Introduction
Sunflower (Helianthus annuus L.), a member of the Asteraceae family, is an important oilseed crop (Anton et al., Reference Anton, Joiţa-Păcureanu and Cucerevii2015; Nasreen et al., Reference Nasreen, Khan, Zia, Ishaque, Uddin, Arshad and Rizvi2015). As in other crops, seed germination and stand establishment are key processes that affect crop production (Ishibashi et al., Reference Ishibashi, Tawaratsumida, Zheng, Yuasa and Iwaya-Inoue2010; Miransari and Smith, Reference Miransari and Smith2014). In sunflower crops, seed germination may be seriously impaired by dormancy, thus affecting emergence and seedling establishment (Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000) which might compromise agronomic yield (Roselló et al., Reference Roselló, Vigliocco, Andrade, Riera, Calafat, Molas and Alemano2016). Seed dormancy is regarded as the failure of an intact viable seed to complete germination under favourable conditions (Bewley, Reference Bewley1997). Seed dormancy is a complex trait because it is determined mainly by genetic factors but with a substantial environmental influence during seed development (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Bodrone et al., Reference Bodrone, Rodríguez, Arisnabarreta and Batlla2017). Sunflower, unlike other crops, has a relative short domestication history which has not been able to ostensibly remove seed dormancy (Rodríguez et al., Reference Rodríguez, Toorop, Benech-Arnold and Kermode2011), even though this crop has an important intraspecific variability for this trait (Subrahmanyam et al., Reference Subrahmanyam, Kumar and Ranganatha2002).
The sunflower achene is a fruit, although it is commonly referred to as seed, and consists of a ‘true’ seed and adhering pericarp; in turn, the seed consists of a seed coat and a thin layer of endosperm surrounding the embryo (Seiler, Reference Seiler and Schneiter1997). The seed develops from a fertilized ovule, whereas the mature pericarp is exclusively of maternal origin, as it is derived from the ovary wall (Esau, Reference Esau1977). Dormancy can be imposed on the embryo and/or by the embryo-covering tissues (i.e. pericarp, seed coat and/or endosperm) (Corbineau et al., Reference Corbineau, Bagniol and Côme1990; Le Page-Degivry and Garello, Reference Le Page-Degivry and Garello1992).
Sunflower achenes display relative dormancy and its expression shows strong dependence on the incubation temperature (Black et al., Reference Black, Bewley and Halmer2006). Inhibition of germination at low incubation temperatures (i.e. 10–15°C) can result from embryo dormancy and/or coat-imposed dormancy (Bodrone et al., Reference Bodrone, Rodríguez, Arisnabarreta and Batlla2017), while inhibition of germination at high temperatures (i.e. 25–30°C) is imposed by the coats (Corbineau et al., Reference Corbineau, Bagniol and Côme1990; Dominguez et al., Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016). Dormancy at harvest is frequently a combination of embryo dormancy and coat-imposed dormancy (Corbineau et al., Reference Corbineau, Bagniol and Côme1990). Dry after-ripening terminates embryo dormancy relatively quickly; however, coat-imposed dormancy may require weeks or even months of dry after-ripening to be removed (Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sánchez2004).
The embryo-covering tissues can impose dormancy through interference with gas exchange (e.g. entry of oxygen and/or carbon dioxide exit) (Corbineau and Côme, Reference Corbineau, Côme, Kigel and Galili1995). Rolletschek et al. (Reference Rolletschek, Borisjuk, Sánchez-García, Gotor, Romero, Martínez-Rivas and Mancha2007) measured the oxygen concentration in developing sunflower achenes using oxygen-sensitive microsensors and reported markedly reduced values within the embryo compared with those measured in the micropylar region (i.e. 1 vs 180 µM). This impediment to gas exchange which results in oxygen restriction to the embryo (i.e. hypoxia) might, in turn, interfere with the metabolism of germination inhibitors (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006). Indeed, it is known that the activity of the enzyme ABA 8´-hydroxylase committed to abscisic acid (ABA) catabolism (Cutler et al., Reference Cutler, Rose, Squires, Loewen, Shaw, Quail, Krochko and Abrams2000) is severely reduced at oxygen concentrations below 10% (Krochko et al., Reference Krochko, Abrams, Loewen, Abrams and Cutler1998). Washing the sunflower achenes in a sodium hypochlorite solution before incubation (a dormancy-relieving treatment) produced a reduction in embryo ABA content, presumably by increasing oxygen levels and ABA catabolism in the embryo (Dominguez et al., Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016). Besides, the hypoxia condition may increase embryo sensitivity to ABA, as demonstrated by Benech-Arnold et al. (Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006) using barley embryos.
The imposition of hypoxia to the embryo by the pericarp might be through different mechanisms. For example, Lenoir et al. (Reference Lenoir, Corbineau and Côme1986) ascribed the high consumption of oxygen by the barley hull to the oxidation of phenolic compounds mediated by high polyphenol-oxidase activity. Respiration of microorganisms growing on the surface of the pericarp (as on the surface of any other fruit) may be also involved in the oxygen diffusion limitation to the embryo by competing for this gas (Heydecker and Chetram, Reference Heydecker and Chetram1971).
The fact that pericarp-imposed dormancy in these achenes is expressed at high but not at low incubation temperatures (below 20°C) could be explained, then, through different, non-exclusive, features: (i) hypoxia is more intense at high incubation temperatures because, in addition to increased embryo respiration rate, the pericarp withholds more oxygen at high temperatures [i.e. higher polyphenol oxidase activity as occurs in barley (Lenoir et al., Reference Lenoir, Corbineau and Côme1986), higher respiration of microorganisms (Heydecker and Chetram, Reference Heydecker and Chetram1971) and/or a reduction of oxygen solubility in the aqueous phase (Hoang et al., Reference Hoang, Bailly, Corbineau and Leymarie2013)]; (ii) this low oxygen availability favours ABA accumulation through catabolism impairment (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006); (iii) embryos are more sensitive to hypoxia and/or ABA at high incubation temperatures (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006).
In this work we explored the above-mentioned possibilities using a sunflower inbred line with pericarp-imposed dormancy (Dominguez et al., Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016). We evaluated the effect of different oxygen concentrations combined with different incubation temperatures on (i) the germination of achenes and seeds, (ii) embryo ABA content throughout the incubation period, and (iii) seed sensitivity to ABA. Our experimental approach included using a mixture of antibiotics to evaluate their effect on achene germination and its microbial communities assessed through bacterial colony counts, community-level physiological profiles (CLPP) of carbon-source utilization and Shannon's diversity index (H index) for bacteria and fungi. The results are aimed to generate a physiological model of functioning of sunflower achenes with pericarp-imposed dormancy with implications for the management of this problem on an agronomic scale. Moreover, to investigate whether this model could be extended to other genotypes displaying pericarp-imposed dormancy, we incorporated in the analysis achenes from a sunflower commercial hybrid for comparison.
Materials and methods
Plant material
Sunflower achenes of an oil-producing parental line were received from Dow AgroSciences (Ruta 8, km 264, CP 2720, Colón, Buenos Aires, Argentina) soon after harvest (6.6% moisture content) and were immediately stored at –30°C to preserve the initial dormancy level (Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Bogatek, Corbineau and Bailly2008; Bazin et al., Reference Bazin, Batlla, Dussert, El-Maarouf-Bouteau and Bailly2011). This inbred line is used as a male parent for pollen donor of many commercial hybrids and develops long-lasting dormancy (M. Gerbaldo, Dow AgroSciences, personal communication). An additional lot of achenes of a sunflower commercial hybrid (not named due to confidentiality with the seed company) displaying pericarp-imposed dormancy were used to complement some of the studies. In the present work ‘achene’ refers to a seed and adhering pericarp, and ‘seed’ refers to a seed coat, a thin layer of endosperm and embryo, following Seiler (Reference Seiler and Schneiter1997) (see Fig. S1). In previous work we determined in the inbred line (Dominguez et al., Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016) and the commercial hybrid (authors’ unpublished data) that the pericarp is the only embryo-covering tissue that imposes dormancy and its expression was evident at high incubation temperatures (i.e. 25–30°C) but not at low temperatures (i.e. 10–15°C).
Achene and seed germination at different oxygen concentrations and incubation temperatures
Achenes and seeds of the inbred line and of the commercial hybrid were incubated at oxygen concentrations of 3, 5, 10, 15 and 21%, at 12 and 30°C. Incubation of seeds under concentrations less than 21% O2 (i.e. hypoxia) was to simulate the effect of oxygen deprivation imposed on the embryo by the pericarp, whereas incubation of achenes under concentrations less than 21% O2 was an attempt to generate a double restriction to the availability of oxygen. Twenty-five seeds or achenes in four replicates were incubated for each treatment (i.e. combinations of oxygen concentration and incubation temperature). Seeds or achenes were incubated in plastic trays (9 cm width × 6 cm depth × 4 cm height) over one filter paper with a layer of cotton underneath moistened with 55 ml of distilled water.
Oxygen concentration lower than 21% was modulated inside a transparent semi-sealable plastic chamber (35.56 cm width × 30.48 cm depth × 15.24 cm height, with water in the base, model C-274, BioSpherix, USA) using an oxygen controller (PRO-OX model 110, BioSpherix, USA). This oxygen controller had a nitrogen gas input (connected to a nitrogen gas tube), a nitrogen gas output (connected to the semi-sealable chamber) and an oxygen sensor (placed inside the semi-sealable chamber). Initially, the oxygen concentration inside the semi-sealable chamber was 21% oxygen (i.e. the oxygen concentration in the atmosphere). To achieve and maintain the oxygen concentration less than 21% inside the chamber, the oxygen controller recorded the oxygen concentration inside the chamber (through an oxygen sensor) and this controller allowed nitrogen gas to enter the chamber. Therefore, the selected oxygen concentration was achieved and maintained by forced displacement of the oxygen (present inside the chamber) with a continuous flow of nitrogen gas (i.e. via dilution of oxygen). The trays of this treatment were placed inside the semi-sealable chamber. In contrast, the oxygen concentration of 21% (i.e. oxygen concentration in the atmosphere) was performed in semi-covered clear plastic containers (21 cm width × 13 cm depth × 12 cm height, with water in the base). The trays of this treatment were placed inside the containers. The containers and the semi-sealable chamber were placed inside an incubator set with the selected temperature (12 or 30°C). Germination was scored at the end of the experiment (10 and 7 days at 12 and at 30°C, respectively).
Pericarp structure
In the inbred line and the commercial hybrid three portions from the central part of different mature achenes were embedded in paraffin and serially cut at 10–12 µm with a Minot-type rotary microtome. Sections were stained with safranin-fast green and mounted in Canada balsam (Johansen, Reference Johansen1940), to observe pericarp structure. Sections were photographed with a Zeiss Axioplan optical microscope (Oberkochen, Germany) and analysed with Zeiss AxioCam ERc 5s software (Jena, Germany). Three measurements of the number of cell layers of sclerenchymatic tissue and the sclerenchyma thickness were made on each of six achenes per genotype.
ABA content of seeds during incubation under different oxygen concentrations and temperatures
Twenty-five seeds from the inbred line were incubated in plastic trays (9 cm width × 6 cm depth × 4 cm height) over one filter paper with a layer of cotton underneath moistened with 55 ml of distilled water, at oxygen concentrations of 5 and 21%, at 12 and 30°C. Oxygen concentration of 5% was attained with the same system described above. Different sampling periods were carried out for ABA determination (every 8 h and 4 h from the start of the incubation at 12 and 30°C, respectively, until onset of germination of the first seed). Three replicates were incubated per treatment and per sampling period. In each sampling period, fifteen seeds per replicate (from the 25 seeds from replicate) per treatment were sampled at random for ABA determination. Upon sampling these seeds were dissected into embryonic axes and cotyledons (with its corresponding fraction of seed coat and endosperm), frozen in liquid nitrogen, and stored at –80°C until processing. Each sample was then lyophilized, powdered, weighed and stored at –30°C until quantification of ABA content with a radioimmunoassay, as described by Steinbach et al. (Reference Steinbach, Benech-Arnold, Kristof, Sánchez and Marcucci-Poltri1995) using the mono-clonal antibody AFR MAC 252 (Quarrie et al., Reference Quarrie, Whitford, Appleford, Wang, Cook, Henson and Loveys1988) and tritiated ABA (Amersham Biosciences, UK).
In parallel with this experiment for ABA determination, another experiment was carried out to measure seed germination as a reference for ABA sampling. Four replicates were incubated (as in the case of the seeds for ABA determination) at oxygen concentrations of 5 and 21%, at 12 and 30°C. Germination was scored daily for 10 and 7 days at 12 and at 30°C, respectively.
Sensitivity to ABA in seeds incubated under different oxygen concentrations and temperatures
Twenty-five seeds in four replicates of the inbred line were incubated in plastic trays (9 cm width × 6 cm depth × 4 cm height) over one filter paper with a layer of cotton underneath moistened with 55 ml of distilled water or ABA solutions. ABA (Sigma-Aldrich, USA) at concentrations of 0 (i.e. distilled water), 1, 10 and 100 µM ABA were used, and incubation was performed under oxygen concentrations of 5 and 21% and at 12 and 30°C. Oxygen concentration of 5% was attained with the same system described above. Germination percentage was scored at the end of the experiment (10 and 7 days at 12 and at 30°C, respectively).
The measurement of oxygen consumption at different incubation temperatures with and without antibiotics
Previous work has indicated that the outer surface of the pericarp is heavily colonized with microorganisms, while no bacteria can be detected in the enclosed embryo (Kutschera, Reference Kutschera2002; Schauer and Kutschera, Reference Schauer and Kutschera2008). This experiment was aimed to quantify oxygen consumption during incubation of isolated pericarps, as an estimator of their microbial-community's activity. Six isolated pericarps in four replicates of the inbred line were incubated in vials of 4 cm3 with 2 ml of distilled water (control) or antibiotics (mixture of fungicides and bactericides) at 10 and 30°C; six isolated pericarps in four replicates of the commercial hybrid were also incubated at 30°C in both incubation media. Mixture of antibiotics included a commercial fungicide (0.75 µl/ml of Ritiram Carb Plus containing Thiram 35% and Carbendazim 15%) and three bactericides (gentamycin 0.1 mg/ml, ampicillin 0.4 mg/ml, and spectinomycin 0.2 mg/ml), and water until reaching final volume. These drugs have different modes of action, and their combination is expected to have an inhibitory effect on most fungi and bacteria. To prevent oxygen diffusion, the vials were closed with a rubber stopper with Vaseline and the edge of the vial was wrapped with parafilm. The oxygen concentration was measured in the incubation medium of each vial at the start of the incubation (0 h) and 24 h afterwards, using an oxygen microsensor (Unisense, Denmark) that is a miniaturized Clark-type oxygen sensor (i.e. an electrochemical oxygen microsensor). The oxygen consumption in the incubation medium (for each treatment) was calculated as the difference between the oxygen concentration at time 0 h and after 24 h of incubation; this value was then adjusted to the volume of incubation media (2 ml) and expressed as relative to the fresh weight of pericarp samples within each vial. The difference in the oxygen consumption between absence and presence of antibiotics for each genotype and temperature was used as an estimator of the activity (i.e. respiration) of pericarp-microbial communities and its possible involvement in dormancy expression.
The effect of using antibiotics on achene germination and its microbial communities at high incubation temperature
To elucidate whether enhanced microbial activity was related to germination impairment at high incubation temperature, we assessed the effect of inhibiting microbial proliferation on achene germination. Four replicates with 25 achenes of both inbred line and commercial hybrid were incubated in 9-cm Petri dishes on two discs of filter paper moistened with 6 ml of distilled water or antibiotics (as described above). Final germination percentage was determined after 10 days of incubation.
In parallel, the same experimental system, but with an incubation of 24 h at 30°C, was used to study the achene-associated microbial communities assessed through bacterial colony counts, community-level physiological profiles (CLPP) of carbon-source utilization and Shannon's diversity index (H index) for bacteria and fungi. For the bacterial colony counts, after 24 h of incubation of three replicates, five achenes from each replicate were rinsed separately in 1 ml of sterile physiological solution for 5 min, and serial dilutions (1×10–1 to 1×10–5) were prepared using more sterile physiological solution. A 30 µl volume of each dilution was plated in LB-agar and kept at 30°C for 24 h until colonies were visible. Moreover, for CLPP of carbon-source utilization and H index for bacteria and fungi, after 24 h of incubation of four replicates, achenes, filters and incubation media (i.e. distilled water or antibiotics) from each replicate were transferred to a 200 ml flask with 50 ml of physiological solution (0.9% NaCl). Each flask was shaken for 30 min and a 10−1 dilution was made to perform CLPP of carbon-source utilization and H index. Community-level physiological profiles of bacterial and fungal communities were obtained as described by Di Salvo and García de Salamone (Reference Di Salvo and García de Salamone2012). The 23 carbon sources included in the microplates were: arginine, glutamine, glycine, phenylalanine, proline, histidine, cellobiose, dextrose, maltose, coumaric acid, xylose, fructose, glycerol, mannitol, lactic acid, malic acid, citric acid, oxalic acid, salicylic acid, benzoic acid, Tween 20, putrescine and itaconic acid. For bacterial microplates, 50 µl per well of tetrazolium violet (0.0025%) was included as redox dye indicator which inhibits fungal growth, and no antibiotics were added. For fungal microplates, 50 µl per well of bactericides prepared as described above, were added and redox dye indicator was not included. Each well was inoculated with 50 µl from the 10−1 dilution. Microplates were incubated at 30°C for 96 h and each one contained four samples. Absorbance values taken at 96 h were obtained with a microplate reader Multiskan EX™ (Labsystems, Finland) at 590 nm. Functional diversity was analysed using Shannon's diversity index (H index), which was obtained from absorbance values of CLPP microplates (Di Salvo et al., Reference Di Salvo, Ferrando, Fernández-Scavino and García de Salamone2018).
Statistical analyses
Analysis of variance, Tukey's test, t-test and discriminant analysis (with P ≤ 0.05) were performed using InfoStat 2014 (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2014). For the figures, GraphPad Prism 4.0 (Motulsky, Reference Motulsky2003) and InfoStat 2014 (Di Rienzo et al., Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2014) were used.
Results
Achene and seed germination at different oxygen concentrations and incubation temperatures
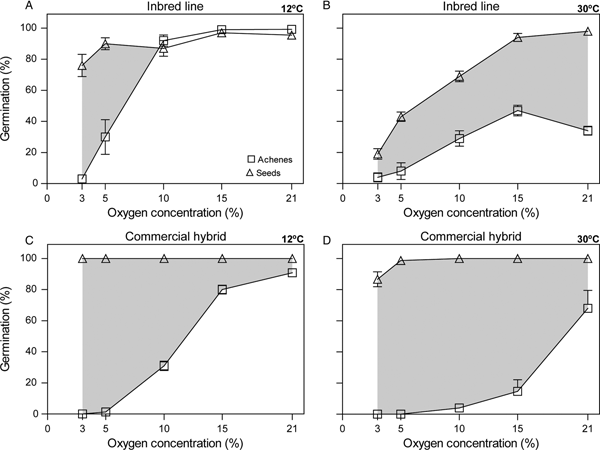
Achenes of both the inbred line and commercial hybrid reached almost 100% germination under air (i.e. 21% O2) at 12°C, but germination dropped significantly in both genotypes when incubation was performed at 30°C (Tukey's test with P ≤ 0.05; Fig. 1). In contrast, seeds (i.e. achenes without pericarp) of the inbred line and of the commercial hybrid reached values around 100% germination under air both at low and high incubation temperatures (Fig. 1), demonstrating the absence of embryo dormancy, and that the seed coat and endosperm did not inhibit germination. It was clear then that both genotypes displayed pericarp-imposed dormancy under air and its expression was evident only at high incubation temperature.

Fig. 1. Final germination percentage of achenes and seeds after 10 days of incubation at 12°C for the inbred line (A) and for the commercial hybrid (C) and after 7 days at 30°C for the inbred line (B) and for the commercial hybrid (D) in distilled water at different oxygen concentrations. Bars represent standard error of the mean for four replicates. Where no bars are shown, the value of standard error of the mean is less than the size of the symbol. Shading indicates the difference in mean germination percentage between incubation without pericarp (i.e. seeds) and with it (i.e. achenes) for the range of oxygen concentrations.
When achenes were subjected to decreasing oxygen concentrations, the germination of both genotypes decreased with increasing hypoxia, although achenes of the commercial hybrid appeared to be more sensitive to hypoxia; this was so irrespective of the incubation temperature (Fig. 1). Also in both genotypes, the decrease in the achene germination under hypoxia was higher at 30°C than at 12°C (Fig. 1).
When seeds were incubated under decreasing oxygen concentrations, the two genotypes displayed remarkable differences in their behaviour. The germination of seeds from the inbred line incubated under increasing hypoxia remained at high values at 12°C (i.e. above 76%) (Fig. 1A); however, incubation at 3% O2 almost completely suppressed germination at 30°C, resembling the behaviour of achenes incubated at this same temperature but under air (Fig. 1B). Clearly, seeds of the inbred line were very sensitive to hypoxia at 30°C but much less sensitive at 12°C. In contrast, germination of seeds from the commercial hybrid incubated under increasing hypoxia remained high at both 12 and 30°C, even at 3% O2 (Fig. 1C,D). These results show that seeds of the commercial hybrid displayed very low sensitivity to hypoxia at both temperatures.
Taken together, these results indicate that these genotypes have different embryo sensitivities to hypoxia (assessed as germination of pericarp-less seeds) especially at high incubation temperature (i.e. 30°C). Embryo sensitivity to hypoxia when incubation was performed at 30°C explained pericarp-imposed dormancy expressed at 30°C in the inbred line (germination of both achenes and seeds decreased at a similar rate with hypoxia) but not in the commercial hybrid (where the decrease in achenes germination with hypoxia was not accompanied by a similar decrease in seeds germination). Indeed, as lower than 3% oxygen values were possibly required to inhibit embryo and seed germination in the commercial hybrid, a very strong effect of the pericarp becomes evident as deduced also from the high sensitivity of achenes to hypoxia displayed by this genotype when compared with the inbred line.
Pericarp structure
To see whether the high sensitivity of achenes from the commercial hybrid to hypoxia (compared with achenes from the inbred line) was through pericarp structure, we assessed the number of cell layers of sclerenchymatic tissue and the sclerenchyma thickness in the two genotypes (see Fig. S2 for a visual assessment). Both measurements were significantly higher in the commercial hybrid than in the inbred line (Tukey's test with P ≤ 0.05; Table 1).
Table 1. Number of cell layers of sclerenchymatic tissue and sclerenchyma thickness of achenes from the inbred line and the commercial hybrid

Values are means ± standard error of three measurements made on each of six achenes. *Treatment means within each column followed by the same letter are not significantly different using Tukey's test (with P ≤ 0.05).
ABA content of seeds during incubation under different oxygen concentrations and temperatures
Only the inbred line (and not the commercial hybrid) showed high embryo sensitivity to hypoxia at high temperature (assessed as germination of pericarp-less seeds). Therefore, to evaluate whether this enhanced sensitivity to hypoxia was through an interference with ABA catabolism that should result in ABA accumulation, we measured ABA content throughout the incubation period and until the onset of germination. ABA content of seeds from the inbred line incubated under hypoxia (i.e. 5% O2) and air (i.e. 21% O2) was assessed in embryonic axes and cotyledons, separately. ABA content in cotyledons was 4-fold higher than in embryonic axes and this difference persisted during incubation at both 12 and 30°C (Fig. 2A,B). However, hypoxia did not produce consistent differences in ABA content compared with incubation under air both for cotyledons and for embryonic axes (Fig. 2A,B). Moreover, ABA content remained fairly constant throughout incubation under both temperatures. Seed germination dynamics showed differences between the two oxygen concentrations at both 12 and 30°C (Fig. 2C,D); however, the final germination percentage of seeds was similar at 12°C (at the 10th day) for the two oxygen concentrations but lower for 5% O2 at 30°C (at the 7th day) (Fig. 2C,D). These results indicate that hypoxia does not interfere with ABA metabolism in the inbred line, and suggest that expression of pericarp-imposed dormancy at 30°C is not mediated by an increase in ABA content.

Fig. 2. ABA content assessed in embryonic axes and cotyledons at 5 and 21% O2 during incubation of seeds from the inbred line in distilled water at 12°C (A) and 30°C (B). Germination percentages of seeds incubated in distilled water at 5 and 21% O2 at 12°C (C) and 30°C (D); horizontal bars indicate the sampling period for ABA content of 5% O2 (filled bar) and 21% O2 (open bar). Bars represent standard error of the mean for three replicates, each measured in duplo (A and B), and for four replicates (C and D). Where no bars are shown, the value of standard error of the mean is less than the size of the symbol.
Sensitivity to ABA in seeds incubated under different oxygen concentrations and temperatures
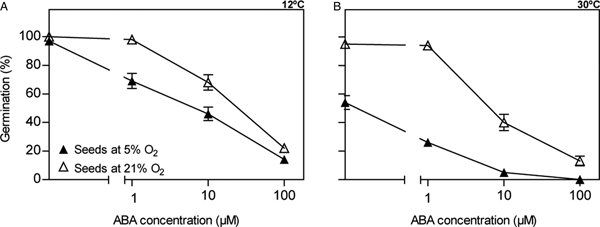
To evaluate whether this enhanced sensitivity to hypoxia at 30°C was through an increased embryo sensitivity to ABA, we measured germination of seeds incubated with different ABA concentrations, under two oxygen concentrations (5 and 21%) and under two temperatures (12 and 30°C). Germination of seeds from the inbred line incubated in the presence of different ABA concentrations decreased with increasing ABA concentrations at both 5 and 21% O2 (Fig. 3); germination in water was high and similar for both oxygen concentrations at 12°C, but at 30°C seed germination was inhibited at 5% O2 relative to 21% O2 (Fig. 3). At 12°C, 100 µM ABA was necessary to nearly completely suppress germination under hypoxia and air; however, at 30°C, 10 µM ABA was enough for suppressing germination under hypoxia and 100 µM ABA for doing so under air (Fig. 3). Incubation at 30°C increased sensitivity to ABA at 21% O2 compared with 12°C; this was evident when comparing germination values at 10 µM ABA (Fig. 3). These results show that hypoxia increases sensitivity to ABA as well as high incubation temperature. However, no synergistic effect between hypoxia and incubation temperature could be detected (i.e. interaction was not significant, t-test with P ≤ 0.05).

Fig. 3. Final germination percentage of seeds from the inbred line after 10 days of incubation at 12°C (A) and 7 days at 30°C (B) in ABA solutions (logarithmic scale on the x-axis) at concentrations of 0 (i.e. distilled water), 1, 10 and 100 µM ABA at 5 and 21% O2. Bars represent standard error of the mean for four replicates. Where no bars are shown, the value of standard error of the mean is less than the size of the symbol.
The measurement of oxygen consumption at different incubation temperatures with and without antibiotics
The isolated pericarps from the inbred line at 10°C did not show detectable oxygen consumption either in the control or in the treatment with antibiotics (Fig. 4A), suggesting that the activity of pericarp-microbial communities is negligible at this temperature and, consequently, should not add to any existing limitation in terms of oxygen availability to the embryo. In contrast, at 30°C, the control treatment of both genotypes had values of oxygen consumption higher than those obtained with the treatment with antibiotics (Fig. 4), thus suggesting that, at high incubation temperature, the microbial activity is high and possibly consumes oxygen that is not available to the embryo. This possibility is strongly supported by the fact that application of the treatment with antibiotics reduced the oxygen consumption to a quarter and a ninth in pericarps from the inbred line and from the commercial hybrid, respectively (Fig. 4).

Fig. 4. Oxygen consumption (nmol O2 per mg FW pericarp) in the incubation media by isolated pericarps of the inbred line at 10 and 30°C (A) and isolated pericarps of the commercial hybrid at 30°C (B) after 24 h of incubation. Incubation media were distilled water (i.e. control) and water plus antibiotics in a commercial fungicide (0.75 µl per ml of Ritiram Carb Plus containing Thiram 35% and Carbendazim 15%) and three bactericides (gentamycin 0.1 mg/ml, ampicillin 0.4 mg/ml, and spectinomycin 0.2 mg/ml). Bars represent standard error of the mean for four replicates. Where no bars are shown, the value of standard error of the mean is less than the line width.
The effect of using antibiotics on achene germination and its microbial communities at high incubation temperature
To assess the possibility that the oxygen consumption at high incubation temperatures due to microbial-community's activity on the pericarp surface is likely to play a role in pericarp-imposed dormancy, this activity was inhibited using antibiotics (mixture of fungicides and bactericides). The effect of the treatment with antibiotics on the achene germination at 30°C was different depending on the genotype (Table 2). In the inbred line, the treatment with antibiotics increased germination but not so much as to determine a significant difference in achene germination compared with its control (i.e. distilled water) (Tukey's test with P ≤ 0.05; Table 2). In contrast, for the commercial hybrid, the treatment with antibiotics improved achene germination significantly (Tukey's test with P ≤ 0.05; Table 2).
Table 2. Final germination percentage of achenes of the inbred line and the commercial hybrid after 10 days of incubation at 30°C in distilled water (i.e. control) or antibiotics

Values are means ± standard error of the mean for four replicates. *Treatment means within each column followed by the same letter are not significantly different using Tukey's test (with P ≤ 0.05).
The same experimental system, but with an incubation of 24 h at 30°C, was used to count bacterial colonies and estimate the CLPP and H index of bacterial and fungal communities associated with the achenes of the different treatments. The bacterial colonies/cm2 for the 1×10–4 dilution differed significantly between control and antibiotic-treated achenes for each genotype (Tukey's test with P ≤ 0.05; Table 3). Moreover, the bacterial load was significantly higher in the commercial hybrid than in the inbred line for the same dilution (Tukey's test with P ≤ 0.05; Table 3).
Table 3. Bacterial colonies per cm2 for the 1 × 10−4 dilution for the inbred line and the commercial hybrid after 24 h of incubation at 30ºC

Three replicates with 25 achenes of both inbred line and commercial hybrid were incubated in 9-cm Petri dishes on two discs of filter paper moistened with 6 ml of distilled water or antibiotics. After 24 h of incubation, five achenes from each replicate were rinsed separately in 1 ml of sterile physiological solution for 5 min, and serial dilutions (1×10–1 to 1×10–5) were prepared using more sterile physiological solution. A 30 µl volume of each dilution was plated in LB-agar and kept at 30°C for 24 h until colonies were visible. Bacterial colonies per cm2 were counted for the 1×10–4 dilution. Values are means ± standard error of three replicates. *Treatment means within each column followed by the same letter are not significantly different using Tukey's test (with P ≤ 0.05).
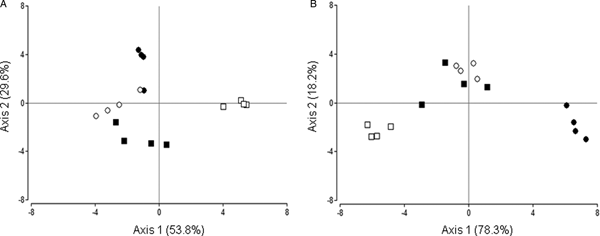
Also, the CLPP were different at 96 h of incubation for each genotype (Fig. 5). In the discriminant analysis of the CLPP of bacterial communities, the axis 1 and 2 explained the 83.4% of the total variation (Fig. 5A). Bacterial communities clustered on the right of axis 1 used preferentially cellobiose, histidine, putrescine, glycine, maltose and glycerol as carbon sources, while bacterial communities clustered on the left used preferentially fructose, glutamine and dextrose. Bacterial communities clustered on the bottom of axis 2 used preferentially proline as carbon source. The discriminant analysis showed that the bacterial communities differed greatly between both genotypes, mainly along axis 1 for the control treatment. Antibiotics also had a profound impact on bacterial community-profiles, which can be seen as a displacement along the axis 1 for the inbred line and axis 2 for the commercial hybrid. Moreover, the H index for bacterial communities differed significantly between control and antibiotic-treated achenes in the case of the inbred line (Tukey's test with P ≤ 0.05; Table 4) but not for the commercial hybrid (Tukey's test with P ≤ 0.05; Table 4).

Fig. 5. Discriminant analysis of the CLPP of bacterial (A) and fungal (B) communities on achenes from two genotypes, treated (or not) with antibiotics and tested in four replicates. Squares correspond to the inbred line and circles to the commercial hybrid; white and black symbols indicate control and treatment with antibiotics, respectively. Data used for the analysis corresponded to 96 h absorbance values. Total explained variance by each axis is shown in parenthesis.
Table 4. Functional diversity of the bacterial and fungal communities analysed using the H index, which was obtained from absorbance values of CLPP microplates, for both genotypes (inbred line and commercial hybrid) and incubation treatments (with and without antibiotics)

Values are means ± standard error of the mean for four replicates. *Treatment means within each column followed by the same letter are not significantly different using Tukey's test (with P ≤ 0.05).
Regarding discriminant analysis of CLPP of fungal communities, both axis 1 and 2 explained the 96.5% of the total variation (Fig. 5B). Fungal communities clustered on the right of axis 1 used preferentially histidine and maltose as carbon sources, while fungal communities clustered on the left used preferentially itaconic acid, citric acid, glycerol and cellobiose. Fungal communities clustered on the top of axis 2 used preferentially lactic acid as carbon source, while fungal communities clustered on the bottom used preferentially glycine and glutamine. The discriminant analysis showed that the fungal communities differed greatly between genotypes, both along axis 1 and 2, when comparing control treatments. Also, for each genotype, the fungal communities were affected by treatment with antibiotics, which produced changes along both axes (mainly axis 1). No significant differences in the H index for fungal communities obtained for control and antibiotics-treated achenes for each genotype (Tukey's test with P ≤ 0.05; Table 4).
Discussion
Attaining rapid and uniform seedling emergence is of paramount importance to crop performance (Paparella et al., Reference Paparella, Araújo, Rossi, Wijayasinghe, Carbonera and Balestrazzi2015). For this reason, the seed market is attempting to increase seed quality standards (Paparella et al., Reference Paparella, Araújo, Rossi, Wijayasinghe, Carbonera and Balestrazzi2015) with the removal of dormancy as a priority in species that still have it. Dormancy is determined by the genotype and the environmental conditions during seed development and its expression depends mainly on the environmental conditions during seed imbibition (Hoang et al., Reference Hoang, Sechet, Bailly, Leymarie and Corbineau2014). The present study attempted to shed light on the physiological mechanisms behind pericarp-imposed dormancy in sunflower achenes and its expression at high incubation temperatures. Our study was based on the behaviour of one inbred line displaying pericarp-imposed dormancy at warm temperatures, but a commercial hybrid also displaying this type of dormancy was included as a means for comparison to see the extent to which the physiological model derived from the inbred line could be generalized. We focused on the imbibition environment (i.e. temperature) and considered oxygen availability to the embryo (Gay et al., Reference Gay, Corbineau and Côme1991), ABA content and sensitivity (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006) and respiration of the pericarp-microbial communities (Heydecker and Chetram, Reference Heydecker and Chetram1971) as features, not only mentioned in the literature as likely to be part of these physiological mechanisms, but also proposed from our own previous studies carried out with the same inbred line (Dominguez et al., Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016).
The germination of achenes and seeds of both genotypes was evaluated under different oxygen concentrations and temperatures. Achenes of both genotypes displayed pericarp-imposed dormancy under air (i.e. 21% O2), which was expressed only at high incubation temperature (Fig. 1) as had been reported previously (Dominguez et al., Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016). Under air, the removal of the pericarp allowed germination at 30°C in both genotypes (Fig. 1B,D). However, when seeds (without the pericarp) were incubated under hypoxia, germination from the inbred line fell markedly with increasing hypoxia at 30°C to the point that the germination of these seeds at 3% O2 resembled that of achenes incubated at 30°C but under air (Fig. 1B). In contrast, seeds from the commercial hybrid displayed very low sensitivity to hypoxia at any temperature (Fig. 1C,D) with an oxygen threshold value for germination inhibition below 3% O2. In other words, both genotypes displayed similar pericarp-imposed dormancy expressed at high incubation temperature but, in the inbred line, this expression seems to be driven through exacerbated embryo sensitivity to hypoxia, whereas in the commercial hybrid, the pericarp arises as a severe restraint when incubation is performed at high temperature. The latter is particularly supported by the fact that germination of achenes from the commercial hybrid incubated under hypoxia was more severely inhibited than achenes from the inbred line (Fig. 1B,D). Interestingly, the pericarp may play a different role in sunflower germination: Andrade et al. (Reference Andrade, Riera, Lindstrom, Alemano, Alvarez, Abdala and Vigliocco2015) observed in a non-dormant sunflower line that pericarp presence may improve germination as it provides structural protection.
Sensitivity to hypoxia has been studied in seeds of other species. For example, in muskmelon, seed sensitivity to hypoxia increases at low incubation temperatures, even when the oxygen solubility in aqueous phase and its availability to the embryo is known to be higher than at high temperatures (Edelstein et al., Reference Edelstein, Corbineau, Kigel and Nerson1995). The physiological processes behind the sensitivity to hypoxia at low temperatures differ between genotypes of muskmelon and are mostly unknown (Edelstein and Welbaum, Reference Edelstein and Welbaum2011), as are the mechanisms that operate differentially between the inbred line and the commercial hybrid.
To evaluate if the high sensitivity to hypoxia shown by achenes from the commercial hybrid at high temperature is due to a severe physical restriction exerted by the pericarp, the pericarp structure was assessed. The number of cell layers of sclerenchymatic tissue and the sclerenchyma thickness were significantly higher in the commercial hybrid than in the inbred line (Table 1).
Only the inbred line (and not the commercial hybrid) showed high embryo sensitivity to hypoxia at high temperature (assessed as germination of pericarp-less seeds). Therefore, to evaluate whether this enhanced sensitivity to hypoxia was through an interference with ABA catabolism that should result in ABA accumulation, we measured ABA content of seeds throughout the incubation period and until the onset of germination and found no changes in ABA content. Our results showed that hypoxia (i.e. 5% O2) did not interfere with ABA content in this genotype either at low or high incubation temperature (Fig. 2A,B). Although there is evidence that hypoxia interferes with ABA catabolism in different ways (i.e. by deregulation of the ABA 8´-hydroxylase enzyme; Cutler et al., Reference Cutler, Rose, Squires, Loewen, Shaw, Quail, Krochko and Abrams2000), our results did not show higher ABA accumulation when incubation was performed under hypoxia as shown previously in barley (Benech-Arnold et al., Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Mendiondo et al., Reference Mendiondo, Leymarie, Farrant, Corbineau and Benech-Arnold2010). On the contrary, these results are consistent with those obtained by Dominguez et al. (Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016) which show that the presence of the pericarp does not promote ABA accumulation in the embryo compared with seeds (without the pericarp); moreover, in this previous work, ABA accumulated to higher values in imbibed seeds compared with intact achenes throughout incubation at 30°C. Also to evaluate whether the enhanced embryo sensitivity to hypoxia at 30°C of only the inbred line was through an increased embryo sensitivity to ABA, we measured germination of seeds incubated with different ABA concentrations under two oxygen concentrations (5 and 21%) and at two temperatures (12 and 30°C). Increasing ABA concentration in the incubation medium consistently reduced germination of seeds from the inbred line slightly more at 30°C than at 12°C when incubation was performed in air (21% O2) (Fig. 3), as observed by Dominguez et al. (Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016). Incubation under hypoxia (5% O2) at 30°C reduced seed germination as shown in Fig. 1B and this appears to be mediated by an increased sensitivity to ABA compared with 21% O2 (Fig. 3B). Taken together, these results suggest that high sensitivity to hypoxia displayed at 30°C by seeds of this inbred line is mediated, at least in part, by enhanced responsiveness to ABA (Fig. 3B), without any substantial changes in ABA metabolism (Fig. 2B). The effect of high incubation temperature on increased sensitivity to ABA could be mediated by hypoxia inside the embryo, as higher temperature increases metabolic rate (and oxygen consumption) and reduces gas solubility.
The increased sensitivity to hypoxia observed in seeds from the inbred line when incubated at 30°C might explain dormancy expression in achenes incubated at this temperature (Fig. 1B). However, dormancy was also expressed in achenes from the commercial hybrid incubated at 30°C even when the seeds exhibited much lower sensitivity to hypoxia than seeds of the inbred line (Fig. 1B,D). Therefore, pericarp-imposed dormancy occurring only at high incubation temperature appears to involve, particularly in the commercial hybrid but possibly also in the inbred line, an enhancement of oxygen withholding by the pericarp, which should increase oxygen deprivation to the embryo to the point reported by Rolletschek et al. (Reference Rolletschek, Borisjuk, Sánchez-García, Gotor, Romero, Martínez-Rivas and Mancha2007). According to the literature this might be due to: (i) enhanced polyphenol-oxidase activity (Lenoir et al., Reference Lenoir, Corbineau and Côme1986); (ii) enhanced microbial activity (Heydecker and Chetram, Reference Heydecker and Chetram1971). In this work we focused on the possibility of enhanced microbial activity. The sunflower pericarp is a natural host for a variety of microbial communities, which can survive as spores (fungi) or endospores (bacteria) for years until hydration takes place. Therefore, at first, we evaluated the oxygen consumption in isolated pericarps incubated in the absence and presence of antibiotics (mixture of fungicides and bactericides) for both genotypes. We observed that the oxygen consumption in water (as incubation medium) with isolated pericarps from the inbred line was markedly increased when incubation was performed at 30°C in comparison with that of isolated pericarps incubated at 10°C (Fig. 4A). However, when the treatment with antibiotics was applied to isolated pericarps from both genotypes incubated at high temperature, the oxygen consumption was reduced dramatically, suggesting that the consumption was mostly due to the inhibition of the activity of pericarp-microbial communities, which is enhanced at high incubation temperatures in the absence of antibiotics (Fig. 4). Other mechanisms as, for example, some enzyme-mediated reactions taking place in the pericarp (i.e. polyphenol oxidase) should not be ruled out as part of this oxygen deprivation mechanism (as has been observed in some cereal grains such as barley and oat). However, as the treatment with antibiotics reduced oxygen consumption to an important extent (and these compounds are not expected to interfere with polyphenol oxidase activity), enhanced microbial activity arises as the central feature to explain the observed increase in oxygen consumption in isolated pericarps incubated at 30°C in absence of antibiotics (Fig. 4).
Moreover, when we used antibiotics to evaluate their effect on pericarp-imposed dormancy (assessed as achene germination), the treatment with antibiotics increased achene germination of the commercial hybrid and the inbred line, although the increment was not statistically significant for the inbred line (Table 2). This suggests that high oxygen consumption at high incubation temperature due to enhanced microbial activity possibly limits achene germination. To the best of our knowledge, this is the first work to suggest that the activity of pericarp-microbial communities impairs achene germination at high incubation temperatures. However, the microbial communities can affect the germination by different pathways. For example, the hard pericarp of Lepidium didymum controls germination and this pericarp-imposed mechanical dormancy is released by the activity of fruit-associated common fungi (fungal colonization of the outer pericarp) (Sperber et al., Reference Sperber, Steinbrecher, Graeber, Scherer, Clausing, Wiegand, Hourston, Kurre, Leubner-Metzger and Mummenhoff2017). Also, associations between seeds and seed-associated microorganisms, especially seed endophytic bacteria, could be beneficial for germination and seedling establishment (Truyens et al., Reference Truyens, Weyens, Cuypers and Vangronsveld2014). In the present work, the apparent failure of the treatment with antibiotics to totally overcome dormancy in achenes of the inbred line (Table 2) might be related to the hyper-sensitivity to hypoxia displayed by its embryos at 30°C (Fig. 1B). Indeed, the low but nevertheless existing oxygen consumption in antibiotic-treated pericarps from the inbred line at 30°C (Fig. 4A), could still be sufficient to impose some level of hypoxia that can be sensed by these embryos. Increased metabolic activity due to high incubation temperature (and, consequently, increased oxygen demand), together with less oxygen solubility in water, might be also instrumental for imposing this hypoxia condition. On the other hand, in the commercial hybrid, in which embryos showed very low sensitivity to hypoxia (Fig. 1D), the treatment with antibiotics significantly increased germination (Table 2). These results suggest that, in this genotype, the activity of pericarp-microbial communities could be responsible for dormancy expression at high incubation temperature, adding to the already strict physical constraint to oxygen diffusion imposed by a thicker pericarp. Indeed, the number of cell layers of sclerenchymatic tissue and the sclerenchyma thickness were significantly higher in the commercial hybrid than in the inbred line (Table 1). The activity of pericarp-microbial communities together with a thicker pericarp structure would explain why, in spite of less sensitivity to hypoxia of the embryos, achenes of the commercial hybrid showed a higher sensitivity to hypoxia than those of the inbred line (Fig. 1).
Our experiments also included an assessment of microbial communities associated with the sunflower pericarp, through bacterial colony counts. The application of antibiotics strongly reduced the bacterial colony counts for each genotype (Table 3); moreover, in agreement with the observed more severe oxygen deprivation to the embryo exerted by the pericarp, the bacterial load in the pericarp was significantly higher in the commercial hybrid than in the inbred line. We also delved into microbial communities through community-level physiological profiles (CLPP) of carbon-source utilization and Shannon's diversity index (H index) for bacteria and fungi with the aim of detecting similarities between genotypes in terms of their pericarp-associated microbial communities. The CLPP differed between the two genotypes (Fig. 5), in accordance with Nelson et al. (Reference Nelson, Simoneau, Barret, Mitter and Compant2018) for other seed crops. The effect of antibiotics applied to the incubation medium also had a strong impact on fungal and bacterial community profiles in both genotypes (Fig. 5). Therefore, the reduced oxygen consumption observed in presence of antibiotics could be due to both a reduction of total microbial proliferation together with changes in specific composition. These changes at the microbiological level could explain the observed decrease in oxygen consumption in presence of antibiotics which, in turn, enhanced germination at 30°C possibly through hypoxia attenuation. Despite the fact that carbon sources into the microplates do not represent the same conditions as in the germination tests, the CLPP provides information about potential functionality of microbial communities (Preston-Mafham et al., Reference Preston-Mafham, Boddy and Randerson2002). Moreover, the H index differed significantly between control and antibiotic-treated achenes in the case of the inbred line for bacteria. These results suggest that each genotype had a different bacterial load and different bacterial and fungal communities, as reflected by their contrasting pattern of oxygen consumption. This work attempted to evaluate the effect that microbial communities have on oxygen consumption and germination at 30°C, but not to characterize these communities beyond the information that can be derived from CLPP analysis. Differences in the composition of microbial communities could be explained by the plant genotype itself (e.g. seed size, seed anatomy) but also by abiotic factors (e.g. field management practices, harvesting methods, seed processing and storage) (Barret et al., Reference Barret, Briand, Bonneau, Préveaux, Valière, Bouchez, Hunault, Simoneau and Jacques2015). Barret et al. (Reference Barret, Briand, Bonneau, Préveaux, Valière, Bouchez, Hunault, Simoneau and Jacques2015) worked with seed samples from various plants belonging to different varieties, species, genera and families, and they demonstrated that the structure of the seed microbiota seems to be indeed driven by abiotic factors, such as the geographic location of the production region and the harvesting year. In the present work, the load and microbial diversity described here only corresponds to these two genotypes belonging to a given production lot under a certain environment and, therefore, cannot be extrapolated to other situations (reason for which a characterization of these communities at a specific level would be useless).
Seed coating treatments in sunflower usually include fungicides to avoid the fungal infestation (as recommended by Viswanathan, Reference Viswanathan1996), but not bactericides aimed specifically to bacteria. The long-known struggle between fungi and bacteria involves the production of natural toxic chemicals aimed at each other. If the production of natural antibiotics produced by fungi is eliminated by applying fungicides (as commonly used in commercial seed) an imbalance towards proliferation of bacteria might occur, thus resulting in faster oxygen uptake and enhancing dormancy at high temperatures at which bacteria divide faster.
In summary, our results show that both genotypes express pericarp-imposed dormancy at high incubation temperature. However, it is not possible to derive a single physiological model for both genotypes. The genotypes displayed different oxygen threshold values for germination inhibition, being lower for the commercial hybrid than for the inbred line. In the inbred line, the main limitation for achene germination was imposed by the high embryo sensitivity to hypoxia, which is enhanced at high incubation temperature which, in combination with enhanced oxygen consumption in isolated pericarps, resulted in dormancy expression. In achenes from the commercial hybrid, in contrast, the main limitation to germination resulted mostly from the presence of the pericarp that represents a severe restraint when the activity of pericarp-microbial communities is possibly enhanced by a high incubation temperature.
Acknowledgements
We thank Silvina Enciso for excellent technical assistance with ABA determinations.
Supplemental material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258519000060.
Figure S1. Photographs of an achene of sunflower from the inbred line (A) and from the commercial hybrid (B) and its components: seed (embryo and endosperm + seed coat) and pericarp.
Figure S2. Transversal sections from the central part of achenes from the inbred line (A) and the commercial hybrid (B). s, sclerenchyma. Scale bars = 50 µm.
Financial support
This work was supported by Dow AgroSciences and Consejo Nacional de Investigaciones Científicas y Técnicas.