Introduction
The reproductive characteristics of alien plant species have been recognized repeatedly as important traits involved in their invasive success (Roy, Reference Roy, Di Castri, Hansen and Debussche1990; Williamson, Reference Williamson1996; Cox, Reference Cox2004). Beyond fecundity and dispersal capacities, the fate of seeds after they have reached a new site is important in determining the outcome of invasion (Moravcova et al., Reference Moravcova, Pysek, Pergl, Perglova and Jarosik2006). The seed bank plays a key role in population dynamics of annual plants and, in particular, in those that are invasive. Several studies pinpointed the large persistent seed banks of successful invasive plants (Pyke, Reference Pyke1990; Honig et al., Reference Honig, Cowling and Richardson1992). However, few studies have attempted to investigate the role and the dynamics of the seed bank in invasive species (Krinke et al., Reference Krinke, Moravcova, Pysek, Jarosik, Pergl and Perglova2005; Yakimowski et al., Reference Yakimowski, Hager and Eckert2005; Shen et al., Reference Shen, Liu, Baskin, Baskin and Cao2006).
For plant species with a persistent seed bank, spreading germination over time, until favourable conditions are reached, is an efficient mechanism to reduce the hazardous effect of severe environmental conditions (Kalamees and Zobel, Reference Kalamees and Zobel2002). Among these plants, the best-documented examples of species that accumulate large persistent soil seed banks are the ruderal plants of arable fields (Grime, Reference Grime2001). The buried seeds of ruderal plants have the capacity to survive for long periods and to germinate in large numbers when the conditions (i.e. mostly disturbance) are favourable. Many invasive plants are ruderals and are well adapted to disturbed conditions due to their long-lived seed banks (Grime, Reference Grime2001; Prinzing et al., Reference Prinzing, Durka, Klotz and Brandl2002). For example, in France, most American exotic species are associated with disturbed areas, such as field crops, set-asides, roadsides, sand dunes and riversides (Maillet and Lopez-Garcia, Reference Maillet and Lopez-Garcia2000).
In the present paper, we investigate the heterogeneity of the spatial distribution of the seed bank of Ambrosia artemisiifolia L. (Asteraceae) and the consequences of disturbance for the dynamics of the species. This species is one of the most important invasive plants in Europe and particularly in France (Muller, Reference Muller2004; Sheppard et al., Reference Sheppard, Shaw and Sforza2006). A. artemisiifolia was introduced for the first time from North America to France in 1863 through seed crops (Chauvel et al., Reference Chauvel, Dessaint, Cardinal-Legrand and Bretagnolle2006). The species is an annual ruderal weed with a 6-month life cycle from spring to autumn (Bassett and Crompton, Reference Bassett and Crompton1975). It colonizes spring crops (sunflower, soybean, maize), intercrops following winter crops (barley, wheat), and set-asides or abandoned fields. Populations of A. artemisiifolia also spread in other human-disturbed habitats, such as roadsides, building sites, abandoned gravel pits or any waste places, and even in semi-natural disturbed areas, such as riverbanks. Furthermore, this species is a threat to human health due to abundant allergenic pollen release (Déchamp and Méon, Reference Déchamp and Méon2002; Laaidi et al., Reference Laaidi, Laaidi, Besancenot and Thibaudon2003).
On average, each A. artemisiifolia plant produces about 300–6000 seeds, with a maximum of 18,000 for the biggest plants (Fumanal et al., Reference Fumanal, Chauvel and Bretagnolle2007). The seeds of A. artemisiifolia are achenes (i.e. hard coat involucre protecting a soft seed), 2.5 mm broad and 3.5 mm long (Bassett and Crompton, Reference Bassett and Crompton1975). We use the more convenient term of ‘seed’ instead of the more botanically accurate term ‘achene’. A. artemisiifolia forms a persistent soil seed bank (Thompson et al., Reference Thompson, Bakker and Bekker1997). The dormant mature seeds released by plants in autumn persist in the soil during the winter and germinate in spring. The seeds that do not germinate in spring re-enter dormancy due to secondary dormancy mechanisms (Bazzaz, Reference Bazzaz1970). Previous long-term seed burial studies showed that seeds of A. artemisiifolia can remain viable in the soil for decades (Toole and Brown, Reference Toole and Brown1946; Stoller and Wax, Reference Stoller and Wax1974). The overall seed-bank densities of A. artemisiifolia in field-crop habitats have been analysed previously (Raynal and Bazzaz, Reference Raynal and Bazzaz1973; Bigwood and Inouye, Reference Bigwood and Inouye1988; Gross, Reference Gross1990; Rothrock et al., Reference Rothrock, Squiers and Sheeley1993; Webster et al., Reference Webster, Cardina and White2003). However, a fine-scale analysis of the spatial heterogeneity and its variation among different habitat types is necessary to understand the population dynamics and the invasiveness of this species.
The aim of the present study was to investigate fully the seed-bank variations and dynamics of A. artemisiifolia across the different environments where it develops in France. For several populations growing in different habitats, we investigated: (1) the size and the respective proportions of dormant, non-dormant and dead seeds composing the soil seed bank; (2) the spatial pattern (vertical and horizontal) of seed distribution in the soil seed bank; (3) the relationship between seed-bank densities and seedling recruitment; and (4) the effects of soil disturbance or vegetation removal on seedling recruitment.
Materials and methods
Plant populations
Nine populations of A. artemisiifolia were studied in 2006 for both seed-bank dynamics and seedling recruitment (Table 1). These populations were chosen from three different habitat types (field crops, set-asides and wastelands; sites described in detail below) where A. artemisiifolia commonly develops in France. The populations chosen contained at least 5000 individual plants. Characteristics of soil texture and the A. artemisiifolia plants are provided in Table 1. The field-crop populations (Fc1, Fc2, Fc3, Fc4) were annually ploughed to a depth of 20 cm, whereas the other habitat types did not experience soil disturbance. The field-crop populations Fc1, Fc3 and Fc4 were organized with a 3-year crop rotation of spring crops (bean, pea, sunflower, maize), and Fc2 was a field crop used for bird food (sunflower or oats/buckwheat). The two set-aside populations, Sa1 and Sa2, were respectively a field previously cultivated in sunflower and abandoned for 10 years, and a field previously cultivated in maize and abandoned for 11 years (mowed annually). Finally, the wasteland sites were recent building sites (Wl1, Wl2) and a gravel pit abandoned for 40 years (Wl3). The population Wl3 was annually flooded (2–3 m deep) from winter to the end of spring.
Table 1 Locations, habitat descriptions and characteristics of the nine French populations of Ambrosia artemisiifolia analysed in the present study. Soil textures were defined according to the United States Department of Agriculture (Natural Resources Conservation Service) soil taxonomy system (http://soils.usda.gov/technical/classification/taxonomy/). The density of flowering plants per m2 and mean plant height were recorded using 10 quadrats (0.5×0.5 m) in 2006, and mean fecundity per plant was estimated using a linear regression relationship between plant dry mass and number of seeds produced (data not shown)

N, number of soil cores sampled.
* Populations used for spatial analysis of soil seed bank.
† Populations used in soil disturbance and vegetation removal experiments.
The populations were located from north, in Burgundy, to the south, Rhone-Alps region, along the major French distribution area of A. artemisiifolia (Table 1, Fig. 1). The mean daily temperatures measured in 2005 during the winter (December–March) ranged from − 6.3°C/+14°C (min/max) for northern populations to − 3°C/+14.4°C (min/max) for southern populations. During the summer (June–September) temperatures ranged from 11.3°C/26.6°C (min/max) for northern populations and 12.9°C/27.6°C (min/max) for southern populations. The annual sum of rainfall was 576 mm in the northern populations and 658 mm in the southern populations.

Figure 1 Location of the nine French populations of Ambrosia artemisiifolia studied in 2006.
Among the populations studied, two nearby (Fc4, Sa2) or adjacent (Fc3, Sa1) field-crop and set-aside populations, characterized by fundamental differences in soil disturbance, were selected to analyse the spatial distribution of the seed bank. Furthermore, the two set-aside populations (Sa1, Sa2) were also used to study the effects of soil disturbance and vegetation removal on above-ground seedling recruitment.
Soil seed bank
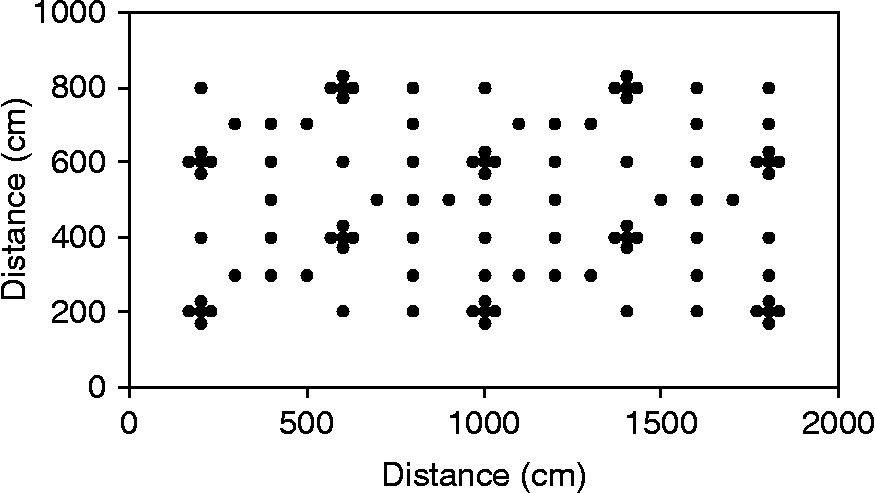
The objective of the first experiment was to quantify the horizontal spatial distribution of the seed bank at two different depths in the soil. One hundred soil cores were collected at four sites (Fc3, Fc4, Sa1, Sa2). To create the maximum class of distances for spatial analysis, the soil cores were sampled at two spatial scales, according to a semi-regular sampling design using intercalate crosses (Fig. 2). Soil cores were sampled in a 200 m2 area for populations Fc3, Fc4 and Sa2 (10 × 20 m, Fig. 2) and population Sa1 (5 × 40 m, same half-design as in Fig. 2 multiplied by 2). The configuration of the area sampled in population Sa1 was different from the others due to its particular configuration (an edge of Fc3). Thus, the minimum distance among soil cores provided in the experimental design was 30 cm.

Figure 2 Semi-regular sampling design with intercalate crosses used to evaluate the soil seed bank of Ambrosia artemisiifolia populations. Black points represent the location of the soil cores sampled.
The objective of the second experiment was to provide an overall description of the seed-bank density at different soil levels in all nine populations. Five additional populations were then sampled using 20 (Fc1, Fc2, Wl2, Wl3) or 10 (Wl1) soil cores randomly taken throughout a 200 m2 sampling area.
Soil samples were collected before natural seed germination in spring, when dormancy was naturally removed at the end of winter. Each soil core sample was collected using a soil hand bore, 5 cm in diameter, at two different depths [the upper soil layer L1 (0–5 cm) and the lower soil layer L2 (5–20 cm)]. The sampled depth of the upper soil layer was chosen at 0–5 cm because it represents the active layer of germination (Munier-Jolain et al., Reference Munier-Jolain, Chauvel and Gasquez2002) and corresponds to the maximum germination depth for A. artemisiifolia (Gebben, Reference Gebben1965). The lower soil layer was chosen at 5–20 cm because it corresponds to the soil ploughing depth in field crops.
The soil cores were washed individually with water to separate the A. artemisiifolia seeds from the substrate, using two sieves, the upper one with 5 mm holes to eliminate coarse particles and the lower one with 1.5 mm holes, small enough to retain A. artemisiifolia seeds. The sieving technique is more convenient than seedling quantification by germination experiments and particularly suitable for A. artemisiifolia due to its relatively large seed size (Gross, Reference Gross1990; Forcella et al., Reference Forcella, Wilson, Renner, Dekker, Harvey, Alm, Buhler and Cardina1992; Thompson et al., Reference Thompson, Bakker and Bekker1997). The seeds were collected, counted and then put on moist germination paper in individual Petri dishes (10 cm diameter) containing 3 ml sterile water for 15 d (25°C, photoperiod of 16 h d− 1). The number of seeds in each Petri dish varied according to seed numbers recovered from each sample core. The number of seeds that germinated (non-dormant) per core sample was then recorded.
At the end of the germination experiment, the seeds that did not germinate were crushed to distinguish living seeds (dormant) showing an intact white embryo from dead seeds (empty seeds or seeds with brown/black embryo). The number of dormant plus non-dormant seeds counted was used to calculate the number of living seeds, and the number of living plus dead seeds was used to calculate the total seed bank. In order to compare the seed densities among populations and between soil layers, the seed counts were extrapolated to the same surface unit of 1 m2 and then averaged to the same depth unit of 1 cm of soil.
Kinetics of dormancy
To quantify the kinetics of secondary dormancy of A. artemisiifolia soil seed bank in natural conditions, 20 soil cores from population Fc2 were sampled every month from February to July 2006 at two different soil depths (L1 and L2). Soil cores were analysed using the procedure described above.
Seedling recruitment
In all the populations studied, seedling densities were quantified using ten quadrats of 0.25 m2 (0.5 × 0.5 m plots of standard size) randomly positioned close to the soil sampling points, during the spring of 2006. The seedling recruitment rate was then calculated as the proportion of seedlings emerging from the upper soil seed bank (L1). Seed-bank densities per quadrat were evaluated using three surrounding soil cores.
Disturbance effects
The effects of two disturbance treatments (soil disturbance or vegetation removal) on the germination of the upper soil seed bank (L1) of A. artemisiifolia were analysed on the two set-aside populations (Sa1, Sa2). Ten soil-disturbed and vegetation-removed quadrats of 1 m2 were positioned randomly within the sampling area close to soil seed bank core positions. The soil-disturbed quadrats were soil-disturbed in the upper soil layer (0–5 cm depth) using a spade, with the total elimination of vegetation. In the vegetation-removed quadrats, the vegetation was cut at the soil level without any perturbation of the soil. As previously quantified in non-disturbed quadrats (controls), the number of seedlings was counted in a central sub-quadrat of 0.25 m2 in each treated square of 1 m2 to avoid border effects, and the rate of recruitment was calculated as described above.
The floristic composition was analysed, and the total percentage of soil plant coverage was estimated to characterize the role of vegetation in the A. artemisiifolia seed bank recruitment in these two set-aside habitats. The number of taxa was characterized by the species richness (S, the number of species found), and the diversity (H) was calculated using Shannon's diversity index (Begon et al., Reference Begon, Harper and Townsend1990):
where p i is the relative cover of each species, calculated as the proportion of cover of a given species (C i) to the total plant cover (C) in the site.
Statistical analysis
A sub-sample of 20 cores was taken randomly from the 100 regular core samples collected for the spatial analysis of Fc3, Fc4, Sa1, Sa2, to compare the overall seed-bank densities among the nine populations.
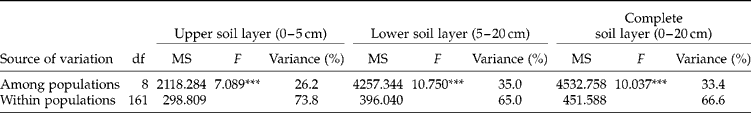
The dormant, non-dormant, living, dead and total seeds of A. artemisiifolia seed bank densities per square metre of surface and centimetre of depth were compared among populations and between soil layers using two-way ANOVAs with fixed effects. The variation of the living seed number among and within populations was examined for L1, L2 and complete (L1+L2) soil layers using one-way ANOVAs for random effect, with the proportion of variance among and within populations expressed as percentages (Sokal and Rohlf, Reference Sokal and Rohlf1981). The relationship among living seed numbers in L1 and L2 was evaluated using the Pearson product–moment correlation for each population.
Moran's I statistics and correlograms were used to study the spatial structure of the seed bank. This analysis conserves the spatial information content of the field sampling by making use of information compiled for the location of each sample point (Sokal and Oden, Reference Sokal and Oden1978). Thus, spatial autocorrelation was used to assess autocorrelation of the seeds among the soil cores. The use of Moran's I to evaluate spatial autocorrelation has been adapted to spatial seed bank description (Dessaint, Reference Dessaint1991). Moran's I statistics were calculated for each distance class and were used for correlogram representation. Correlograms for populations Fc3, Fc4, Sa2 were calculated for 12 distance classes (classes 1–4 with a distance of 0.5 m, classes 5–12 with a distance of 2 m), and for 15 distance classes for population Sa1 (classes 1–4 with a distance of 0.5 m, classes 5–12 with a distance of 2 m, and classes 13–15 with a distance of 5 m), according to the initial sampling matrix design. The sizes of distance classes were selected to have enough pairs of points for individual Moran's I calculations. Moran's I statistic varies from − 1 to +1, and is approximately equal to zero when no trend is present in the spatial pattern (the distribution is randomized). Moran's I is positive (positive autocorrelation) when the values in nearby cores tend to vary in the same direction as the mean, indicating that similar values tend to occur in clusters (aggregate spatial pattern) for a given class of distances. Moran's I is negative when the values in nearby cores, for a class of distances, vary in opposite directions from the mean. The test of significance of Moran's I per distance class was performed against the randomization null hypothesis. Complementary to Moran's I correlograms, the spatial pattern of the seed-bank distribution, as regards the two soil layers studied, was assessed by interpolated contour maps (Legendre and Fortin, Reference Legendre and Fortin1989).
The seedlings emerged per quadrat were compared among populations using one-way ANOVA for random effects, with the proportion of variance among and within populations expressed as the percentage of total variance. The seedling recruitment was compared among populations using a chi-square test. The relationship between living seeds m− 2 in L1 (mean of all sampled cores surrounding the quadrats) and seedlings emerged m− 2 (mean per quadrat) was evaluated within each population, using the Pearson correlation.
The seedling recruitment rates in the disturbance experiment were compared among treatments within the two populations studied (Sa1, Sa2) using chi-square tests.
Before performing statistical analyses, count data were checked for normality and homogeneity of variance, and were square root transformed (Sokal and Rohlf, Reference Sokal and Rohlf1981), with the addition of 0.5 to all data in order to avoid zeros (Yamamura, Reference Yamamura1999). Statistical analyses were performed using SYSTAT 11 software for Windows with a significance level of α = 0.05, and the spatial analysis of the seed banks was performed using PASSAGE 1.0 software (Rosenberg, Reference Rosenberg2001; available at .
Results
Soil seed bank
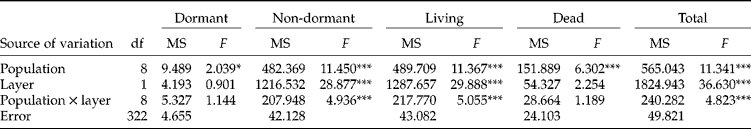
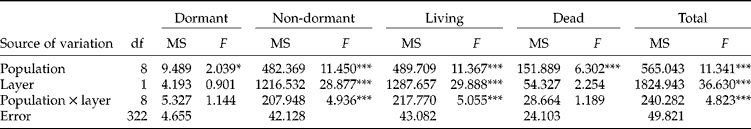
The mean ( ± SE) living seed densities of the complete soil seed banks (L1+L2, 0–20 cm depth) of A. artemisiifolia ranged from 536 ( ± 194) to 4477 ( ± 717) seeds m− 2 among populations. The density of living seeds m− 2 (Table 2) in L1 (0–5 cm) was lower than in L2 (5–20 cm) for field-crop populations (Fc1, Fc2, Fc3, Fc4), whereas the opposite pattern was found for the others (Sa1, Sa2, Wl1, Wl2, Wl3). The population Sa2, with 96% of living seeds distributed in the upper soil layer, was also different from the other set-aside or the wastelands. The majority of living seeds were non-dormant (67–100%) when the soil seed bank was sampled, early in spring (Table 2, Fig. 3). The proportion of dead seeds in the seed banks (Fig. 3) was highly heterogeneous among populations (4–74%). The dormant, non-dormant, dead, living and total seed densities per m2 by centimetre of depth were significantly different among the nine populations tested. The non-dormant, living and total seed densities per m2 by centimetre of depth were significantly different between the two soil layers, whereas no significant differences were detected when looking for dormant and dead seed densities (Table 3). However, a significant interaction between populations and depths was also found for non-dormant, living and total seed densities, indicating that both effects were not independent of each other. The contribution to the total variance of among-population variations for living seed densities per m2 of the upper (L1), lower (L2) and complete (L1+L2) soil seed banks were lower than the contribution of within-population variations (Table 4).
Table 2 Mean (±SE) dormant, non-dormant, living, dead and total Ambrosia artemisiifolia seeds m−2 within the upper soil layer (L1, 0–5 cm) and the lower soil layer (L2, 5–20 cm) of nine French populations. Means were calculated on 20 replicates per layer for all the populations, except for Wl1 where ten replicates were used. Living seeds are those dormant and non-dormant, and total seeds include living and dead seeds


Figure 3 Proportions of non-dormant (white bars), dormant (hatched bars) and dead seeds (black bars) of Ambrosia artemisiifolia within the upper soil layer (L1, 0–5 cm) and the lower soil layer (L2, 5–20 cm) for the different populations studied. The proportion of living seeds corresponds to the proportions of non-dormant and dormant seeds.
Table 3 Two-way ANOVAs on the dormant, non-dormant, living, dead and total seed densities m−2 by centimetre of depth of Ambrosia artemisiifolia among populations and soil layers (L1 and L2)
*P < 0.05; ***P < 0.001.
Table 4 One-way random ANOVAs on the living soil seed bank of Ambrosia artemisiifolia among and within populations from the upper soil layer (L1, 0–5 cm), from the lower soil layer (L2, 5–20 cm), and from the complete soil layer (L1+L2, 0–20 cm). The estimates of variance components are expressed as percentages

***P < 0.001.
A significant correlation between densities of living seeds from the upper soil layer and the lower soil layer was detected for the population Wl2 (r = 0.62, P = 0.002), whereas no correlation was found for the other populations (P>0.05).
Kinetics of dormancy
Seed dormancy was broken at least 2 months before natural germination of A. artemisiifolia (i.e. February). Only 2% of the total A. artemisiifolia soil seed bank (0–20 cm) sampled in February in population Fc2 was dormant. The proportion of dormant seeds remained small until May (from 2 to 6%) and increased to 79% until middle July. The acquisition of seed dormancy was quite similar for the two soil layers (L1 and L2), even though the dormant seed proportion in L1 increased faster than in L2 (data not shown).
Spatial analysis of soil seed bank
The interpolated contour maps of the upper (L1) and lower (L2) living seed banks of A. artemisiifolia showed an aggregated distribution for the two field crops (Fc3, Fc4) and set-asides (Sa1, Sa2) analysed (Fig. 4). The patches shown on interpolated maps did not clearly match between the two soil layers (L1 and L2), except for the Sa1 populations, where some similarity occurred. Obviously, this similarity could be mostly explained by the total absence of disturbance of soil and/or of vegetation in the Sa1 population, compared to the others annually ploughed (Fc3, Fc4) or mowed (Sa2). In each population, different sizes of seed patches can be observed, but no particular trend seemed to occur when comparing populations from different habitat types, disturbance regimes or soil layers. Furthermore, the surface where there are no seeds (Fig. 4) is the highest in Sa2 and the lowest in Sa1. This observation may suggest that the undisturbed (soil and vegetation) population Sa1 was the least heterogeneous of all the populations. Moran's I indices of autocorrelation were mostly significant in the upper soil layer of three populations (Sa1, Sa2 and Fc3) (Fig. 5). However, for the population Fc4, most of the Moran's I indices of both soil layers were significant. The significant positive value of Moran's I (P < 0.001) for the first distance classes, followed by negative significant values for longer classes, indicated the occurrence of a gradient of densities of seeds only for the lower soil layer of Fc4 population (Legendre and Fortin, Reference Legendre and Fortin1989). Moran's I was not significant for the first distance classes for the other populations, except for Sa1 in the upper soil layer.

Figure 4 Linear interpolated maps of the spatial distribution of Ambrosia artemisiifolia living seed bank in the upper soil layer L1 (left panels) and lower soil layer L2 (right panels), for the field-crop populations Fc3 and Fc4, and set-aside populations Sa1 and Sa2. Keys on the right of each diagram represent the scale of seed numbers.

Figure 5 Moran's I correlograms of the living seed bank of Ambrosia artemisiifolia in the upper (L1, 0–5 cm, circles) and the lower (L2, 5–20 cm, squares) soil layers for field-crop populations Fc3 and Fc4, and set-aside populations Sa1 and Sa2. White symbols, Moran's I not significantly different from 0; black symbols, Moran's I significantly different from 0.
Seedling recruitment
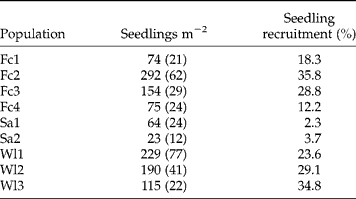
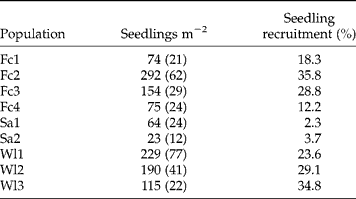
The mean ( ± SE) number of seedlings emerged per m2 (Table 5) ranged from 23 ( ± 12) to 292 ( ± 62) and was significantly different among populations (F = 7.09, P < 0.001). The lowest seedling densities were observed in the set-aside populations (Sa1, Sa2). The seedling densities were quite similar for the two field crops Fc1 and Fc4 that were managed similarly, and were lower than the two other field crops (Fc2, Fc3) and wastelands (Wl1, Wl2, Wl3). As observed previously for seed-bank densities, the majority of the variation in seedling density (58.5%) was found within populations. The seedling recruitment (emergence from the upper living soil seed bank) ranged from 2.3 to 35.8% and was also lower in the two set-aside populations (Table 5). However, significant differences among populations were observed (χ2 = 984.604, P < 0.001).
Table 5 Mean (±SE) seedlings emerged per m2 and the seedling recruitment rate of Ambrosia artemisiifolia for the nine French populations studied. Means were calculated on ten quadrats
A positive correlation between the L1 seed bank and seedling numbers was found only for the two field-crop populations, Fc3 (r = 0.66, P = 0.034) and Fc4 (r = 0.81, P = 0.004), whereas no correlation was found for the seven remaining populations (P>0.05).
Disturbance effects on seed-bank recruitment
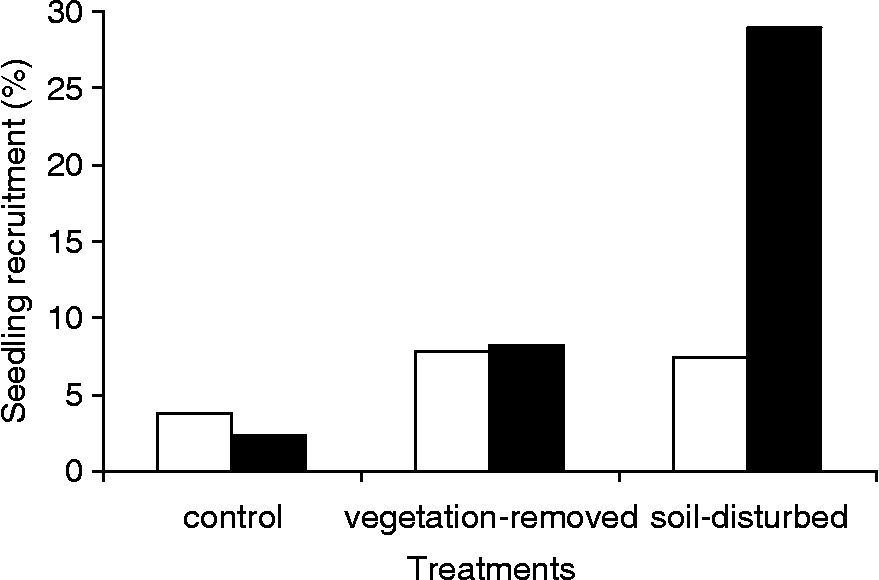
Seedling recruitment was significantly different among treatments in both Sa1 (χ2 = 940.572, P < 0.001) and Sa2 (χ2 = 10.572, P = 0.006) populations. However, different patterns were observed between the two populations (Fig. 6). The average seedling recruitment of vegetation-removed and soil-disturbed quadrats in Sa2 were twofold higher than control ones (χ2 = 9.346, P = 0.002; χ2 = 8.125, P = 0.004). However, seedling recruitment of vegetation-removed quadrats in Sa2 was not significantly different from soil-disturbed ones (χ2 = 0.046, P = 0.830). In Sa1, the average seedling recruitment in vegetation-removed quadrats was fourfold higher than control quadrats (χ2 = 97.252, P < 0.001), while the average seedling recruitment in soil-disturbed quadrats was twelve-fold higher than control quadrats (χ2 = 740.620, P < 0.001). The seedling recruitment in soil-disturbed quadrats was fourfold higher than in vegetation-removed quadrats (χ2 = 388.931, P < 0.001).

Figure 6 Seedling recruitment rates of Ambrosia artemisiifolia in the two set-aside populations, Sa2 (white bars) and Sa1 (black bars), according to disturbance treatments.
The species richness and Shannon diversity index were about twofold higher for Sa2 (S = 29, H = 2.069) than for Sa1 (S = 15, H = 1.222), but the total coverage of habitat by vegetation was lower for Sa2 (C = 90%) than for Sa1 (C = 100%). The dominant species in Sa2 community belonged to Poaceae, Plantaginaceae and Asteraceae families [50% covered by Vulpia myuros (L.) C.C. Gmelin and Plantago lanceolata L., 20% covered by Erigeron annuus (L.) Pers. and Crepis foetida L.], while the dominant species in Sa1 belonged to the Poaceae family [75% covered by Elytrigia repens (L.) Nevski and Poa pratensis L.].
Discussion
The present data are the first quantitative estimates of the A. artemisiifolia seed bank in different environments in its area of introduction. The density of living seed bank in the 0–20 cm soil layer varied among populations, by a magnitude of up to eight, on average from 536 to 4477 seeds m− 2. The minimum variance within habitat types was observed for field crops and the maximum for the set-aside populations. Such heterogeneity in the A. artemisiifolia seed bank within sites has been reported previously (Bigwood and Inouye, Reference Bigwood and Inouye1988; Rothrock et al., Reference Rothrock, Squiers and Sheeley1993). These variations can be explained by historical or ecological differences experienced by the populations, such as human uses and soil disturbance, population age, or even nutrient status of the soil, which influence plant size and seed rain (Krinke et al., Reference Krinke, Moravcova, Pysek, Jarosik, Pergl and Perglova2005). The importance of the seed bank is directly connected to the number of residual living seeds in the soil, to the seed rain of previous years, and to seed losses by predation or seed decay (Harper, Reference Harper1977). Moreover, the differences found could be due to the plasticity in fecundity and vigour of plants, and to the variation in genetic make-up (Krinke et al., Reference Krinke, Moravcova, Pysek, Jarosik, Pergl and Perglova2005). The total living seed-bank density also varied within populations, and such variation was higher than the variation observed among populations. The propensity of A. artemisiifolia to develop in disturbed and heterogeneous habitats may explain the differences in plant and seed-bank distribution within habitats.
The density of A. artemisiifolia living seeds found in the present study was higher than previously published data from populations growing in North American field crop habitats (Raynal and Bazzaz, Reference Raynal and Bazzaz1973; Bigwood and Inouye, Reference Bigwood and Inouye1988; Rothrock et al., Reference Rothrock, Squiers and Sheeley1993; Webster et al., Reference Webster, Cardina and White2003). As for other invasive species, Eupatorium adenophorum Sprengel (Shen et al., Reference Shen, Liu, Baskin, Baskin and Cao2006) and Heracleum mantegazzianum Sommier & Levier (Krinke et al., Reference Krinke, Moravcova, Pysek, Jarosik, Pergl and Perglova2005), our data support the hypothesis that the large seed bank of A. artemisiifolia could be a crucial feature of its invasive success. The large seed bank of A. artemisiifolia and its persistence over time could provide this species with a high invasive potential through the possibilities of dispersion both in space and time. Therefore, even if only a few seeds persist in the seed bank, it can potentially generate a new population after a long time.
The proportions of living seeds were lower in L1 than in L2 for field-crop populations, whereas the opposite pattern was found for non-field crop populations. The higher proportion of seeds in L1 in non-field-crop habitats is explained by the annual seed rain and the absence of soil disturbance as in field crops. Even if the seed bank in field crops is expected to be more homogeneous than in non-field crops, as the seeds are buried by ploughing, we should expect more seeds in the deeper layer. Gross (Reference Gross1990) found a similar pattern in an annually ploughed field where A. artemisiifolia seeds, like the other species tested, were more abundant in the lower soil layer (5–15 cm) than in the upper soil layer (0–5 cm). The seed-bank distribution in depth has an influence on the renewal and the persistence of annual weed populations (Forcella et al., Reference Forcella, Benech-Arnold, Sanchez and Ghersa2000; Ghersa and Martinez-Ghersa, Reference Ghersa and Martinez-Ghersa2000). According to Gebben (Reference Gebben1965), the amount of germination of A. artemisiifolia depends on different factors, such as the burial depth of seeds and the maximum length of elongation of the hypocotyls. Site management can influence the seed distribution in the soil and population replacement (Dyer, Reference Dyer1995), as agricultural management tends to bury deeply a large proportion of the seed bank.
Seed burial can be an advantage for short-term weed management, by reducing seedling density. However, a stock of buried seeds with prolonged viability creates a latent problem, and can promote a new infestation by the weed following ploughing. The buried seeds of A. artemisiifolia are less sensitive to environmental extremes (Béres and Hunyadi, Reference Béres and Hunyadi1984) compared to seeds near the soil surface, which suffer from meteorological factors during the winter. Buried seeds are also less apt to be predated, as most seed predators are surface foragers (Fenner and Thompson, Reference Fenner and Thompson2005). Finally, the burial of A. artemisiifolia seeds prevents their germination because of the dark, the low fluctuation in temperatures and the high carbon dioxide concentrations (Bazzaz, Reference Bazzaz1970). Therefore, agricultural practices that bury A. artemisiifolia seeds deeply will not be suitable for long-term management.
The average percentage of dormant seeds ranged from 0 to 18% according to the population, but no pattern was detected among habitat types or within populations among soil layers. In general, the variation of dormant seed proportions can be related to environmental conditions or mother plant stress during seed maturation (Baskin and Baskin, Reference Baskin and Baskin1998). These low values of dormant seeds indicated that the majority of A. artemisiifolia seeds were able to germinate before natural germination time in spring. Furthermore, 98% of the total seedbank (0–20 cm) in the Fc2 population were non-dormant 2 months before the natural germination period in the field. Therefore, management practices applied early in spring using tillage, followed by seedling destruction, should be useful to reduce the A. artemisiifolia seed bank. However, following a period in which conditions are favourable for germination, the majority of A. artemisiifolia seeds (79%) became progressively dormant until summer. These results confirmed previous findings of Béres and Hunyadi (Reference Béres and Hunyadi1984) on natural populations of A. artemisiifolia growing in Hungarian fields. Furthermore, the seeds within the two soil layers exhibited similar seed dormancy patterns.
Similar to the vertical distribution of seeds, the horizontal spatial distribution of A. artemisiifolia seeds was also heterogeneous within the four populations studied. According to Dessaint et al. (Reference Dessaint, Chadoeuf and Barralis1991), the seeds of weed species are generally aggregated around the mother plant that produced them. However, the amounts of seeds found in a particular area may depend on several factors, such as the mother plant traits (affecting the number, the size and the shape of seeds), the activity of dispersal agents and the spatial heterogeneity of parent plants in the habitat (Dessaint et al., Reference Dessaint, Chadoeuf and Barralis1991). Finally, the spatial pattern of seeds in the soil seed bank depends on their predation, longevity and the level of soil disturbance. The two soil layers, among the four populations analysed, showed an aggregated spatial distribution of A. artemisiifolia seeds, confirming what was previously supposed (Bigwood and Inouye, Reference Bigwood and Inouye1988; Rothrock et al., Reference Rothrock, Squiers and Sheeley1993). An aggregated soil seed bank of A. artemisiifolia was observed in habitats that did or did not experience soil disturbances. Such aggregate structure of a seed bank is common (Bigwood and Inouye, Reference Bigwood and Inouye1988; Dessaint et al., Reference Dessaint, Chadoeuf and Barralis1991; Benoit et al., Reference Benoit, Derksen and Panneton1992). Furthermore, a gradient was observed for the lower soil layer of the field crop population Fc4. The spatial structures between the two soil layers did not match very well, except for the set-aside Sa1. The description of spatial heterogeneity, as a functional component of weed populations, is fundamental to derive realistic predictions about weed population behaviour and to model weed population dynamics (van Groenendael, Reference van Groenendael1988), or to design adequate sampling procedures for seed-bank analysis (Wiles and Schweizer, Reference Wiles and Schweizer2002). A spatial study of the seed bank could also be a helpful tool for agricultural management, so as to target the areas for post-emergence management (Wiles and Schweizer, Reference Wiles and Schweizer2002).
Although most seeds sampled after winter were able to germinate immediately (82–100%) in laboratory conditions, the rate of natural seedling recruitment was low (2.3–35.8%). The results obtained in field crops (12.2–35.8%) were consistent with what was found previously for the species in similar habitat types, 6.8–38.2% (Forcella et al., Reference Forcella, Wilson, Renner, Dekker, Harvey, Alm, Buhler and Cardina1992), 42% (Rothrock et al., Reference Rothrock, Squiers and Sheeley1993) and 15–39% (Webster et al., Reference Webster, Cardina and White2003). The similar seedling recruitment of A. artemisiifolia observed in field-crop and wasteland habitats was about ninefold higher than that found in the two set-asides. The decrease of recruitment in the set-asides could be explained by the degree of vegetation coverage. Higher vegetation cover could modify the conditions for germination, i.e. the light requirements needed for seed germination of A. artemisiifolia (Bazzaz, Reference Bazzaz1968). Furthermore, a positive relationship was found between seed-bank and seedling densities in two field-crop habitats only. The same pattern was previously found in A. artemisiifolia (P < 0.001, r 2 = 0.91; Rothrock et al., Reference Rothrock, Squiers and Sheeley1993). However, no significant relationship was found in the remaining habitats. In the non-field-crop populations, the absence of a relationship could be explained by the heterogeneity of either vegetation cover (influencing seedling recruitment) or heterogeneous seed-bank distribution. Furthermore, several studies demonstrated a lack of relationships in non-crop habitats, as in agricultural fields (Thompson and Grime, Reference Thompson and Grime1979; Roberts, Reference Roberts1981; Forcella et al., Reference Forcella, Wilson, Renner, Dekker, Harvey, Alm, Buhler and Cardina1992). Therefore, for management strategy purposes, the knowledge of spatial distribution of the A. artemisiifolia seed bank will not be very helpful, in most cases, to target control efforts on seedlings (site-specific control).
Seedling recruitment in the two set-aside populations was highly enhanced by soil disturbance and vegetation removal. Soil disturbance increased seedling recruitment more strongly than vegetation removal for the population that had the higher vegetation cover, lower species richness and Shannon diversity index, and that was dominated by perennial grasses. In the case of A. artemisiifolia, the limiting factor for population spread seems to be high vegetation cover. Similar results were found in Daucus carota L., with reductions in seedling emergence associated with high cover of perennial grasses in 3-year fallows (Holt, Reference Holt1972). These results highlight the importance of soil disturbance for A. artemisiifolia to be able to persist in abandoned fields. In turn, the main obstacle to A. artemisiifolia persistence seems to be vegetation composed of competitive perennial grasses, rather than a mixture of forbs that are less competitive against A. artemisiifolia. This might also explain the differences in seedling recruitment according to vegetation cover and the lack of relationship previously found between seed bank and seedling densities in the other populations. Gebben (Reference Gebben1965) showed the role of soil disturbance in germination of A. artemisiifolia in plots tilled prior to seed sowing. Furthermore, these results highlight why A. artemisiifolia is most abundant during the first years of secondary succession, when the community is not yet stabilized (Bazzaz, Reference Bazzaz1968). Germination of A. artemisiifolia is closely linked to disturbance, which ensures the availability of resources and reduces the probability of competition with later-successional plant species (Bazzaz, Reference Bazzaz1979). When seeds are brought towards the surface by soil disturbance, they experience reduced CO2 concentrations, and when vegetation is removed, they experience unfiltered light and fluctuating temperatures. These factors promote seed germination in A. artemisiifolia. As in the case of most weedy invasive plants, colonization by A. artemisiifolia of secondary successional or stable habitats may be successful when temporary openings follow disturbance.
The reproductive output of A. artemisiifolia is quite high for a weed with large seeds, and seems to be a crucial feature that makes invasion possible in France and elsewhere in Europe. Based on our results, management strategies should first focus on seed-bank reduction, in particular in field crops. Furthermore, to reduce the spread of the species in non-field crop areas, soil or vegetation disturbance should be minimized to favour natural vegetation competition by the planting of perennial grasses with high soil cover. As Davis (Reference Davis2006) pointed out recently, there is a crucial need to develop weed seed-bank management techniques for summer annual weed species as part of integrated weed management systems. Furthermore, an accurate knowledge of the spatial distribution of seeds could be used to simulate population dynamics of invasive annual plants.
Acknowledgements
This work was supported by the Regional Council of Rhône-Alpes, the Regional Council of Burgundy and by different French agricultural research institutes (ARVALIS, CETIOM) or societies (APRR). G. Louviot is acknowledged for technical assistance. We also thank F. Dessaint and J.-L. Demizieux for their helpful comments on the manuscript.