Introduction
The promotion of germination by light is a common feature of small seeds of many plant families (Millberg et al., Reference Millberg, Anderson and Thompson2000; Barzani et al., Reference Barzani, Quaye, Ohali, Barzilai and Kigel2012; Baskin and Baskin, Reference Baskin and Baskin2014), including cacti (Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011). Seed of six species of Argentine cacti (Lindow-Lopez et al., Reference Lindow-Lopez, Galindez, Aparicio-Gonzalez, Suhring, Rojas-Arechiga, Pritchard and Ortega-Baes2018) and five species of Southern Chihuahuan desert cacti (Mascot-Gomez et al., Reference Mascot-Gomez, Flores, Lopez-Lozano and Yanez-Espinosa2020) hardly germinate or fail to germinate in the dark. Such photoblastism is known for seeds of the cacti Carnegiea gigantea (Alcorn and Kurtz, Reference Alcorn and Kurtz1959), Cereus griseus (Williams and Arias, Reference Williams and Arias1978), Ferocactus histrix (del Castillo, Reference del Castillo1986) and at least 250 other taxa in the family (Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Orozco-Segovia and Vázquez-Yánes1997; Nobel, Reference Nobel1988; Barrios et al., Reference Barrios, Sanchez, Flores and Jurado2020). For example, out of the 30 taxa of cacti occurring in north-eastern Brazil, 22 taxa all from the Cactoideae subfamily were classified as positive photoblastic with no germination in darkness, while the other eight taxa from the Opuntioideae (five taxa) and Pereskioideae (three taxa) subfamilies were indifferent to light (Meiado et al., Reference Meiado, Rojas-Aréchiga, de Siqueira-Filho and Leal2016).
Evidence suggests that seeds of barrel cacti, which tend to be smaller, have a greater preference for germination in the light than the usually larger-seeded columnar species, which may germinate equally well with or without light (Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Orozco-Segovia and Vázquez-Yánes1997; Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011). For temperate woodland herbs, seeds with masses >1.5 mg tended to have less preference for light germination (Jankowska-Blaszczuk and Daws, Reference Jankowska-Blaszczuk and Daws2007). In contrast, this smaller versus larger seed light preference threshold does not appear to apply consistently in the cacti. While two larger-seeded (5–10 mg) Pachycereus species germinate equally well in the light and dark (Yang, Reference Yang1999), in 55 species of the tribe Cacteae, there was evidence of positive photoblastism with seed mass <1.5 mg (45 species) and >1.5 mg (10 species), with no germination under dark conditions (Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Mandujano and Golubov2013). Moreover, in 136 cactus taxa, there is strong covariance between relative light germination (RLG) and log10 seed mass (Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011).
In comparative studies, seed positive photoblastism is often measured as RLG (Millberg et al., Reference Millberg, Anderson and Thompson2000) rather than germination percentage as seed lots can differ in their dormancy level as a result of inter-seasonal variation in environments during seed maturation and other factors. In a study of six cactus species of the Sonoran desert, four smaller-seeded species in Stenocereus and Ferocactus had RLG of 1 and two larger-seeded Pachycereus species had RLG of 0.51 (Yang, Reference Yang1999). In another study of four accessions of three cactus species (Coryphanta clavata, Mammillaria compressa and two varieties of Ferocactus latispinus) from southern Chihuahuan Desert (Mexico), with seed masses between 0.39 and 1.6 mg, three out of the four seed accessions germinated higher in light and only C. clavata had a RLG of 0.5 (Flores et al., Reference Flores, González-Salvatierra and Jurado2016). For 17 cactus species from north-western Córdoba province in Argentina, the RLG was 1 for eight species, and always ≥0.65, irrespective of seed mass (Sosa Pivatto et al., Reference Sosa Pivatto, Funes, Ferreras and Gurvich2014). Similarly, among 55 species of Cacteae tribe, seed RLG was 1, suggesting that the photoblastic response is phylogenetically fixed and coupled with environmental cues that fine tune the germination response (Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Mandujano and Golubov2013). Overall, in the cacti, RLG has been estimated to range from 0.441 (Copiapoa gigantea) to 1 (around 60% of 196 seed lots studied among 136 taxa) (Seal et al., Reference Seal, Flores, Ceroni Stuva, Dávila Aranda, León-Lobos, Ortega-Baes, Galíndez, Aparicio-González, Castro Cepero, Daws, Eason, Flores Ortiz, del Fueyo, Olwell, Ordonez, Peñalosa Castro, Quintanar Zúñiga, Ramírez Bullón, Rojas-Aréchiga, Rosas, Sandoval, Stuppy, Ulian, Vázquez Medrano, Walter, Way and Pritchard2008; Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011).
In many plant species, RLG decreases with increasing seed mass, for example, in 27 temperate forest herbs (Jankowska-Blaszczuk and Daws, Reference Jankowska-Blaszczuk and Daws2007), neotropical pioneer species (Pearson et al., Reference Pearson, Burslem, Mullins and Dalling2002) and in 136 cactus taxa (Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011). As light only penetrates into the soil by a few millimetres (Tester and Morris, Reference Tester and Morris1987) and light quality will be attenuated towards higher wavelengths (Bliss and Smith, Reference Bliss and Smith1985), a light requirement for germination acts as a depth sensing mechanism to enable small seeds to germinate only when close the surface, or from the soil seed bank after tillage or erosion of the upper soil layer (De la Barrera and Nobel, Reference De la Barrera and Nobel2003).
In addition to reacting to the presence/absence of light, small seeds may also be responsive to light quantity; too much light (high irradiance reaction, HIR) can be inhibitory (Carta et al., Reference Carta, Skourti, Mattana, Vandelook and Thanos2017). Often the inhibitory effects of light are associated with light spectral quality, specifically the ratio between red (RED; 660 nm) and far-red (FR; 730 nm) light (RED:FR). Whereas under leaf canopies and leaf litter the filtering of RED light results in a RED:FR of around 0.2 or lower, unfiltered daylight has a ratio of around 1.2 (Federer and Tanner, Reference Federer and Tanner1966; Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002). Small seeds (<1.5 mg) of temperate woodland herbs prefer high-quality microsites with the RED:FR ratio of >0.5 (Jankowska-Blaszczuk and Daws, Reference Jankowska-Blaszczuk and Daws2007). For neotropical pioneer species, the seed germination preference for light quality matches the habitat preference of the adult plant, for example, gap size, with smaller seedlings being less likely to be able to compete in shade conditions (Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002). Thus, the requirement of small seeds of cacti, and other species, for germination in the light can be seen as an important means of preventing germination at times and in places that are unfavourable for seedling establishment.
Habitat conditions can change and the process of germination under favourable light conditions can be adversely affected by a shift in light spectral quality. A decreased ratio of RED:FR may then result in the establishment of induced or secondary dormancy (Bewley and Black, Reference Bewley and Black1994; Baskin and Baskin, Reference Baskin and Baskin2014) as a result of the dark reversion of the FR, active form of phytochrome, P FR, to the red receptive form, P R. Germination stimulation and the counterbalancing inhibition by alternating illuminations, and the reversibility of these triggers, were first observed by Borthwick et al. (Reference Borthwick, Hendricks, Parker, Toole and Toole1952). Interestingly, seeds of some cacti are known to be insensitive to FR; when applied for 12 h d−1 at a constant 25°C, germination occurred in all seven cactus species studied (Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Orozco-Segovia and Vázquez-Yánes1997.) However, for some of the species, the germination level in FR light was significantly lower than that in both the RED and white light treatments. Detailed information on how cactus seeds react to light in terms of germination stimulation and possible subsequent desensitization to light signals by dark incubation is not available (Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011)
C. repandus (L.) Mill. formerly C. peruvianus (L.) Miller is a non-arborescent cactus that grows to around 1 m tall and produces edible fruits of commercial importance (Mizrahi, Reference Mizrahi2014). The species has a water-use efficiency of about 10 times higher than C3 fruit tree species; it can tolerate very high temperatures and low relative humilities and is an ideal fruit crop for semi-arid zones (Mizrahi, Reference Mizrahi2014). The seeds are relatively small (3.2 mg) and have a RLG of 1, similar to that of two other Cereus species (Yang, Reference Yang1999). The temperature control of germination has been elucidated, with an average base temperature for the germination rate, T b, across percentiles of 10.4 ± 0.3 (SD) °C and a thermal time for 50% germination (θ 50) of around 50 °C d (Yang, Reference Yang1999). These are germination trait features that are similar to those of the relatives Polaskia chende and P. chichipe, which have T b of 11.2 and 12, and θ 50 of 65 and 54 °C d, respectively (Ordoñez-Salanueva et al., Reference Ordoñez-Salanueva, Seal, Pritchard, Orozco-Segovia, Canales-Martínez and Flores Ortiz2015). C. repandus is, thus, an ideal model in which to study the sensitivity of cactus seed to light spectral quality. In this paper, we explore (1) the requirement of C. repandus seed germination for light; (2) the differential sensitivity of the seeds to RED and FR light dose among seed lots; (3) the reversibility of the RED light response by FR light; and (4) the kinetics of dark-induced desensitization to the light triggering of germination.
Materials and methods
Seed accessions
Seeds from mature fruits of Cereus repandus (L.) Mill. (syn. Cereus peruvianus) – the Peruvian apple cactus – were provided by the breeding programme of the Ben Gurion University of the Negev, Israel (Batches 1 and 2; B1, B2). Batch 3 (B3) seed was purchased from a commercial supplier (Whitestone Gardens Ltd, UK). Seed dry weight was measured after oven drying (103°C, 17 h) of 15 individual seeds per seed lot. After receipt, the seeds were kept at 45% relative humidity (RH) and 15°C in heat-sealed aluminium foil laminate bags. Seed RH was measured at 21°C using a Michell series 4020 dewpoint hygrometer (B1, B2) or a Rotronic hygrometer (B3) after a 30 min period. Times of storage under these conditions before use varied from a 1 (B2, B3) to 2 years (B1).
Light-mediated germination
An initial screen for germination in light and darkness was carried out at 26°C, within the sub-optimal temperature range for germination rate (Yang, Reference Yang1999), using 50 seeds (n = 2 × 25) per treatment. Seeds were sown on 1% (w/v) agar-water in Petri dishes and incubated in the presence of light (12 h d−1, warm white fluorescent light at around 15 μmol m−2 s−1) or the absence of light, achieved by wrapping dishes in aluminium foil. Germination (>1 mm radicle emergence) was recorded under very low-level green light (Pritchard and Manger, Reference Pritchard and Manger1990). The number of germinated seeds was recorded regularly over a 2- to 3-week period until no more germination occurred.
All other experiments were also performed with seeds on agar-water at 26°C. Before and after light treatment (RED or FR), the Petri dishes were placed in aluminium foil-wrapped containers to exclude light. Light treatments were applied to seeds with the Petri dish lids removed. Red (RED) and far-red (FR) light were generated from a Rank Aldis Tutor No. 2 slide projector using a 655–665 nm (RED) or a 725–735 nm (FR) interference filter. The RED or FR was transmitted through a porthole in the side of a constant temperature (26°C) incubator and deflected through 90° onto the seeds using a glass mirror.
Imbibition time and light sensitivity
Seeds were rehydrated in the dark for varying periods up to 3 d. Immediately before illumination, five seeds of each sample were removed for moisture content (MC) assessment (after surface drying on laboratory tissue paper). Then, the seeds were given various light treatments (n = 3 × 25 seeds per dose). For B3 seeds, with higher initial germination, the RED light dose given was 25 min. For B2 seeds, 100 min RED light was applied. The dishes were re-wrapped and kept at 26°C for germination and scored only under very low-level green light.
Differences in RED light sensitivity between seed batches were explored by imbibing seeds in the dark for 3 d, followed by the exposure of the seeds to RED light for up to 1000 min. Per dose, duplicate dishes of 25 seeds were re-wrapped and kept at 26°C for germination and scored only under very low-level green light.
Loss of RED sensitivity
The loss of RED light sensitivity in seeds was investigated in different ways. Seeds of B2 were imbibed at 26°C in the dark for periods up to 18 d and then exposed to RED light for 100 and 1000 min. Also, B3 seeds were given two different short doses of RED light per day, followed by being held in the dark for the rest of the day. In this case, B3 seeds were rehydrated at 26°C in the dark for 3 d and then treated with RED illumination for either 1 or 5 min daily for a total of 20 min RED light, that is, the treatments were applied over 20 and 4 d, respectively. All post light treatment germination at 26°C was assessed under very low-level green light.
Reversibility of RED by FR
The reversibility of the RED light effect by FR light was assessed in two ways. Seeds of B2 were hydrated for 3 d, irradiated with RED for 1000 min and then exposed to FR for up to 150 min immediately thereafter. Also easier to germinate B3 seeds were hydrated for 3 d, and exposed to RED for 60 min. Then after periods up to 1 d in the dark, the seeds were exposed to FR for 10 min. Dishes were re-wrapped and kept at 26°C for germination and scored only under very low-level green light. Two×25 seeds were used for each of the treatments.
Data analysis
The effects of light and darkness on germination were compared statistically using a two-sample test for the equality of proportions using S-Plus 2000 software (MathSoft, Inc.). RLG calculation followed the method of Millberg et al. (Reference Millberg, Anderson and Thompson2000) as follows: RLG = Gl/(Gd + Gl), where Gl is the germination percentage in light and Gd is the germination percentage in darkness.
All dose dependencies of seed germination to illumination were subjected to probit analysis (to accommodate variation in seed numbers and responders) in Generalized Linear Interactive Modelling (GLIM), with fits for convergence and parallel responses subjected to F-statistics (Crawley, Reference Crawley1993).
Because all light treatments involved duplicates (mainly) or triplicates (rarely) of 25 seeds, the error bars on the figures are shown as binomial confidence limits (95%) for the total seeds sown and responding. In Figs. 1B,C, the MC values are for single determinations per time point per seed batch.
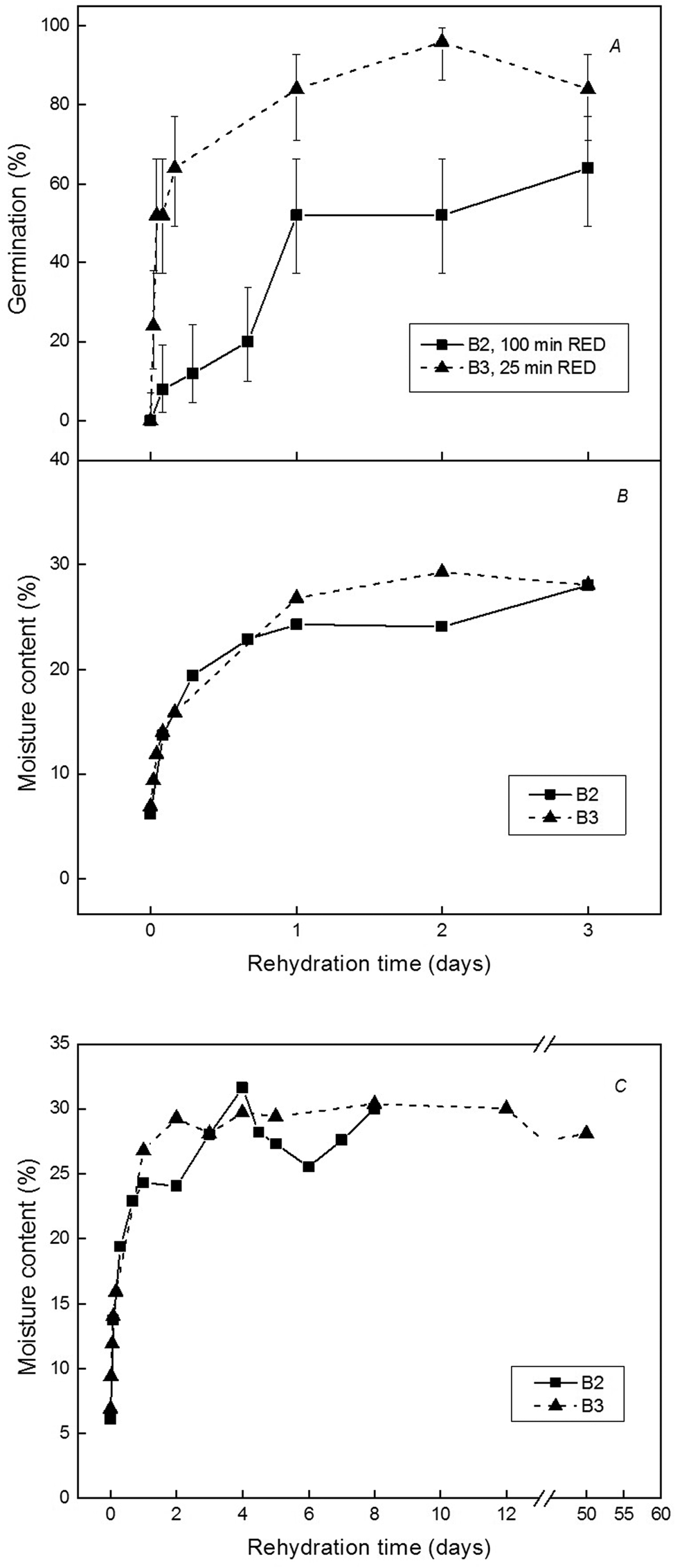
Fig. 1. Effects of imbibition time and exposure time to RED light on the germination of C. repandus B2 (■) and B3 (▴) seeds at 26°C. (A) Mean seed germination after imbibition and exposure to 100 min (B2 seeds) and 25 min (B3 seeds) RED light. Binomial confidence limits (95%) are shown for the total responders based on three replicates of 25 seeds sown. (B,C) Changes in seed MC with shorter-term (≤3 d; B) and longer-term (≥8 d; C) imbibition at 26°C prior to RED illumination for B2 (■) and B3 (▴) seeds. The MC means are for a single determination per time point per seed batch.
Results and discussion
Relative light germination
Seeds of all three batches failed to germinate in the dark and reached 96% (B2) or 98% (B1, B3) germination in 12 h d−1 light. RLG is therefore 1 in all cases.
The triggering of seed germination in cacti by light, that is, positive photoblastism, is well known and is associated with a range of plant traits including shorter stature and lighter seeds. C. repandus is cylindrical and has relatively light seeds (3.2 mg). Other species in the tribe Cereeae also have light seeds, and a RLG averaging 0.89 (n = 12 species) (Yang, Reference Yang1999; Seal et al., Reference Seal, Flores, Ceroni Stuva, Dávila Aranda, León-Lobos, Ortega-Baes, Galíndez, Aparicio-González, Castro Cepero, Daws, Eason, Flores Ortiz, del Fueyo, Olwell, Ordonez, Peñalosa Castro, Quintanar Zúñiga, Ramírez Bullón, Rojas-Aréchiga, Rosas, Sandoval, Stuppy, Ulian, Vázquez Medrano, Walter, Way and Pritchard2008; Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011). Small (0.62–0.77 mg) seeds of Trichocereus terscheckii also germinate in the light but not in the dark (Ortega-Baes and Rojas-Aréchigo, Reference Ortega-Baes and Rojas-Aréchigo2007). These species thus shows a preference for germination close to the soil surface, as light only penetrates to a depth of a few millimetres (Tester and Morris, Reference Tester and Morris1987). The relationship between light preference for germination and smaller seeds has been reported previously for European herbs from a variety of habitats (Millberg et al., Reference Millberg, Anderson and Thompson2000; Jankowska-Blaszczuk and Daws, Reference Jankowska-Blaszczuk and Daws2007), pioneer trees from semi-deciduous tropical forest and cacti, with around 60% of a broad range of seed lots among 136 taxa having RLG of 1 (Seal et al., Reference Seal, Flores, Ceroni Stuva, Dávila Aranda, León-Lobos, Ortega-Baes, Galíndez, Aparicio-González, Castro Cepero, Daws, Eason, Flores Ortiz, del Fueyo, Olwell, Ordonez, Peñalosa Castro, Quintanar Zúñiga, Ramírez Bullón, Rojas-Aréchiga, Rosas, Sandoval, Stuppy, Ulian, Vázquez Medrano, Walter, Way and Pritchard2008; Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011). However, less is known for light-responsive cactus seeds about the spectral requirements for the trigger, which has implications for understanding of the importance of nurse plants, and shading, in the natural regeneration process in cacti (Ordoñez-Salanueva et al., Reference Ordoñez-Salanueva, Seal, Pritchard, Orozco-Segovia, Canales-Martínez and Flores Ortiz2015). This specific requirement for light might also serve to stop the seed from germinating within the developing fruit before endozoochorous dispersal (Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011), although this is not the case in all cacti as vivipary has been reported for at least 53 species (Cota-Sánchez, Reference Cota-Sánchez2004; Cota-Sánchez et al., Reference Cota-Sánchez, Reyes-Olivas and Abreu2011; Aragón-Gastélum et al., Reference Aragón-Gastélum, Flores, Yáñez-Espinosa, Reyes-Olivas, Rodas-Ortiz, Robles-Díaz and González2017).
Onset of RED light sensitivity is related to the seed imbibition period
The imbibition rates for B2 and B3 seeds were very similar, with MCs increasing rapidly to around 25% fresh weight (FW) basis by 24 h and remaining around 30% from 2 to >8 d. (Fig. 1). During water uptake, the sensitivity of seeds to RED light increased. Few seeds responded to RED when applied around 10% MC, about half responded at an average seed MC of 15%, and at 25% MC, all seeds capable of germination responded to RED. Thus, as the seeds were hydrated in the transitional region of sorption zones II and III, they became sensitive to light-stimulated germination, similar to the heat shock response of Carica papaya seeds (Wood et al., Reference Wood, Pritchard and Amritphale2000). What was noticeable was the difference in RED light sensitivity among seed batches with B2 requiring 100 min RED to achieve around 60% germination compared with only 25 min for B3 to be saturated with RED and achieve higher germination (around 90%) (Fig. 1A).
Germination is dependent on RED light
With the sufficient dose of RED light seed germination in all three batches of C. repandus reached 88–96% (Fig. 2). However, the single dose required to promote a certain level of germination varied between batches, indicating different initial competencies for germination. While B2 required a dose of around 100 min RED to attain around 50% germination, the other two batches needed <10 min RED. Thus, the requirement for RED light for the germination of B3 and B1 seed was more than one order of magnitude lower than B2. Pre-storage time could have impacted the germination response, as B1 had been stored the longest (2 years) and had the highest initial germination level without RED treatment (Fig. 2, closed circles). However, parallel RED dose-response lines for the three batches suggest that the variation in pre-storage time did not affect the overall sensitivity of seeds to increasing RED (Fig. 2).
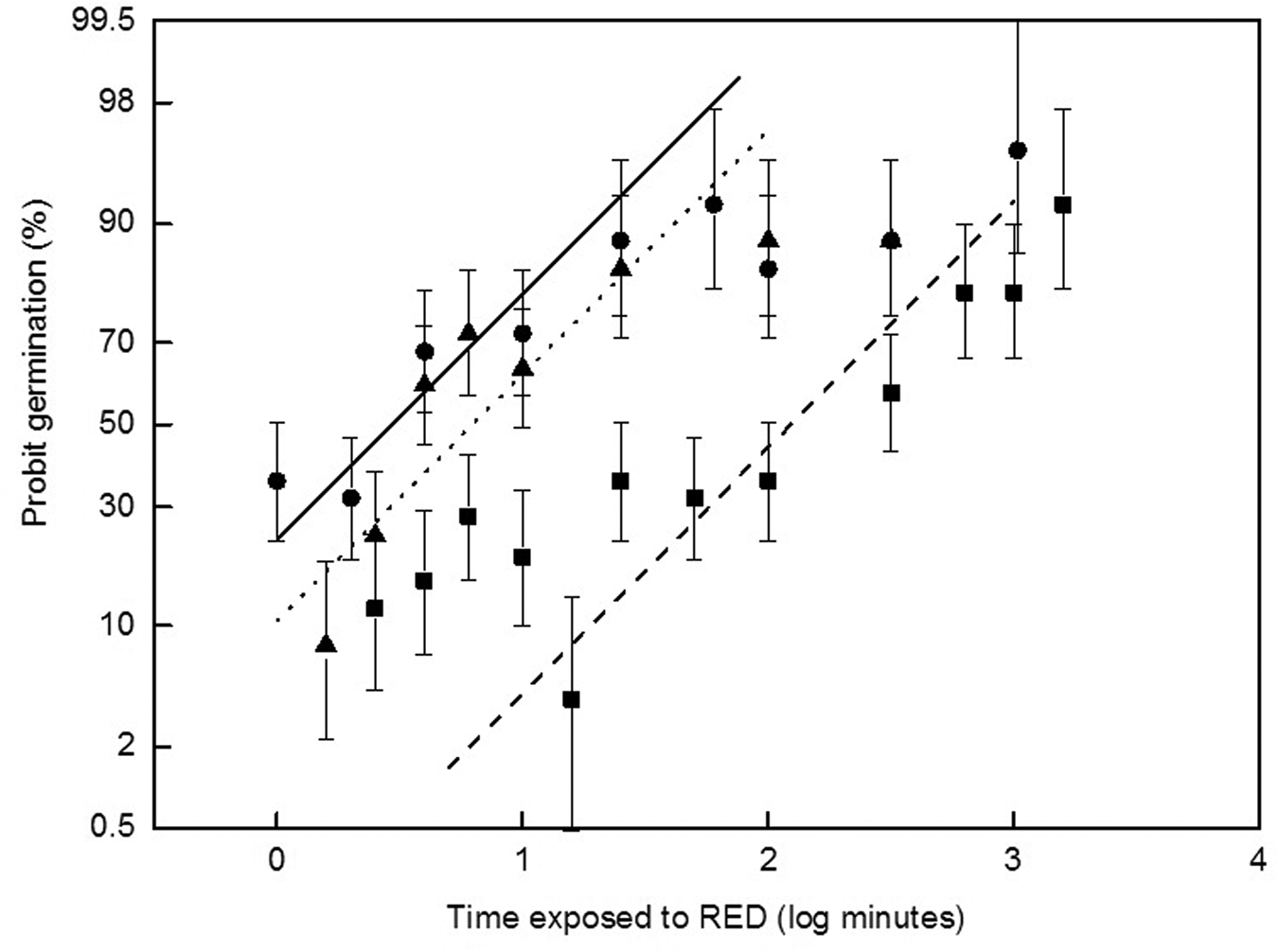
Fig. 2. Effects of RED light dose (log min) on seed germination (probability scale) for C. repandus B1 (⬤), B2 (■), B3 (▴). Probit germination increased by 1.571 ± 0.059 per log dose (min) for each seed batch, which had zero dose germination levels. Further explanation for fitting parallel lines is given in the text. Binomial confidence limits (95%) are shown for the total responders based on two replicates of 25 seeds sown per light dose.
Intra-specific variability in seed lot quality with respect to the seed proportion that easily germinates is well known, and is often a function of the maternal environment during development, for herbaceous (Baskin and Baskin, Reference Baskin and Baskin2014) and tree (Daws et al., Reference Daws, Lydall, Chmielarz, Leprince, Matthews, Thanos and Pritchard2004) seeds. In addition, B2 seed was stored for the shortest period of time (1 year) prior to experimental use and post-harvest handling can affect the depth of any primary dormancy. In the case of Stenocereus queretaroensis seeds, storage in the dark at 25°C and 42% RH increased germination competence to 85% after around 15 months (De la Barrera and Nobel, Reference De la Barrera and Nobel2003), similar to that of B1 which had been stored dry for 2 years before use.
A linear relationship was observed between seed germination (on a probit scale) and exposure time to RED for the seed batches B1, B2 and B3 (Fig. 2). Fitting parallel lines did not significantly affect the goodness of fit compared to free-fitting [F = 1.22; tabulated F(2, 28) 3.34; r 2 = 0.850], such that probit germination increased by 1.571 ± 0.059 per log dose (min). This suggests that although the initial competency levels for germination were different (intercepts 4.266 ± 0.101, 1.715 ± 0.124 and 3.752 ± 0.116 probit for B1, B2 and B3, respectively), the seed sensitivity (i.e. the distribution of responses per dose) to the RED dose was the same across seed batches of this species. Thus, germination capability increased by 1 probit per 0.64 log min of RED dose in all three seed batches.
High sensitivity to increasing the RED light level has been observed in the cactus S. queretaroensis, for which seed germination approximately doubled per log dose (De la Barrera and Nobel, Reference De la Barrera and Nobel2003). As C. repandus seed germination increased about fourfold per log dose (Fig. 2), it appears that these seeds are relatively more sensitive to RED light than S. queretaroensis. The fine-tuning of the seed response to light is modulated by PIF (phytochrome[phy]-interacting factor) transcription factors, which are pivotal in cellular signalling and promote shade-avoidance through regulated gene expression (Leivar and Quail, Reference Leivar and Quail2011). When phy molecules are activated by light, the rapid degradation of PIF proteins is induced. Other pathways converge to regulate PIF, however, including the gibberellin pathway which contributes to the lowering of seed physiological dormancy. In Arabidopsis, for example, PIF1 promotes the expression of two gibberellic acid (GA)-repressor (DELA) genes that decrease the sensitivity of the seed to GA (Leivar and Quail, Reference Leivar and Quail2011).
Dark incubation reduces light sensitivity
The long-term retention of light-sensitive seeds in the dark often results in the desensitization to light (or the induction of secondary dormancy; Bewley and Black, 1994). To see if this was also the case for C. repandus, B2 seeds were left in the dark for greater than the usual period (3 d prior to RED). Two conditions of RED stimulation, 100 min and 1000 min, were applied and dark incubation times of up to 18 d were used.
Lengthened dark imbibition decreased light sensitivity significantly (Fig. 3). For example, germination was reduced from around 92 to 20% when dark incubation time increased from 2 to 18 d prior to irradiation with 1000 min RED. When seeds were only exposed to RED for 100 min, germination fell from 52% after the shortest dark incubation time of 2 d to 20% after the seeds were incubated in the dark for 8 d. A negative linear relationship between probit germination and dark incubation time was observed for both RED doses. GLIM analysis showed an insignificant difference between the parallel model and free-fitting models (F = 0.79; tabulated F(1,10) 4.96; r 2 = 0.863), such that probit germination = 7.331–0.242x (1000 min RED) and probit germination = 5.709–0.242x (100 min RED). The reduced sensitivity of C. repandus seeds to RED light after long-term (1–2 weeks) exposure to darkness suggests that buried seeds could enter the soil seed bank (Fig. 3).

Fig. 3. Effect of dark incubation time prior to RED light irradiation on the germination (probability scale) of C. repandus B2. Seeds were imbibed for up to 18 d prior to the application of RED light for 1000 min (■) or 100 min (▫). Binomial confidence limits (95%) are shown for the total responders based on two replicates of 25 seeds sown. The fitted parallel lines indicate a one probit reduction in germination per 0.242 dose of dark imbibition, that is, every 4.1 d.
The effect of multiple, short doses of RED light and long periods of darkness per day on seed germination was also assessed. B3 seeds were rehydrated in the dark for 3 d and exposed to 1 or 5 min of RED per day, cumulatively for 20 min, with germination proceeding in the dark. When 1 min of RED was provided daily, germination increased to around 40% by 15 d, but there was no further increase by the 20th day (Fig. 4). Germination promotion did not reach the full level for this seed lot. A germination increase was greater when 5-min daily exposures to RED were used. A single 5-min treatment on the first day resulted in 48% germination, with the trajectory for germination promotion similar to that of a 1-min daily dose (Fig. 4). However, four daily 5-min treatments (i.e. 20 min cumulatively) raised germination to 92%, close to the maximum for this seed lot (Fig. 4).

Fig. 4. Effect of cumulative exposure to RED light on the germination of B3 seeds. Seeds were imbibed in the dark for 3 d and then RED light applied for 1 min (▴) and 5 min (▵) per day to total exposure time of 20 min. Thus, the experiments ran for 20 and 4 d, respectively. Binomial confidence limits (95%) are shown for the total responders based on two replicates of 25 seeds sown.
A single dose of 100 min of RED light are sufficient to saturate the light requirement in B3 seeds, which then germinate to a high level in the dark (Fig. 2). The seeds are clearly highly sensitive to RED light, as the dose effect can be reduced to just 20 min at 5 min a day for 4 d. However, shortening the exposure to RED light to 1 min, a day only partially promotes subsequent germination (Fig. 4). Nonetheless, the seeds show signs of a low fluence response (Cone et al., Reference Cone, Jaspers and Kendrick1985; De Petter et al., Reference De Petter, van Wiemeersch, Rehty, Dedonder, Fredericq and De Greef1988), similar to that of S. queretaroensis seeds that respond over a 10-d period to a minimum light level of 0.15 μmol m−2 s−1 for 10 h d−1 (De la Barrera and Noble, Reference De la Barrera and Nobel2003). Such sensitivity to high-quality light acts as a gap-sensing mechanism that also enables the rapid emergence of the seedling (Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002). When the daily RED dose is insufficient to stimulate all seeds to germinate (i.e. <5 min), the long periods of darkness between RED applications are presumed to be sufficient to permit the reversion of the active form of phytochrome (P FR) to the inactive form (P R), and some of the seeds fail to germinate potentially because of the induction of secondary dormancy (Cone et al., Reference Cone, Jaspers and Kendrick1985; De Petter et al., Reference De Petter, van Wiemeersch, Rehty, Dedonder, Fredericq and De Greef1988). Similarly, seeds of eight out of 11 species of cacti did not germinate to a significantly higher level when the same set of seeds was transferred from dark to light, suggesting that darkness had triggered secondary dormancy (Flores et al., Reference Flores, Jurado and Arredondo2006). The molecular framework for such rapid and time-lapse responses to light could be modulated by the interaction between de-etiolated 1 (DET1), which is an evolutionary conserved protein and central repressor of light-induced germination, constitutive photomorphogenic 10 (COP10), long hypocotyl in FR 1 (HFR1) and PIF1 (Shi et al., Reference Shi, Wang, Mo, Tang, Zhong and Deng2015).
As postulated for S. queretaroensis (De la Barrera and Nobel, Reference De la Barrera and Nobel2003), only with erosion of the soil surface or tilling would C. repandus seeds likely accumulate sufficient light to germinate. This response has the advantage that the seeds could await rainfall in a habitat, in which rainfall events are far apart. Eventual emergence therefore depends on the seeds having the ability to survive. Seeds used in this study, which were stored for up to 2 years before experimentation, nonetheless had initial (on receipt) germination competency of at least 96%, indicating, as with S. queretaroensis (De la Barrera and Nobel, Reference De la Barrera and Nobel2003), that the seeds have good longevity. Nonetheless, it would be interesting to know precisely how temperature and humidity affect seed longevity in C. repandus.
RED light effect is reversible by FR light
The involvement of phytochrome in the RED light response was confirmed by reversing the effect with FR light using seed lots B2 and B3.
For B2 seeds, the greater the dose of FR given to seeds immediately after 3-d hydration followed by exposure to RED for 1000 min, the less effect RED had on germination. While control (1000 min RED) seeds reached 80% germination, irradiation with FR for 1.5 min (0.18 log min), 15 min (1.176 log min) and 150 min (2.18 log min) reduced the germination to 48, 34 and 26%, respectively. GLIM analysis revealed a significant (r 2 = 0.986, P < 0.05) dependency of (decreased) germination on the FR of −0.488 ((±0.215) probit per log min, suggesting that FR was very effective at increasing the requirement for RED. Numerous PIFs are known to repress light responses in plants, including PIF8 (Oh et al., Reference Oh, Park, Song, Bae and Choi2020). PIF8 protein accumulates more in Arabidopsis in FR light than in darkness or RED light through a mechanism that includes the inhibition of COP1 by phyA (Oh et al., Reference Oh, Park, Song, Bae and Choi2020). In seeds, the FR light response depends on phyA being transported from the cytoplasm to the nucleus by shuttle proteins FAR RED ELONGATED HYPOCOTYL 1 (FHY1) and FHY1-like (FHL) (Sheerin and Hiltbrunner, Reference Sheerin and Hiltbrunner2017). In the nucleus, light-activated phytochromes suppress the E3 ubiquitin ligase complex CUL4/DDB1COP1/SPSA and inactivate PIF, although additional mechanisms must exist that shift the action of phyA to FR (Sheerin and Hiltbrunner, Reference Sheerin and Hiltbrunner2017). For example, in Arabidopsis seeds, SPATULA gene expression is repressed under FR light in a PIF1-dependent manner (Vaistij et al., Reference Vaistij, Barros-Galvao, Cole, Gilday, He, Li, Harvey, Larson and Graham2018).
As FR clearly desensitized the seeds to RED light, we next investigated how quickly seeds could escape the effect of FR completely (Fig. 5). B3 seeds were hydrated for 3 d in the dark and then given 60 min RED light, which in the control promoted germination to >90% and then subjected seeds to 10 min FR light up to 2 d afterwards. The application of FR within 10 min of RED was sufficient to reduce germination to <10% (Fig. 5). As the time between the RED and FR pulse increased the reversibility of RED by FR was reduced, such that applying FR 24 h after RED failed to stop germination in 60% of the seeds. However, when 48 h were allowed to elapse between RED and FR, FR failed completely to stop the progress of germination; the seeds reached 100% (data point not shown in Fig. 5). The dependency of germination on escape time since RED illumination was described for B3 seed as: Germination (probability) 3.41 (±0.019) + 0.078 (±0.011) dose, in hours (r 2 = 0.945, P < 0.01) (Fig. 5).

Fig. 5. Effect of FR light on C. repandus B3 seed germination (probability scale). After rehydration for 3 d, seeds were exposed to RED for 60 min and, then after a range of escape times (hours), exposed to FR light for 10 min. Responsivity to FR light decreased by one probit of germination per 0.078 dose, that is, 12.8 h. Binomial confidence limits (95%) are shown for the total responders based on two replicates of 25 seeds sown. A data point at 48 h and 100% germination is not shown in the graph but was included in the probit analysis, by convention, as 99.9% germination.
Presumably, C. repandus seed burial or deep shading by a nurse plant cumulatively reduces seed sensitivity to light and supports the formation of a soil seed bank, as is relatively common in small seeds of many cacti, for example, 0.6 mg seeds of P. chende (Flores et al., Reference Flores, Jurado, Chapa-Vargas, Ceroni-Stuva, Dávila-Aranda, Galíndez, Gurvich, León-Lobos, Ordóñez, Ortega-Baes, Ramírez-Bullón, Sandoval, Seal, Ullian and Pritchard2011; Ordoñez-Salanueva et al., Reference Ordoñez-Salanueva, Orozco-Segovia, Canales-Martinez, Seal, Pritchard and Flores-Ortiz2017). However, if the seeds receive sufficient RED light and time to initiate the germination process before burial, the stimulatory effects of RED light are irreversible by FR light. This time interval is evidently around 1–2 d (Fig. 5). At 26°C in the light (12 h d−1), C. repandus seed germination is visible within 3 d (Yang, Reference Yang1999), and germination processes prior to radicle emergence must be far advanced by 2 d, as the seeds will have been at full hydration from 24 h onwards (Fig. 1). The commitment to complete germination quickly is a feature of small seeds of desert plants in order to maximize the likelihood of seedling establishment (Baskin and Baskin, Reference Baskin and Baskin2014). This temporal control of the interplay between the effects of RED and FR light on cactus seed germination involving phytochrome might also be temperature-dependent. The biologically active Pfr state can convert to Pr in a light-dependent thermal relaxation process called dark reversion (Mancinelli, Reference Mancinelli1994). As phyB inactivation is proportional to temperature in the dark, phytochromes potentially also function as thermal timers that integrate temperature information overnight (Jung et al., Reference Jung, Domijan, Klose, Biswas, Ezer, Gao, Khattak, Box, Charoensawan, Cortijo, Kumar, Grant, Locke, Shafer, Jaeger and Wigge2016). Although much is known about how temperature influences cactus seed germination (Yang, Reference Yang1999; Seal et al., Reference Seal, Daws, Flores, Ortega-Baes, Galíndez, León-Lobos, Sandoval, Ceroni Stuva, Ramírez Bullón, Dávila-Aranda, Ordoñez-Salanueva, Yáñez-Espinosa, Ulian, Amosso, Zubani, Torres Bilbao and Pritchard2017; Barrios et al., Reference Barrios, Sanchez, Flores and Jurado2020), the possible modulating and concurrent role of phytochromes in both the thermal and photon responses of cactus seeds appears to be unexplored.
Conclusion
In conclusion, the germination in three seed lots of C. repandus are revealed to be highly sensitive to RED light in a classical phytochrome-mediated response that is reversible by FR light. The preference for high RED light may enable the small seeds to germinate preferentially in high-quality microsites, rather than open sites within which seedlings can die faster than under nurse plants due to high solar gain (Flores et al., Reference Flores, Briones, Flores and Sánchez-Colón2004). As seeds used in this study were obtained from a breeding programme of the Ben Gurion University of the Negev, Israel, and from a commercial supplier, some differences in response could be possible for wild-collected material.
Acknowledgements
X-Y. Y. acknowledges financial support from the Chinese Academy of Sciences, Xishuangbanna Tropical Botanical Garden, Jilin Agricultural University, PR China. X-Y. Y. and H.W.P. received funding from the Millennium Seed Bank Project and The Royal Botanic Gardens, Kew, receives grant-in-aid from Defra.
Conflicts of interest
None declared.








