels in the incubation medium. Consistently, more ABA is able to leak to the medium from seeds with a thin seed coat. The thickness of the seed coat seems to explain variation in seed dormancy between accessions, while its variation among environments was consistent only for one of the two evaluated accessions.
Acknowledgements
We thank Gabriela Zarlavsky for histological sections, Silvina Enciso for ABA determinations, Martín León for technical assistance, Roberto Benech-Arnold and IFEVA (Institute for Physiological and Ecological Research Applied to Agriculture – UBA – CONICET) for providing us with working facilities and INTA Base Bank of Germplasm for help in experiments.
Financial support
This work was supported by a fellowship from INTA (National Institute of Agricultural Technology), Argentina.
Conflict of interest
None.









