Introduction
Germination is initiated when a quiescent dry seed takes up water (imbibition) and terminates with the elongation of the embryonic axis (Bewley and Black, Reference Bewley and Black1994; Bewley, Reference Bewley1997). The end of seed dormancy (dormancy types and duration differ between species) is dependent upon a threshold stimulus that varies widely amongst individuals (Bewley, Reference Bewley1997). The germination process has been described as an interplay of genetic, environmental and seed processing effects (Apostolides and Goulas, Reference Apostolides and Goulas1998; Sadeghian and Yavari, Reference Sadeghian and Yavari2004). Imbibition, the initial step, is facilitated by moist aggregates, water films surrounding soil particles, as well as water vapour. Additional influences include soil aggregate size and distribution, strength of the top soil and the presence of a soil crust. Currently, there is a knowledge gap in our understanding of the relationships and interactions between soil physical properties and environmental factors and their subsequent effect on germination, emergence and establishment in plants, which we outline in this review. We propose that seed–soil contact, an important, yet frequently ignored factor, influences germination and constant yield.
Sugar beet is the second largest global source of sugar besides sugar cane and of high importance, especially for European countries, where climate conditions are unsuited for sugar cane. Globally 277 million tons of sugar beet were produced in 2016 with Europe producing 69.6% (FAOSTAT, 2018). Sugar beet has a small seed in comparison with other crops such as wheat and maize and has been reported as being highly susceptible to changes in climate (e.g. temperature and rainfall) and soil physical condition (e.g. compaction and crust formation) due to its low emergence force. In particular it can be significantly affected by varying soil moisture conditions (Rinaldi et al., Reference Rinaldi2005).
This review aims to highlight the key factors influencing successful germination and crop development in sugar beet with a specific focus on seed–soil contact and the interaction between soil physical properties and environmental conditions. We outline that field conditions on the day of germination initiation significantly influence the productivity of the early seedling. We explain how typical field management techniques can impact on soil conditions and the subsequent impact for the emerging seedling. We also highlight the latest state of the art in imaging techniques and modelling approaches that are being applied to research in this area to improve the predictability of germination.
The concept and importance of seed–soil contact
The concept of seed–soil contact is based on the notion that seeds should be able to absorb water from water films and moist aggregates that are in direct contact with the seed for imbibition and, ultimately, for germination. The importance of the area of contact in combination with the soil matric potential for germination was initially described by Sedgley (Reference Sedgley1963) and Manohar and Heydecker (Reference Manohar and Heydecker1964). The wetted area of contact has been found to be one of the factors controlling germination using Medicago tribuloides seeds (Sedgley, Reference Sedgley1963). Increasing area of contact results in an enhanced germination rate (Manohar and Heydecker, Reference Manohar and Heydecker1964). This was tested by drilling holes of different diameters into an acrylic glass layer (i.e. Perspex) and allowing different parts of the seed to be in contact with varying areas of moist soil. Acknowledging that this was one of the first studies on seed–soil contact, seeds receiving the same treatment (i.e. hole size) were most likely to be exposed to different soil contact areas due to the heterogeneity of soil aggregates creating differently sized air pockets in between aggregates touching the seed. The micropyle (120 µm × 80 µm in size) has been suggested as the main point of water uptake in pea seeds (Manohar and Heydecker, Reference Manohar and Heydecker1964). The orientation of the seed would therefore be a major influence for all seed–soil contact experiments if it was not in direct contact with a moist aggregate.
Pre-soaking seeds (M. tribuloides and Lactuca sativa) reduces the importance of the soil matric potential (Collis-George and Hector, Reference Collis-George and Hector1966). The area of contact is considered important at high matric potentials for the later germinating seeds based on observations of different calculated wetted areas. Thus, the influence of area of contact decreases with a reduction of water potential. As field water potentials are often below water potentials used in laboratory experiments such as those carried out by Sedgley (Reference Sedgley1963) and Manohar and Heydecker (Reference Manohar and Heydecker1964), the area of contact is probably less important at field scale. A reduction in germination rate and water uptake with a decreasing hydraulic conductivity was reported based on a fixed seed–soil contact (Hadas and Russo, Reference Hadas and Russo1974a,Reference Hadas and Russob). This work introduced the concept of seed–soil contact under laboratory conditions but was not extended to field scale. The main concept we consider is that the size and shape of soil aggregates in the seedbed impact on the establishment of crop seedlings and are responsible for seed–soil contact.
Preparation of the soil seedbed
Centuries of development in agricultural practice have informed our current techniques for sowing seeds. Farmers aim for uniform crop establishment, which can ultimately enhance yield, help to reduce soil nutrient leaching and increase the ability of the crop to compete with weeds (Håkansson et al., Reference Håkansson, Myrbeck and Etana2002). Several abiotic factors including temperature, sowing depth and soil moisture are important to achieve optimal germination conditions for the seed. A soil temperature of above 3°C has been proposed as the germination initiation temperature for sugar beet; however, at temperatures below 5°C the germination rate can be slow (Gummerson, Reference Gummerson1986; British Beet Research Organisation (BBRO), 2017). The base temperature for the adjusted thermal time (accumulated days above a base temperature adjusted for the specific plant species) is higher than for ryegrass (base temperature of 1.0–2.0°C) and clover (base temperature of 0.0–1.5°C) but lower compared with maize (base temperature of 7.0–9.0°C) (Moot et al., Reference Moot2000; Trudgill et al., Reference Trudgill, Squire and Thompson2000). These temperature requirements make sugar beet an ideal spring sown crop once the temperature rises above the base temperature. Under shallow sowing conditions, seeds experience a higher temperature; however, the temperature decreases with increasing soil water content (Ferraris, Reference Ferraris1992).
Heavy rainfall within 48 hours after drilling can have negative effects on sugar beet germination (BBRO, 2017). Rainfall often results in slumping of a bare seedbed to some degree, i.e. soil structural collapse and thereby altering the intended seedbed structure as well as influencing the seed–soil contact. As slumping increases soil bulk density and compaction, porosity decreases. In this case an increase in seed–soil contact could therefore reach a critical level due to reduced oxygen availability, although this is hard to assess due to the opacity of soil. At high soil moisture contents, oxygen limitation can occur as the percentage of water-filled pores increases at the expense of air-filled pores. Oxygen limitation, however, has been reported to have a limited influence on germination, certainly lower than the considerable negative influence of waterlogging (Håkansson et al., Reference Håkansson2012). It is also likely that oxygen limitation does not influence the germination initiation as the embryo is confined to the pericarp and therefore limited to external oxygen supply. A reduced sugar beet establishment has been found to be due to poor drainage and a water level above seeding depth (Durrant et al., Reference Durrant1988).
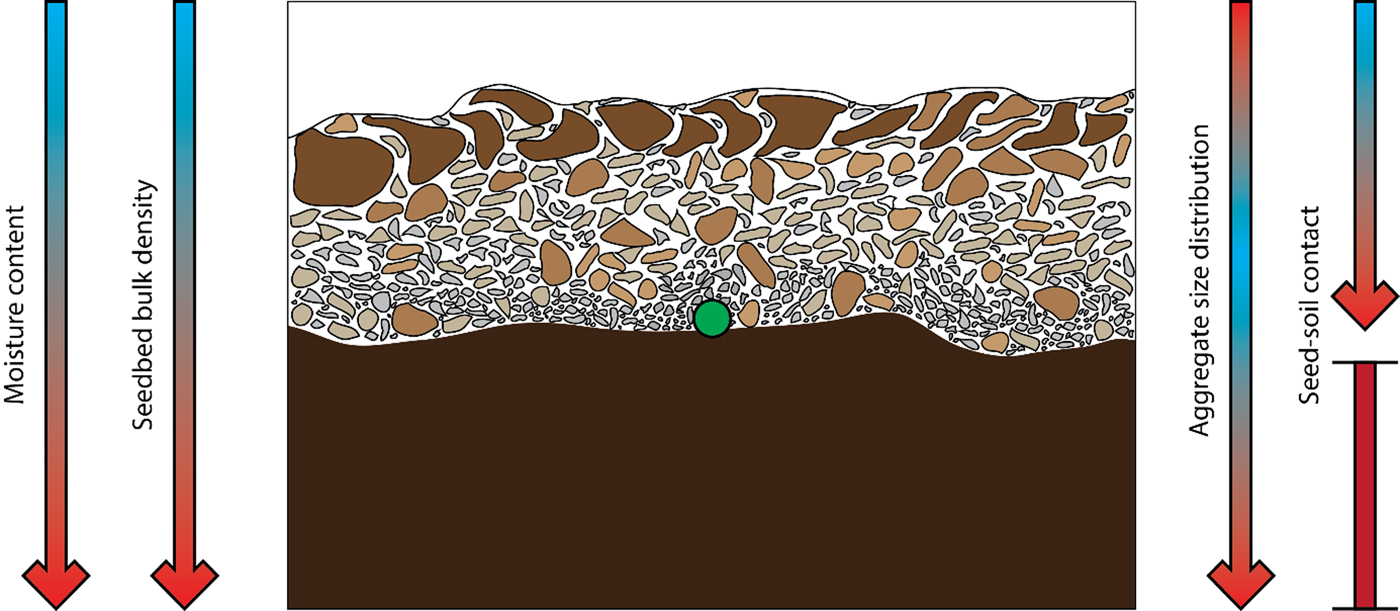
Crusting of the topsoil may occur in some soils, especially finer textured soils, which reduces the chance of emergence for weaker seedlings (Aubertot et al., Reference Aubertot2002). Sugar beet is highly susceptible to variations in soil physical conditions in the field due to the low seedling emergence force (i.e. force of the hypocotyl) of 0.15 N (Souty and Rode, Reference Souty and Rode1993). Previous work has recommended that the physical stress should not exceed a weight equivalent to a force of 0.10 N for at least 50% of the seedlings to successfully emerge (Souty and Rode, Reference Souty and Rode1993). Seedbed preparation has therefore to be executed at specific times to avoid crust formation due to rainfall within the first few days after drilling. As sugar beet seeds are also heavily susceptible to water stress under drought conditions, seed priming (pre-germinating the seeds in the presence of small amounts of water) is used to enhance the drought tolerance for sub-optimal conditions whereas a prolonged steeping (a type of priming including an acid steeping step) process increases the tolerance even further (Durrant and Mash, Reference Durrant and Mash1991). Seedbed preparation is a crucial step for sugar beet farmers not only due to the influence of weathering on the seedbed but also as seedling emergence is influenced by soil physical properties (e.g. soil texture, bulk density and water content), climate, tillage, and drilling procedures (Aubertot et al., Reference Aubertot1999). Soil compaction (a decrease in pore space and increase in bulk density) poses a serious problem for the sugar beet industry as conventional field preparation techniques result in subsoil compaction, reducing root development and yield (Marinello et al., Reference Marinello2017). The ideal conditions for a seedbed are thought to consist of both fine and coarse aggregates to prevent erosion (erosion prevention facilitated by a proportion of coarse aggregates) and to ensure sufficient soil–seed and soil–root contact (improved contact facilitated by a proportion of fine aggregates) whilst minimizing compaction which represents a challenge to the farmer (Fig. 1) (Braunack and Dexter, Reference Braunack and Dexter1989).
Fig. 1. Schematic representation of a typical sugar beet seedbed covering a dense layer. The aggregate size decreases from top to bottom, whereas the moisture content increases. Seed–soil contact is high at the lowest point of the seedbed and too high within the dense sublayer due to compaction. The green symbol indicates the ideal positioning of the seed, being slightly incorporated into the firm sublayer and in contact with a high abundancy of small aggregates (adapted from Hakansson et al., 2002).
A seedbed has previously been defined as a loose and shallow managed surface layer (Håkansson et al., Reference Håkansson, Myrbeck and Etana2002). The surface layer is ideally prepared to a depth of 5–7 cm with a minimum of 30% aggregates below 3 mm for improving the moisture availability around the seed (BBRO, 2017). Aggregate size and position above the seed in the seedbed influences the emergence probability of the seedling (Bouaziz and Bruckler, Reference Bouaziz and Bruckler1988; Souty and Rode, Reference Souty and Rode1993; Boiffin et al., Reference Boiffin, Jensen, Schjnning and Madsen1994) as well as the soil aggregate roughness (Richard and Dürr, Reference Richard and Dürr1997; Aubertot et al., Reference Aubertot1999). This is likely to be due to the limited emergence force of the young sugar beet seedling. Increasing bulk density and aggregate size results in a delay of seedling emergence, as shown for wheat by Nasr and Selles (Reference Nasr and Selles1995). A higher abundance of aggregates >5 mm has been reported within the 0–3 cm layer compared with the 3–10 cm layer using tillage techniques segregating aggregate classes and being preferable for seedbeds (Kritz, Reference Kritz1983). Soil aggregate size has a significant impact on the seed–soil contact. Testing different aggregate size classes to simulate different seed–soil contacts has been used to identify accelerated germination for the finest seedbed aggregate sizes (tested on peanut seeds) (Khan and Datta, Reference Khan and Datta1987). This is attributed to increased seed–soil contact and thus enhanced water availability. The increase in germination and emergence time can also be attributed to a change in hydraulic conductivity, soil–water diffusivity, the soil moisture flux, the thermal conductivity and oxygen flux. However, the treatments used by Khan and Datta (Reference Khan and Datta1987) consisted of >70% aggregates within the specific size class, which leaves up to 30% of smaller aggregates within each treatment. Assuming that a third of the aggregates were smaller, we hypothesize that these probably filled the larger pores in the coarser treatments therefore influencing the seed–soil contact to the point that it is difficult to conclude which factor had the strongest impact. The presence of larger aggregates has also been reported to result in detrimental effects with an exponential decrease in emergence found using aggregates >10 mm incorporated into the seedbed (Dürr and Aubertot, Reference Dürr and Aubertot2000). Seedbeds composed mainly of larger aggregates are not suitable for most agricultural purposes due to reduced establishment caused by reduced seed–soil contact and also due to the limiting emergence force of the seedling. However, they do offer the benefit of protection against erosion (Lyles and Woodruff, Reference Lyles and Woodruff1962; Keller et al., Reference Keller, Arvidsson and Dexter2007; Obour et al., Reference Obour2017). A balance is therefore needed between the ratio of larger aggregates for reducing erosion and smaller aggregates to improve the establishment rate, but not exceeding a critical level determined by the emergence force (Boiffin, Reference Boiffin, Callebaut and de Boodt1986; Duval and Boiffin, Reference Duval, Boiffin, Jensen, Schjnning and Madsen1994; Håkansson et al., Reference Håkansson, Myrbeck and Etana2002).
Soil aggregate size can influence soil water content through the provision of macropores between aggregates and micropores within aggregates, as well as soil physical properties (Dürr and Aubertot, Reference Dürr and Aubertot2000). Field management techniques, particularly those concerned with seedbed preparation, significantly influence aggregate size distributions with small aggregated seedbeds providing a higher contact area between soil aggregates and the sugar beet seed, and therefore improving water transfer (Bruckler, Reference Bruckler1983; Schneider and Gupta, Reference Schneider and Gupta1985; Braunack and Dexter, Reference Braunack and Dexter1989; Braunack, Reference Braunack1995; Dürr and Aubertot, Reference Dürr and Aubertot2000).
A firm adjacent basal sublayer consisting of soil with a higher bulk density was recommended as preferable for Swedish soils (Håkansson et al., Reference Håkansson, Myrbeck and Etana2002). However, an open porous soil structure with larger aggregates is the current recommendation by the British Beet Research Organisation (BBRO, 2017). The structure of the lower layer of soil is generally not tilled which can result in a drought stress as root growth can be restricted. The incorporation of the sugar beet seeds within the dense sublayer, however, could enable access to a higher moisture content through an increased contact area between the seed and the soil (Gummerson, Reference Gummerson1989). The idea of accessing a higher water source through an adjacent layer is an interesting one as the seed would benefit from both the fine seedbed as well as the water source. However, this would require sowing at a higher precision than is currently employed in most field cases as slight unevenness of the seed surface would result in misplacement of the seed. Therefore, the seed would either be placed within the fine seedbed or deep within the compacted sublayer which would have a negative impact on emergence time. Current recommendations aim for an even seedbed, as unevenness may lead to yield loss due to reduced establishment and increased harvester losses (BBRO, 2017). Additionally, the BBRO (2017) highlight that the timing and procedure of cultivation management techniques can reduce the final yield by 30% under sub-optimal conditions.
These previous studies highlight that water- and temperature-related environmental factors have a very significant influence on seed germination and plant growth, whereas the soil physical factors, which directly affecting seed–soil contact and chance of emergence, can be adjusted and influenced to a larger extent through appropriate cultivation and management techniques.
Cultivation and management techniques
Structural variations in the seedbed are primarily caused by tillage operations and drilling machinery (man-made) or by wetting–drying/freeze–thaw cycles and biological actions (natural) (Aubertot et al., Reference Aubertot1999). Seedbeds are commonly prepared into a fine and homogenous state using tillage operations such as harrowing, ploughing, discing or by tines (Obour et al., Reference Obour2017). Reduced tillage techniques in comparison with conventional tillage, reduces the number of passes through the fields and the intensity and depth (usually the upper 5 cm) of cultivation (Halvorson and Hartman, Reference Halvorson and Hartman1984). Fields managed under no tillage conditions prepare the seedbed via the action of the soil biota and wetting and drying cycles (Tisdall, Reference Tisdall1994; Degens, Reference Degens1997; Romaneckas et al., Reference Romaneckas2009). Dense soil surface layers commonly found on no-tillage managed fields can adversely affect establishment due to a low emergence force (Koch, Reference Koch2009), although literature in this area is sparse. Strip tillage procedures are used for partial or complete removal of the soil surface layer by tilling narrow strips to control erosion (for both wind and water), reduce evaporation and avoid loss of soil organic matter (Jabro et al., Reference Jabro2014). Similar yields for sugar beet have been reported compared with intensive tillage. One-third of the sugar beet grown in the USA is managed by strip tillage as the number of passes is reduced from five (conventional tillage) to one (strip tillage) and therefore fuel usage is reduced as well (Evans et al., Reference Evans, Stevens and Iversen2010; Cane, Reference Cane2015; Stevens et al., Reference Stevens2015; Tarkalson et al., Reference Tarkalson, Bjorneberg and Moore2016). Strip tillage can, however, increase the time until emergence by up to 5–7 days in a silt loam (Lower Saxony, Germany), probably due to an uneven coarse seedbed in comparison with intensive tillage and reduced tillage (Laufer and Koch, Reference Laufer and Koch2017). Further research is needed concerning the preferred tillage system for optimized seedbed preparation; however, reduced and no-tillage techniques show considerable promise, providing the soil bulk density does not exceed a critical level.
Sugar beet fields in European countries are commonly ploughed in the previous year as clod strength reduction (tilth mellowing) facilitated by weathering is considered to help the seedbed composition throughout the winter period (wetting–drying as well as freeze–thaw cycles) (Utomo and Dexter, Reference Utomo and Dexter1981). The effectiveness of this method of soil breakdown by tilth mellowing is determined by the soil consistency (i.e. resistance to deformation in a wet and dry state) (Larney et al., Reference Larney, Fortune and Collins1988). For heavy textured soils in the UK, ploughing is recommended before the end of October, whereas for lighter textured soils from October onwards is preferable (BBRO, 2017). Light soils (i.e. high sand content) should only be ploughed directly before drilling to avoid drying, slumping and erosion (caused by friable soil structure). Spring cultivations, for creating a level and consolidated seedbed, are thought to be optimal for high seed–soil contact, although this is hypothesized rather than based on actual measurements, and therefore a successful uniform establishment and high yield (BBRO, 2017). Based on these recommendations, farmers need to consider both field conditions (e.g. soil texture, bulk density and soil strength) as well as the average weather conditions (e.g. rainfall, temperature as well as base temperature for the specific crop) to make an informed decision on appropriate field management techniques which adds to the challenge.
Cultivations aim to optimize the structure of the seedbed and therefore ensuring consistent and homogeneous establishment and stand (Håkansson et al., Reference Håkansson, Myrbeck and Etana2002). The ‘Speeding Up Sugar Yield’ (SUSY) project investigated the yield differences between historic production between 2002 and 2006 (10 Mg ha–1; Hanse et al., Reference Hanse2011) and optimal potential (23 Mg ha–1; de Wit, Reference de Wit1953) in the Netherlands. Top yielding farmers typically use less cultivation steps compared with average yield farmers, as well as earlier sowing dates based on the comparison of total yield from previous years (Hanse et al., Reference Hanse2011). Statistical modelling (REML) showed that soil hydraulic conductivity (i.e. a measure of a soil's drainage rate), tillage operation depth as well as soil structure had the highest impacts on obtaining a good yield.
A combination harrow is recommended for a final depth of 5–7 cm; however, only one pass is optimal so as to avoid excessive compaction (BBRO, 2017). Seedbeds are commonly rolled during sowing to increase seed–soil contact using small press-wheels attached to the seed-drill (Sadeghpour et al., Reference Sadeghpour2015). Rolling is a controversial practice in this regard as excess pressure results in high compaction and thus severely reduced establishment (Jaggard, Reference Jaggard1977; Hebblethwaite and McGowan, Reference Hebblethwaite and McGowan1980; Brereton et al., Reference Brereton, McGowan and Dawkins1986), whereas beneficial effects on yield have been reported using single passes with press-wheels, indicating an increase in seed–soil contact while avoiding oxygen limitation (Håkansson et al., Reference Håkansson2011; Arvidsson et al., Reference Arvidsson, Bölenius and Cavalieri2012). Again, the opacity of soil making it hard to visualize seed–soil contact has remained an obstacle to understanding the mechanical processes concerned with seedbed preparation. For many decades, seed–soil contact has been a mere concept and the real influence of compaction of seed–soil contact is largely unknown. The changes in yield after compaction could be due to different causes (i.e. water retention, avoidance of erosion). The current drilling practice, however, does require a slight compaction as a channel in the soil is opened that would leave the seeds exposed without the use of press-wheels. Cultivation techniques in comparison with reduced tillage and no-tillage have been reported to result in a more consistent and high yield; however, being susceptible to compaction due to multiple passes needed for preparing optimal seedbed conditions remains a significant but poorly understood problem.
Impact of soil amendments on seed–soil contact
Without doubt, different management techniques have a variable impact on seed–soil contact and are dependent on the physical force of machinery. An alternative but emerging approach includes the incorporation of other, non-soil materials into the seedbed including plant residue, plastic or glass that alter the contact area of the seed with the soil. Since the increase in adoption of minimum and no-tillage systems, the incorporation of plant residue has become a more regular practice depending on the type of cultivator used (Morris et al., Reference Morris2009). Incorporation of plant residue can serve several functions for the soil including (1) the reduction of soil erosion, (2) the supplementation of plant nutrients, (3) the functionality as a mulch, reducing soil water loss and (4) the modification of soil temperature (Wilhelm et al., Reference Wilhelm, Doran and Power1986). Furthermore, increased aggregate stability has been reported on a 10-year no-tillage site using crop residue management (Karlen et al., Reference Karlen1994). The application of conservation tillage (>30% plant residue cover) can improve important soil quality indicators (e.g. soil structure, aggregation and organic matter) (Rasmussen and Rohde, Reference Rasmussen and Rohde1988; Daughtry et al., Reference Daughtry2006). Besides an improved water availability (Evans and Young, Reference Evans and Young1970; Carson and Peterson, Reference Carson and Peterson1990), the incorporation of plant residue can reduce seed–soil contact (Fowler, Reference Fowler1986; Chambers, Reference Chambers2000; Rotundo and Aguiar, Reference Rotundo and Aguiar2005). This reduction in seed–soil contact is thought to be caused by the seed being positioned directly next to plant residue or the residue creating larger pore spaces than would be there otherwise. The direct contact may also exhibit positive effects for nutrient transfers; however, decomposing plant residues in a moist environment can also attract pathogens which have negative effects on germination and early growth. Additionally, a reduced soil temperature and germination was reported using a straw cover (Børresen and Njoes, Reference Børresen and Njoes1990). A reduced germination efficiency in seeds has been found in the presence of plant residue in direct contact for oilseed rape which was attributed to the reduced seed–soil contact (Morris et al., Reference Morris2009). This negative effect of plant residue was investigated using wheat straw in varying quantities either in direct contact with the seed or incorporated into the soil. Straw residue positioning has been shown to be the primary factor of establishment reduction whereas the impact of the amount of residue was lower and did not reduce establishment significantly, highlighting the impact of the contact area reduced by residue (Morris et al., Reference Morris2009).
An increase in seed longevity has been shown for Bromus pictus seeds placed within a layer of plant litter, but a reduction in germination rate for seeds surrounded by plant litter (no seed–soil contact) (Rotundo and Aguiar, Reference Rotundo and Aguiar2005). A lack of seed–soil contact (for sugar beet and oilseed rape seeds) was shown by placing a seed on wheat residue, resulting in a reduced emergence rate by 30% (this method simulates ‘broadcast sowing’, common for oilseed rape when distributing the seed on the soil surface) (Morris et al., Reference Morris2009). This effect was reversed when placing residue on top of the soil leading to rapid emergence due to the reduced evaporation (simulating an Autocast system that distributes straw above the seeds following sowing from a hopper attached to a combine harvester) (Morris et al., Reference Morris2009). Uneven distribution of straw can therefore result in a patchy establishment with a 50% reduction of biomass growth which was verified using oilseed rape and sugar beet by mixing the residue into the soil or placing it onto the surface (HGCA, 2002; Morris et al., Reference Morris2009). Placement of plant residue is therefore crucial as beneficial effects such as a reduction in evaporation and supply of nutrients can accelerate the emergence rate; however, there can be severe negative impacts. For weaker seedlings like sugar beet, the use of plant residue is only advisable if the seedlings’ emergence force can overcome the surface cover and the residue is not placed in direct contact with the seed.
Traditionally, sugar beet fields have been drilled in the preceding autumn to winter burying all stubbles, depending on the soil type (Ecclestone, Reference Ecclestone2004). However, non-inversion tillage systems retain residue at the soil surface. Furthermore, the position of plant residues in the seedbed can have phytotoxic effects on developing seedlings due to the production of phenolic compounds during their decomposition, especially under anaerobic conditions (Wuest et al., Reference Wuest, Albrecht and Skirvin2000). Besides beneficial effects on soil biochemical properties, significant improvements in yield were shown over a period of 4 years for maize with wheat residue, but incorporation of residue from the same crop used for the following season depressed yield significantly (Sidhu and Beri, Reference Sidhu and Beri1989). However, this is attributed more to the biochemical influences than the seed–soil contact alterations by incorporating chopped residue (likely to have produced inhibiting metabolites).
Alternative research has considered the benefits of waste materials as soil amendments to improve seedling emergence and crop establishment. The effect of fine (<6 mm) and coarse (6–15 mm) glass debris incorporated into the soil or as a mulch material was tested, as the incorporation of glass into soil is a possible option for glass disposal (De Louvigny et al., Reference De Louvigny2002), although concerns regarding a potential chemical and physical alteration of the soil as well as an effect on the growth behaviour of plants have been raised (Ngoya et al., Reference Ngoya, Hensley and Murdoch1997). High glass contents within the soil were achieved by creating a paste made of glass, water and soil which was air dried and cut into aggregates of different sizes. These aggregates have been used within the seedbed (layered with 5 mm of fine soil) or laid on the soil surface. Final sugar beet emergence rate was not significantly reduced, but it slowed when the glass contents as a portion of the soil was >80% (De Louvigny et al., Reference De Louvigny2002). Higher glass–soil contents also resulted in the trapping of seedlings below rough glass surfaces. With the incorporation of high levels of glass (>80%), increased temperature on average of about 2°C per day and significantly increased sowing depth has been reported (De Louvigny et al., Reference De Louvigny2002). While the increased temperature has beneficial effects for accelerated germination, an increased sowing depth would reduce establishment count, especially under water-restricted growth caused by the high glass content. Furthermore, as high glass contents were realized by creating artificial aggregates containing glass, the difference in seed–soil contact cannot be quantified directly but rather the impact on emergence.
Calculation of seed–soil contact
Soil aggregate size distribution from field structured soil can be determined by measuring fractions of the total soil sample size after sieving (Kemper and Chepil, Reference Kemper, Chepil and Black1965) or by the measurement of mass proportions of aggregates within sublayers (Kritz, Reference Kritz1983). Soil embedded in resin can be used to identify aggregate and air space distribution, but this is typically restricted to a 2D view of the soil matrix unless serial sections are collected, which is a laborious process (Protz et al., Reference Protz1987; Bresson and Boiffin, Reference Bresson and Boiffin1990; Dexter, Reference Dexter1991). Quantification of seed–soil contact has proven challenging and field management decisions have been selected based on the assumption of its effect. Only a few approaches have been made that have attempted to estimate seed–soil contact, typically resulting in subjective descriptions such as ‘poor’ or ‘good’.
Until very recently, the best approach to estimate seed–soil contact has been based on simplistic simulations and modelling such as that by Brown et al. (Reference Brown1996) and Zhou et al. (Reference Zhou, Chen and Sadek2014). The influence of aggregate size and macroporosity was simulated using deformable spheres of a uniform size and a rigid disc or sphere as a seed which is only a coarse assumption due to the heterogeneity of soil aggregates and particles (Brown et al., Reference Brown1996). Using a coloured liquid poured over the sample from multiple directions, an increase of contact with decreasing macroporosity was found upon dismantling of the sample (Brown et al., Reference Brown1996). A discrete element method (DEM) by using a distinct sphere as the seed and a randomly generated set of differently sized spheres to represent soil aggregates was used to calculate the area of contact by Zhou et al. (Reference Zhou, Chen and Sadek2014). They found 0–33 contact points with 0–41 mm2 area of contact with varying sowing depths. A soil to seed size ratio of 1.33 and 1.75 was considered as exhibiting the highest contact area. A simulation of rolling using press-wheels increased the modelled seed–soil contact significantly. Both approaches fail to account for the heterogeneity of soil due to varying soil aggregate structures (e.g. size, roughness and tortuosity). An additional challenge is posed by the presence of mineral stones and organic matter in varying sizes and shapes (not considered in models) that can be in direct contact with the seed or create air pockets, reducing the seed–soil contact. Even if those are not in direct contact with the seed but rather in proximity, the hydraulic conductivity and the pore network is amended compared with a modelled pure soil structure.
X-ray computed tomography (X-ray CT) has previously shown great promise for quantifying soil properties like bulk density and porosity (Steude et al., Reference Steude, Hopkins, Anders, Anderson and Hopmans1994; Atkinson et al., Reference Atkinson, Sparkes and Mooney2007, Reference Atkinson, Sparkes and Mooney2009). The application of this imaging approach offers the opportunity to overcome the limitation of soil opacity and actually visualize and measure the seed–soil contact under field conditions. A recent approach using X-ray CT quantified the actual soil matrix and pore space surrounding a sugar beet seed at a resolution of 20 µm (Blunk et al., Reference Blunk2017). An interesting increase in seed–soil contact percentage for round-shaped seeds in comparison with untreated star-shaped sugar beet seeds was reported in the same study (Blunk et al., Reference Blunk2017). Blunk et al. (Reference Blunk2017) developed an imaging method to measure in 3D the precise seed–soil contact based on visualization of the soil aggregates and pore geometry in relation to a sugar beet seed validated on laboratory prepared and field collected samples (Fig. 2). This research has shown how the advancements in imaging technologies can assist us to overcome the limitations associated with the opacity of soil and will undoubtedly provide new data to inform the future modelling approaches to improve their accuracy.
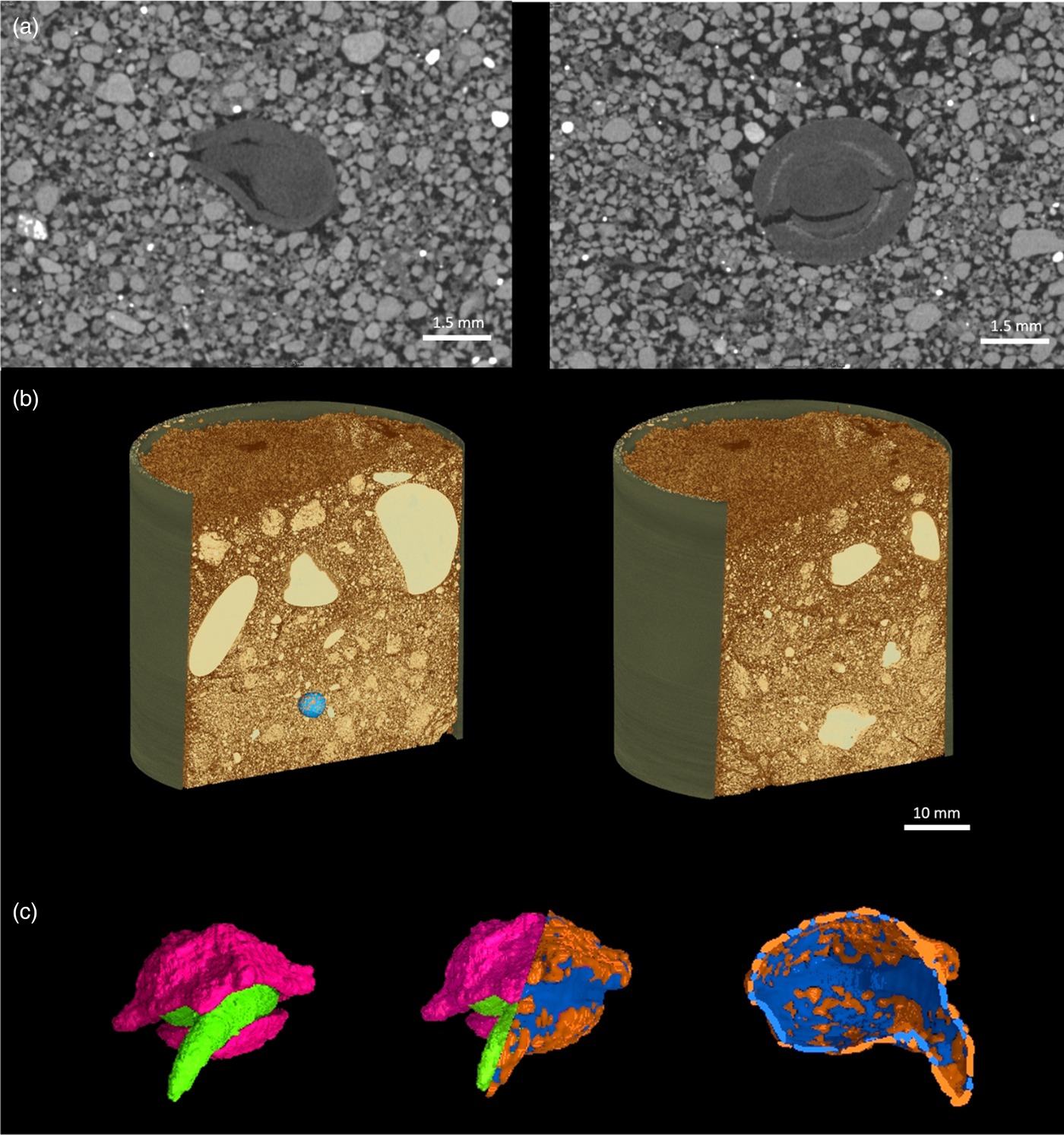
Fig. 2. X-ray CT quantification of seedbed properties. (A) 2D slice of a naked star-shaped seed and a pelleted and coated round-shaped seed within an artificially created seedbed sieved <1 mm. (B) 3D reconstruction of a pelleted and coated round-shaped seed within a field structured seedbed. (C) 3D reconstruction of surrounding soil and air space around a naked star-shaped seed: pink = pericarp; green = embryo; orange = soil; blue = air.
Future perspectives
Seed–soil contact as a concept has been well known for several decades but has lacked direct assessment until recently. Research into its measurement has been limited by the inability to observe it directly but with the recent developments in imaging techniques, seed–soil contact can be investigated at an appropriate resolution and the impact of management techniques on the seedbed and the resulting area of contact assessed. Future research should be able to directly assess the impact of soil management practices on the seed–soil contact that is achieved and the impact on germination. However, a potential problem to the adoption of new agricultural practices is that farmers tend to rely on former experience. BBRO (2017) provide recommendations for the appropriate soil structure of the seedbed, but there is only little quantitative knowledge concerning the effects of the different preparation techniques (e.g. harrow, tine, frost action) under present conditions (e.g. temperature, rainfall, soil moisture, soil texture, previous crop) on the resulting seedbed. Laser range scanners have shown considerable promise for mapping the seedbed surface structure to give indications of the ultimate effect of tillage operations including surface roughness (Jensen et al., Reference Jensen2017). These laser range measurements can also be used to estimate aggregate size distribution which could be extrapolated to estimate seed–soil contact (Jensen et al., Reference Jensen2016) and provide data for future modelling efforts.
Furthermore, the relationship between factors influencing germination, emergence and establishment requires a deeper understanding for choosing appropriate management techniques. Modelling approaches that take multiple factors into account represent a first step in the right direction. The soil quality of establishment (SQE) statistical model (Atkinson et al., Reference Atkinson, Sparkes and Mooney2007, Reference Atkinson, Sparkes and Mooney2009) uses field measurements (e.g. bulk density or shear strength), macrostructure properties and management techniques to predict establishment in wheat; however, it does currently not account for environmental factors like rainfall and temperature. The SUCROS model predicts sugar beet yield based on emergence time, establishment count, leaf area at emergence and leaf area growth rate which are highly dependent on soil texture, weather, seedbed preparation, sowing technique and seed lot characteristics (Spitters et al., Reference Spitters, van Keulen, van Kraalingen, Rabbinge, Ward and van Laar1989; Boiffin et al., Reference Boiffin1992; Dürr et al., Reference Dürr1992; Guérif and Duke, Reference Guérif and Duke1998). SUCROS, however, is a function of thermal time and does not include soil water as a limiting factor (Rinaldi et al., Reference Rinaldi2005). The SIMPLE (SIMulation of PLant Emergence) model, in comparison, is used to predict the effect of tillage and sowing operations for sugar beet (Dürr et al., Reference Dürr2001). This model uses texture, aggregate size distribution, position in the seedbed, sowing depth, soil temperature, rainfall, seed characteristics, germination time and hypocotyl elongation distribution to create a 3D seedbed based on aggregates and seed characteristics and predicts the duration until emergence based on the thermal time of the seed (Dürr et al., Reference Dürr2001). However, a more complex model is needed that adjusts relevant factors based on the relationship towards other factors (e.g. a change in soil compaction affects aggregate size distribution, porosity, hydraulic conductivity, etc.). The basis of this will be more sophisticated seedbed analysis approaches to quantify relevant factors influencing germination, emergence and establishment and their impact on seed–soil contact. Furthermore, quantitative image data generation using X-ray imaging can be used as a basis for modelling approaches and therefore improving the predictability under specific conditions. Further investigations that seek to quantify field structured seedbeds and screening of field environmental conditions are urgently needed to inform the selection of future management techniques especially in the face of environmental and climatic change.
Conclusions
Factors of soil seedbed preparation affecting germination and establishment in sugar beet have received much attention; however, their mutual interactions have not been fully explored. Imbibition, the initial step of germination, is known to be influenced by seed–soil contact which is affected by a variety of soil physical and environmental factors but is challenging to assess, not least due to the inability to observe the seed within the soil due to its opacity. The suite of field management techniques represents the extent of the limited options farmers are able to impose on the field and these are well known to have been shown to be affected by high variability of seed–soil contact. Engineering what might be considered an ‘optimal’ seed–soil contact can only be achieved using appropriate field management techniques at precise times (due to variation between soil texture, climatic conditions and crop). We consider the present soil and environmental conditions on the sowing day and the consecutive two to three days as the decisive factors affecting seedling emergence as the early seedling is dependent on seed reserves and its activation. A non-favourable germination initiation due to poor soil conditions (e.g. seed–soil contact) could affect the seedling early, resulting in a struggle to keep up with seedlings under optimal conditions. Future modelling efforts concerning the interactive network of factors influencing seed–soil contact should be sought to improve the predictability of germination, emergence and establishment based on image derived data. The image data will help to comprehend the impact that tillage operations pose on the seedbed and the actual contact to the seed. Deeper understanding of how plant establishment can be influenced altering seed–soil contact and therefore adjusting management and sowing techniques is fundamental for the improvement of future farming practices.
Financial support
This work was financially supported by Syngenta.