Introduction
Seed traits play a vital role in the successful dispersal and establishment of plant species. For example, traits may be related to dispersal mode: anemochorous seeds, i.e. seeds dispersed by wind, tend to be small, light and may have appendices that favour buoyancy (Magalhães and Mariath, Reference Magalhães and Mariath2012; Barfuss et al., Reference Barfuss, Till, Leme, Pinzón, Manzanares, Halbritter, Samuel and Brown2016). However, although selective pressures are exerted on seed traits to adapt to local environments, seed traits are more conserved than other plant traits such as leaf size and plant height (Ackerly, Reference Ackerly2009), and thus have been used to define taxonomic relationships (Smith and Downs, Reference Smith, Downs, Smith and Downs1974; Magalhães and Mariath, Reference Magalhães and Mariath2012). Thus, seed traits can provide valuable insights into the ecology of a species and its evolutionary history, and this information may also be useful to predict possible effects of changing environmental conditions.
The epiphytic habitat presents a number of challenges as epiphytes establish in a discontinuous mosaic of suitable trees. Among these challenges are effective dispersal and the colonization of a suitable environment for germination and establishment. There is a significant convergence across families, where about 84% of the epiphytic species are anemochorous, a much higher percentage than among terrestrial species (Madison, Reference Madison1977). Published studies on seed traits are sufficient to identify this general strategy, but, for most genera, the studies are not detailed enough to characterize seed trait diversity among species that may show specific traits that favour the colonization of particular habitats.
Tillandsia consists of about 730 species and is the biggest genus of the family Bromeliaceae (The Plant List, 2013). The genus encompasses a wide range of vegetative forms that allowed the colonization of diverse terrestrial and epiphytic habitats (Benzing, Reference Benzing2000). The diversity of forms described in the adult phase is not concomitant with diversity described in seeds. Most of the studies including Tillandsia species have been performed with adults. A few studies have described seed dispersal ability (García-Franco and Rico-Gray, Reference García-Franco and Rico-Gray1988; Mondragón and Calvo-Irabien, Reference Mondragón and Calvo-Irabien2006; Cascante-Marín et al., Reference Cascante-Marín, von Meijenfeldt, de Leeuw, Wolf, Oostermeijer and den Nijs2009), seed morphology and anatomy (Cecchi-Fiordi et al., Reference Cecchi-Fiordi, Palandri, Turicchia, Tani and Di Falco2001; Palací et al., Reference Palací, Brown and Tuthill2004; Magalhães and Mariath, Reference Magalhães and Mariath2012), while there are more studies on germination (Bernal et al., Reference Bernal, Valverde and Hernández-Rosas2005; Scatena et al., Reference Scatena, Segecin and Coan2006; Mondragón and Calvo-Irabien, Reference Mondragón and Calvo-Irabien2006; Valencia-Díaz et al., Reference Valencia-Díaz, Flores-Palacios, Rodríguez-López, Ventura-Zapata and Jiménez-Aparicio2010; Sosa-Luría et al., Reference Sosa-Luría, Chávez-Servia, Mondragón-Chaparro, Estrada-Gómez and Ramírez-Vallejo2012; Marques et al., Reference Marques, Atman, Silveira and de Lemos-Filho2014; Duarte et al., Reference Duarte, de Lemos-Filho and Marques2017; Müller et al., Reference Müller, Albach and Zotz2017).
Seed morphology has been used in taxonomic studies in the Bromeliaceae for a long time; Smith and Downs (Reference Smith, Downs, Smith and Downs1974) chose the presence and position of appendices in the seeds among the primary characters of distinction to delimit three subfamilies. Within Tillandsioideae, Tillandsia seeds have a plumose coma formed by numerous whitish hairs growing from the micropilar region; the apical end consists of trichomes derived from seed coat cells (external integument; Benzing, Reference Benzing2000). This coma, attached to a small seed increases drag force, maintains the seed in the air for a longer time and potentially increases dispersal (Greene and Quesada, Reference Greene and Quesada2005). The seed coat is also composed of dead cells filled with air, which further increases buoyancy (Madison, Reference Madison1977). There are few studies that describe in detail the characteristics of the plumose coma in epiphytic bromeliads (Palací et al., Reference Palací, Brown and Tuthill2004; Scatena et al., Reference Scatena, Segecin and Coan2006; Wester and Zotz, Reference Wester and Zotz2011; Magalhães and Mariath, Reference Magalhães and Mariath2012; Corredor-Prado et al., Reference Corredor-Prado, Schmidt, Steinmacher, Guerra, Bouzon, Dal Vesco and Pescador2014). Considering the diversity of anemochorous species, information on the falling velocity of their seeds is also scarce (Sheldon and Burrows, Reference Sheldon and Burrows1973; Augspurger, Reference Augspurger1986; Matlack, Reference Matlack1987; Greene and Quesada, Reference Greene and Quesada2005).
Tillandsia seeds are ≤5 mm in length and possess a small embryo and starchy endosperm (Scatena et al., Reference Scatena, Segecin and Coan2006; Sosa-Luría et al., Reference Sosa-Luría, Chávez-Servia, Mondragón-Chaparro, Estrada-Gómez and Ramírez-Vallejo2012), which can be variable in size (Martin, Reference Martin1946; Magalhães and Mariath, Reference Magalhães and Mariath2012; Montes-Recinas et al., Reference Montes-Recinas, Márquez-Guzmán and Orozco-Segovia2012). The development of the endosperm relative to the embryo can vary across plant lineages. More basal lineages tend to have a small embryo and large endosperm (Stebbins, Reference Stebbins1974). In seeds with bigger embryos germination tends to be faster and dormancy reduced; bigger embryos are favoured in environments with short growing seasons, where rapid emergence and establishment may be crucial (Vivrette, Reference Vivrette, Arroyo, Zedler and Fox1995; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Vandelook et al., Reference Vandelook, Janssens and Probert2012).
Anemochorous Tillandsia seeds tend to be small, which does not preclude the possibility for some species-specific variation in seed size, a phenomenon that remains unexplored. In general, increased germination and establishment success has been reported for heavier seeds in exchange for reduced dispersal ability (Dalling, Reference Dalling, Guariguata and Catan2002).
In the present study, we set out to evaluate seed, germination and dispersal traits of six species of Tillandsia collected in the seasonally dry forests of the Yucatan peninsula, Mexico. Our objective was to describe the seed form and relate it to its function in the six Tillandsia species, and to emphasize the divergence and convergence among them, which may be taxonomically relevant. We expected the species limited to the dry sites of the peninsula to invest more in seed resources (seed size, seed mass and coma length) in order to survive under higher stress and to have a higher proportion of embryo to endosperm coupled with increased germination rate to promote faster establishment.
Materials and methods
Study species
The six species in this study are grouped in the Tillandsioideae complex; the majority corresponding to the subgenus Tillandsia except for Tillandsia recurvata, which is in the subgenus Diaphoranthema (see Table 1 and Supplementary Fig. S1; Smith and Downs, Reference Smith, Downs, Smith and Downs1974). Tillandsia species are distributed in different vegetation types (Table 1, Supplementary Fig. S2) which present a distribution according to the rainfall gradient from north to south in the Yucatan peninsula, Mexico. Specimens were collected each year from 2012 to 2015 at three sites in the Yucatan peninsula: T. brachycaulos, T. recurvata and T. yucatana were collected in the tropical dry deciduous forest at the Dzibilchaltún National Park (21°05′N; 89°35′W), T. brachycaulos was also collected in the tropical semi-deciduous forest at the Kaxil-Kiuic Biocultural Reserve (20°05′N; 89°32′W) and T. juncea, T. polystachia and T. schiedeana were collected in the tropical semi-evergreen forest of the Calakmul Biosphere Reserve (18°06′N; 89°48′W; Supplementary Fig. S2). The three collection sites have very small differences in temperature, with most environmental heterogeneity being derived from the differences in precipitation. The species represent ample distribution species (T. brachycaulos and T. schiedeana), and species limited to either the dry (T. recurvata and T. yucatana) or moist (T. juncea and T. polystachia) areas of the peninsula (Supplementary Fig. S2). From each species, at least five plants with seed capsules were collected and brought to a greenhouse at the Centro de Investigación Científica de Yucatán (CICY). Seeds were collected once the capsules opened naturally.
Table 1. Ecological characteristics of six Tillandsia species from the Yucatan peninsula
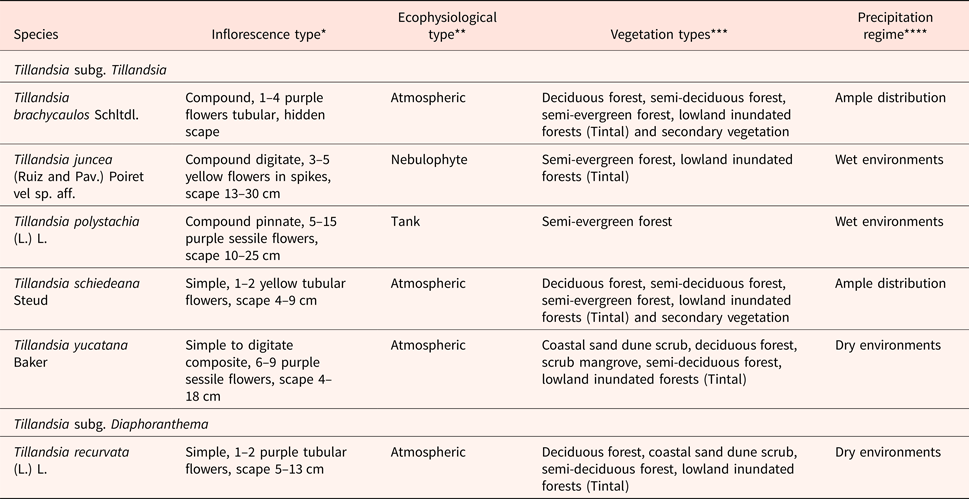
*Davidse et al. (Reference Davidse, Sánchez and Chater1994).
**Pittendrigh (Reference Pittendrigh1948); Benzing (Reference Benzing2000); classification of nebulophytes were defined as in Reyes-García et al. (Reference Reyes-García, Mejia-Chang and Griffiths2012).
***Ramírez et al. (Reference Ramírez, Carnevali and Chi2004); Cach-Pérez et al. (Reference Cach-Pérez, Andrade, Chilpa-Galván, Tamayo-Chim, Orellana and Reyes-García2013).
****Precipitation regime defined according to Supplementary Fig. S2.
Seed anatomy and morphology
Length of seed and plumose coma of a total of 50 seeds per species were measured with a Vernier caliper. Seed mass (without coma) was determined with a digital analytical balance (AND GR 200, Bradford, MA, USA). The outer morphology of seed and coma was studied through photomicrographs using a digital camera coupled to a stereomicroscope (Canon EOS 100D-Stemi SV6, Carl Zeiss, NY, USA). Three seeds with plumose coma per species were given a gold bath for its subsequent observation in scanning electron microscopes (MEB; Jeol, JSM-6360LV, Illinois, USA and Jeol JSM-5310 LV, Utah, USA).
To characterize anatomical structures, five seeds per species were sanded to wear down the hard seed coat and prevent the soft inner seed from collapsing under the microtome. The sanded seeds were fixed in FAA (10% formaldehyde, 5% acetic acid, 50% alcohol ethyl and 35% distilled water) for 24 h and then progressively dehydrated through an ethanol series (30, 50, 70, 85, 96 and 100%, v/v) for subsequent inclusion in paraffin (Merck, Darmstadt, Germany) at 60°C for another 24 h (Márquez-Guzmán et al., Reference Márquez-Guzmán, Wong, Pérez-Pacheco, López-Curto and Murguía-Sánchez2016). Seeds were cut longitudinally into sections of 8 µm with a rotary microtome (American Optical 820, NY, USA) and a Reichert Jung razor (American Optical and Reichert, NY, USA). Longitudinal sections were stained with Safranin-Fast Green, and photographed under a light microscope (Olympus Provis AX-70; Olympus, Tokyo, Japan, software Q capture Pro). The cross-sectional area of the embryo and endosperm was determined using ImageJ software (Rasband, Reference Rasband2014).
Seed terminal velocity
We determined the terminal velocity (V term) of falling seed in still air for 50 seeds of each Tillandsia species. Each seed was dropped in a closed chamber with no air currents from a height of 2 m and the time of fall was timed. Care was taken to ensure that the plumose coma was deployed.
Histochemical characterization
Longitudinal sections were also used to perform histochemical tests for protein, starch and lipids in six seeds of each Tillandsia species. For the best structure visibility, the objective was to obtain the central longitudinal section of each seed, but this was not achieved every time. Sections close to the central one, with an acceptable visibility of the anatomy were sometimes used. For staining, the sections were dehydrated in an ethanol series from 96% alcohol (v/v) to water. To observe proteins and polysaccharides, sections were stained with periodic acid-Schiff reagent and then later with Naphthol Blue Black for 5 min, which dyes proteins in blue and carbohydrates in magenta. To detect starch, sections were stained with Lugol, which dyes starch grains in purple or black. Lipid reserves were stained with Oil Red O (for 25 min), which dyes lipids in an orange or reddish colour. It is noteworthy that the reaction product should be insoluble, which prevents diffusion or migration of the reagents in the solution (Márquez-Guzmán et al., Reference Márquez-Guzmán, Wong, Pérez-Pacheco, López-Curto and Murguía-Sánchez2016). Photomicrographs were obtained with a Photomicroscope (Olympus Provis AX-70; software Q capture Pro).
Germination trials
To quantify germination, we collected 90 seeds per species, except for T. recurvata and T. juncea for which we only had 60 and 35 seeds, respectively. After plumose coma removal, seeds were disinfected in a solution of ethanol (70% v/v) for 2 min, followed by immersion in a solution of sodium hypochlorite (NaClO, 2.5% v/v) for 40 min. Subsequently, the seeds were rinsed in distilled water twice for 5 min to completely remove the remnants of NaClO (Wester and Zotz, Reference Wester and Zotz2011). The seeds were evenly distributed in 9 cm Petri dishes (three per species) on filter paper (Whatman No. 1) with 4 ml of sterile distilled water. We selected environmental conditions for germination that were similar to those in the field, and to previously published studies (Valencia-Díaz et al., Reference Valencia-Díaz, Flores-Palacios, Rodríguez-López, Ventura-Zapata and Jiménez-Aparicio2010; Montes-Recinas et al., Reference Montes-Recinas, Márquez-Guzmán and Orozco-Segovia2012). Petri dishes were kept closed and placed inside a growth chamber (E-30BHO, Percival Scientific Inc., Perry, IA, USA) for 16 days under a photoperiod of 12 h light/12 h dark, at 30 ± 0.5°C and relative humidity of 60 ± 0.5 % (Wester and Zotz, Reference Wester and Zotz2011). We also performed previous germination trials on some of the species to increase the chances of high germinability in our final experiment. Previous trials involving the optimal germination temperature were carried out in T. brachycaulos and T. yucatana, germinating the seeds at 18, 28, 35 and 42°C (n = 90 seeds per species per treatment, distributed evenly in three Petri dishes), and in T. polystachia, T. recurvata and T. schiedeana under the last three temperatures (n = 20–90 seeds per species per treatment, distributed evenly in three Petri dishes). High germination was observed in the range of 28 to 35°C. To determine the effect of seed age on germination, seeds from T. brachycaulos, T. recurvata and T. yucatana (n = 90, 30 per Petri dish) were germinated at 0, 3, 6, 9, 12 and 18 months after the release from capsule, at 26°C. A decrease in germination was observed after 6 months. Results were not conclusive for T. recurvata given the consistently low germinability. We did not use seeds older than 6 months in the current study. The Petri dishes were periodically randomly re-arranged inside the chamber.
Germination was quantified according to Ranal et al. (Reference Ranal, Santana, Ferreira and Mendes-Rodrigues2009). The percentage of germination is reported as germinability and calculated by dividing the number of germinated seeds by the number of seeds sown and multiplying the result by 100. Mean germination rate is calculated as the reciprocal of the mean germination time. Mean germination time (![]() $\bar t$) is calculated as:
$\bar t$) is calculated as:
 $$\bar t = \displaystyle{{\mathop \sum \nolimits_{i = 1}^k n_it_i} \over {\mathop \sum \nolimits_{i = 1}^k n_i}},$$
$$\bar t = \displaystyle{{\mathop \sum \nolimits_{i = 1}^k n_it_i} \over {\mathop \sum \nolimits_{i = 1}^k n_i}},$$where t i is the time from the start of the experiment to the i th observation (measured in days); n i is the number of seeds germinated in the i th time (not the accumulated number, but the number corresponding to the i th observation), and k is the last time of germination.
We define seed germination as ‘visible germination’, i.e. the rupture of the testa and the protrusion of the hypocotyl.
Data analyses
To evaluate the interspecific differences in seed traits, seed length, seed mass, average total seed area, seed terminal velocity and germinability, one-way ANOVAs were used. A nested ANOVA was performed to assess differences in the proportion of embryo and endosperm within the seed. Percentages and ratios were arcsine transformed prior to analysis. Data transformations were performed for the variables that did not fulfil the assumptions of normality and homoscedasticity, for seed terminal velocity decadal logarithms were used. A non-parametric Wilcoxon test was applied when the data did not fulfil the assumptions even when transformed, as was the case for coma length, ratio of coma/seed and mean germination rate. Significant group differences (P < 0.05) were evaluated using Tukey's honestly significant difference test.
Simple regressions were performed to assess the relationship between percentage of germination or coma size and seed mass, as well as for seed mass or coma length and seed terminal velocity. All analyses were performed using the software STATISTICA 7 (Tulsa, OK, USA).
Results
Morphology and anatomy
The coma structure of Tillandsia recurvata differed from the five studied congenerics (Fig. 1A,F,G,L,M,O,Q). Instead of a single umbrella-like structure in the distal part of the coma, it exhibits two umbrellas, located in the proximal and distal sections (Fig. 1L,J). Imbrications of the individual coma hairs differed among the species (Figs. 1B,E,H,K,N,P). The indentations in the junctions between cells of T. brachycaulos and T. juncea were shallow (Fig. 1B and E), while the other four species exhibited more pronounced indentations (Fig. 1H,K,N,P). The symmetry of the indentations also varied. The extended plumose coma covered the seed and the seed coat was covered by dead cells and flakes, creating air pockets (Fig. 1C,D,I).
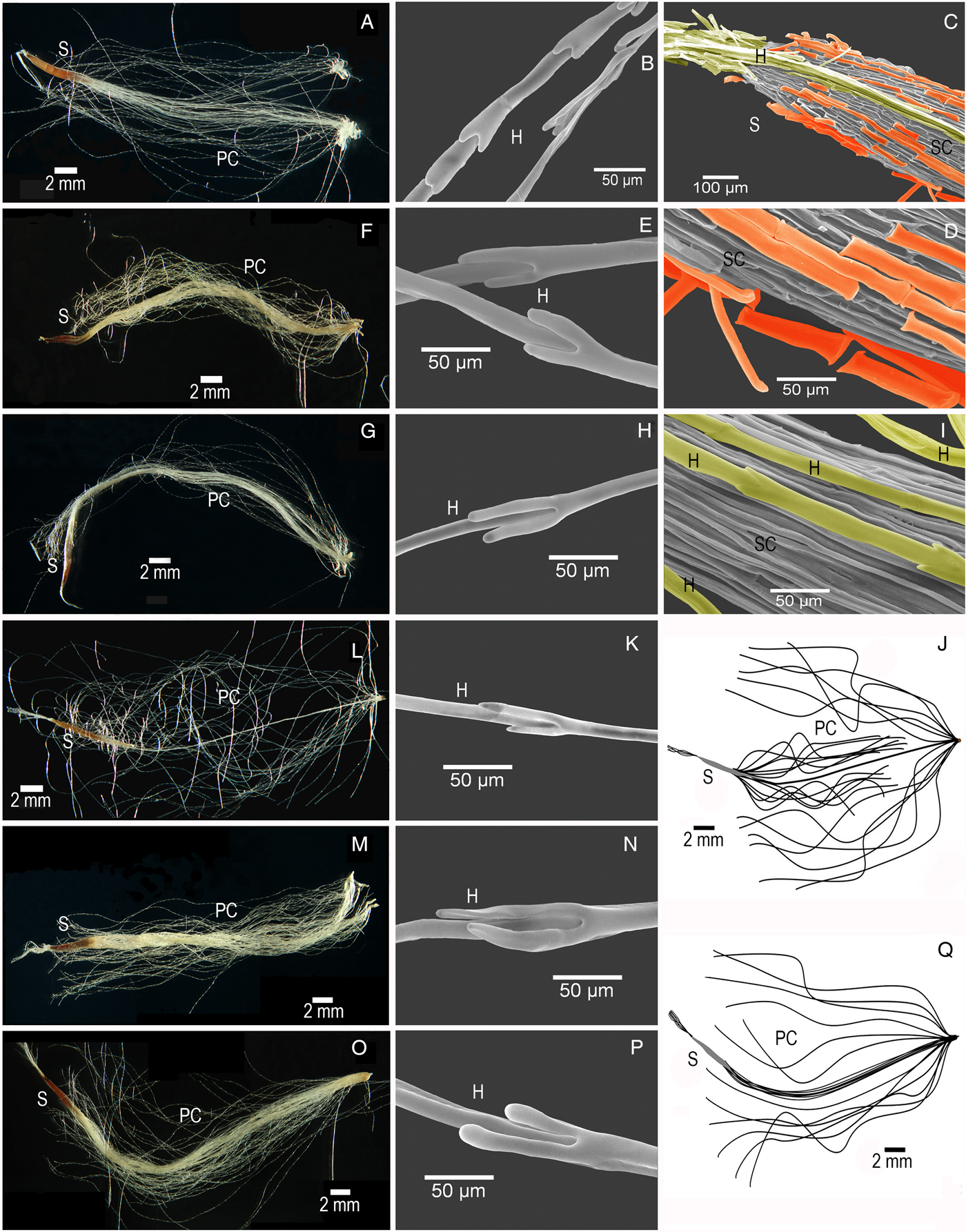
Figure 1. Photomicrographs of seeds of six Tillandsia species. (A–D) Tillandsia brachycaulos. (A) Entire seed. (B) Detail of the hair in plumose coma. (C) Detail of seed with dead cells that cover the seed coat (indicated in orange) and coma hairs attached to the seed coat (indicated in yellow). (D) Detail of seed showing the dead cells covering the seed coat (indicated in orange). (E–F) Tillandsia juncea. (E) Detail of coma hairs. (F) Entire seed. (G–H) Tillandsia polystachia. (G) Entire seed. (H) Detail of coma hairs. (I–L) Tillandsia recurvata. (I) Detail of hairs attached to seed. (J) Schematic representation of the T. recurvata seed with plumose coma deployed. (K) Detail of coma hairs, recurvata seed coat. (L) Entire seed. (M–N) Tillandsia schiedeana. (M) Entire seed. (N) Detail of coma hairs. (O–Q) Tillandsia yucatana. (O) Entire seed. (P) Detail of coma hairs. (Q) Schematic representation of the seed of T. yucatana with plumose coma deployed (the coma structure is similar to that of T. brachycaulos, T. juncea, T. polystachia and T. schiedeana). H, hair; PC, plumose coma; S, seed; SC, seed coat. The scale bar in the schematic representation corresponds to 2 mm for the seeds.
The plumose coma length ranged from 14.6 ± 0.4 mm (mean ± SE, n = 50) in Tillandsia juncea to 30.8 ± 0.4 mm in T. yucatana (Table 2, P < 0.05). Significant differences were found in seed size, even though the range of variation was small. Seeds of T. juncea were the smallest with a length of 4.8 ± 0.1 mm, with the largest seeds (T. brachycaulos, T. yucatana and T. recurvata) averaging 5.0 mm (Table 2, P < 0.05). A positive relationship between coma length and seed mass was found among the six species (r 2 = 0.44, P < 0.05; Fig. 2A), which was most strongly affected by the light seeds and small coma of T. recurvata, and the heavy seeds and large coma of T. yucatana, with the other four species showing intermediate values.
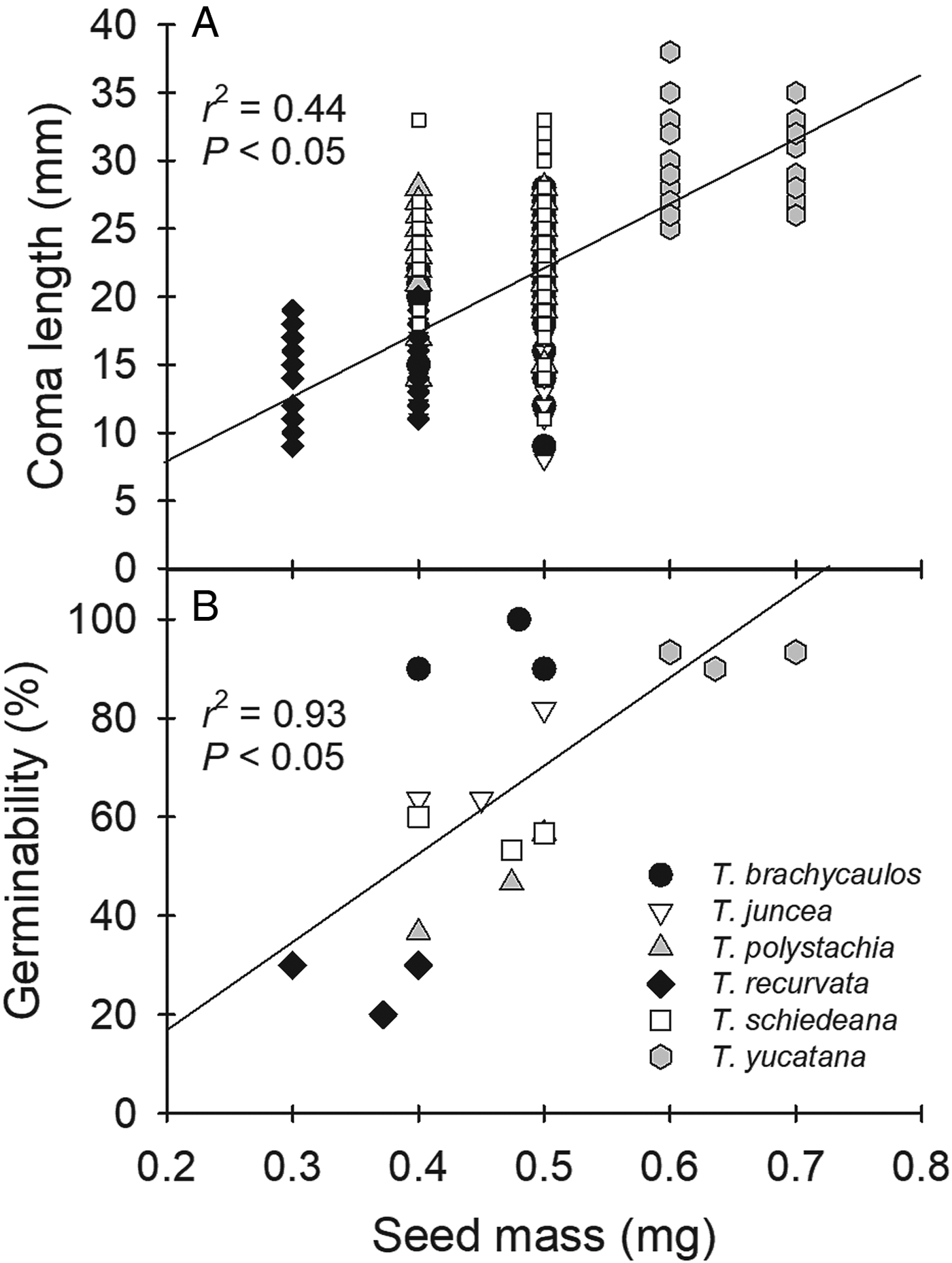
Figure 2. Relation between (A) Coma length (mm) and (B) Germinability (%) and the seed mass (mg) of six Tillandsia species. Data are presented as means ± SE (in some cases SE bars are smaller than the symbol) of seed mass (n = 50 seeds), coma length (n = 50 seeds) and germination (n = 11–30 seeds).
Table 2. Means and standard error of seed and coma length, ratio of coma/seed, seed mass, embryo and endosperm percentage of six Tillandsia species from Yucatan peninsula. Ratio of coma/seed refers to the ratio of coma length to seed length. Error terms are not shown for seed mass because they were all < 0.01 mg.
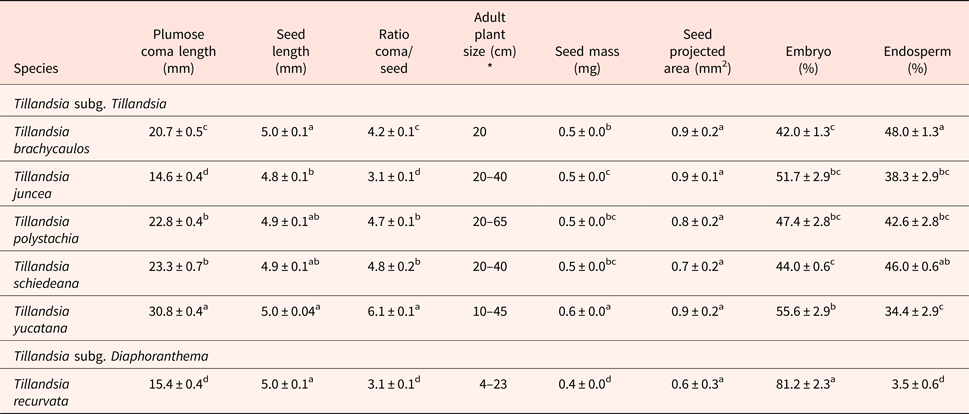
Different letters within columns of plumose coma length, ratio coma/seed (n = 50) indicate significant differences: Wilcoxon test, P < 0.05.
Different letters within columns of seed length, seed mass (n = 50), average total seed area (n = 4) indicate significant differences: one-way ANOVA, P < 0.05.
Different letters within columns of embryo and endosperm percentage (n = 4) indicate statistically significant differences within columns: nested ANOVA, P < 0.05.
*Data obtained from Ramírez et al. (Reference Ramírez, Carnevali and Chi2004).
Seeds of T. recurvata were notably different from those of the other five species. Seed mass was lowest, averaging 0.4 mg, with an embryo that covers the entire seed, without an endosperm (Table 2, P < 0.05). Tillandsia yucatana seeds were the heaviest, averaging almost 50% more at 0.6 mg (Table 2, P < 0.05). The embryo in the species of the subgenus Tillandsia covered 42.0 ± 1.3 to 55.6 ± 2.9% of the area within the seed (Table 2, P < 0.05).
Seed terminal velocity
Seed terminal velocity (V term) varied by ca 15%, ranging from a mean of 0.29 to 0.36 m s–1 (Fig. 3). The lowest V term values were observed in T. recurvata and T. yucatana seeds, while T. polystachia seeds fell fastest (P < 0.05). Surprisingly, terminal velocity did not show a significant correlation with seed mass or coma length (Supplementary Fig. S3).

Figure 3. Terminal velocity of seeds (V term) of six Tillandsia species. Data are means ± SE (n = 50 seeds). Different letters denote significant differences among species (one-way ANOVA, Tukey's test, P < 0.05).
Germination
Germinability was highest in Tillandsia brachycaulos and T. yucatana at 93.3 ± 3.3 and 92.2 ± 1.1%, respectively, after 16 days (Fig. 4A, P < 0.05). The lowest values were observed in T. recurvata with 26.7 ± 3.3%. A positive relationship was obtained between germinability and seed mass among the six species (r 2 = 0.93, P < 0.05; Fig. 2B).

Figure 4. Germination of seeds of six Tillandsia species. (A) Germinability (%) over 16 days. (B) Mean germination rate (day–1). Seed number varied between species according to seed availability at the time of the experiment, n = 11–30 per Petri dish (n = 3). Data are means ± SE; in one case the SE bar is not seen due to its reduced size. Different letters denote significant differences among species (one-way ANOVA and Wilcoxon test, P < 0.05).
Mean germination rate (day–1) was generally fast, but differed among species (Fig. 4B, P < 0.05). Tillandsia juncea had the slowest mean germination rate of 0.1 day–1, while germination was four times faster in T. recurvata (0.4 ± 0.1 day–1) followed by T. yucatana, averaging 0.3 day–1.
We observed differences in the visible germination and post-seminal development of Tillandsia species: in T. recurvata the seed coat seems to tear longitudinally as the embryo elongates (Fig. 5J,K) and there was a progressive thickening of the hypocotyl, which subsequently developed the scutellum. In the other five species, the seed coat broke transversely and the protruding hypocotyl located between the haustorium and the embryo could be observed within 8 days (Fig. 5B,F); after 15 days, the developed scutellum was observed, and the seed coat containing the endosperm remained visible at the side of the scutellum (Fig. 5C,G). The scutellum grew more slowly in T. recurvata and the first leaf developed after 80 days (Fig. 5K,L), while in the other species, we observed two to three leaves after 35 days (Fig. 5D,H).
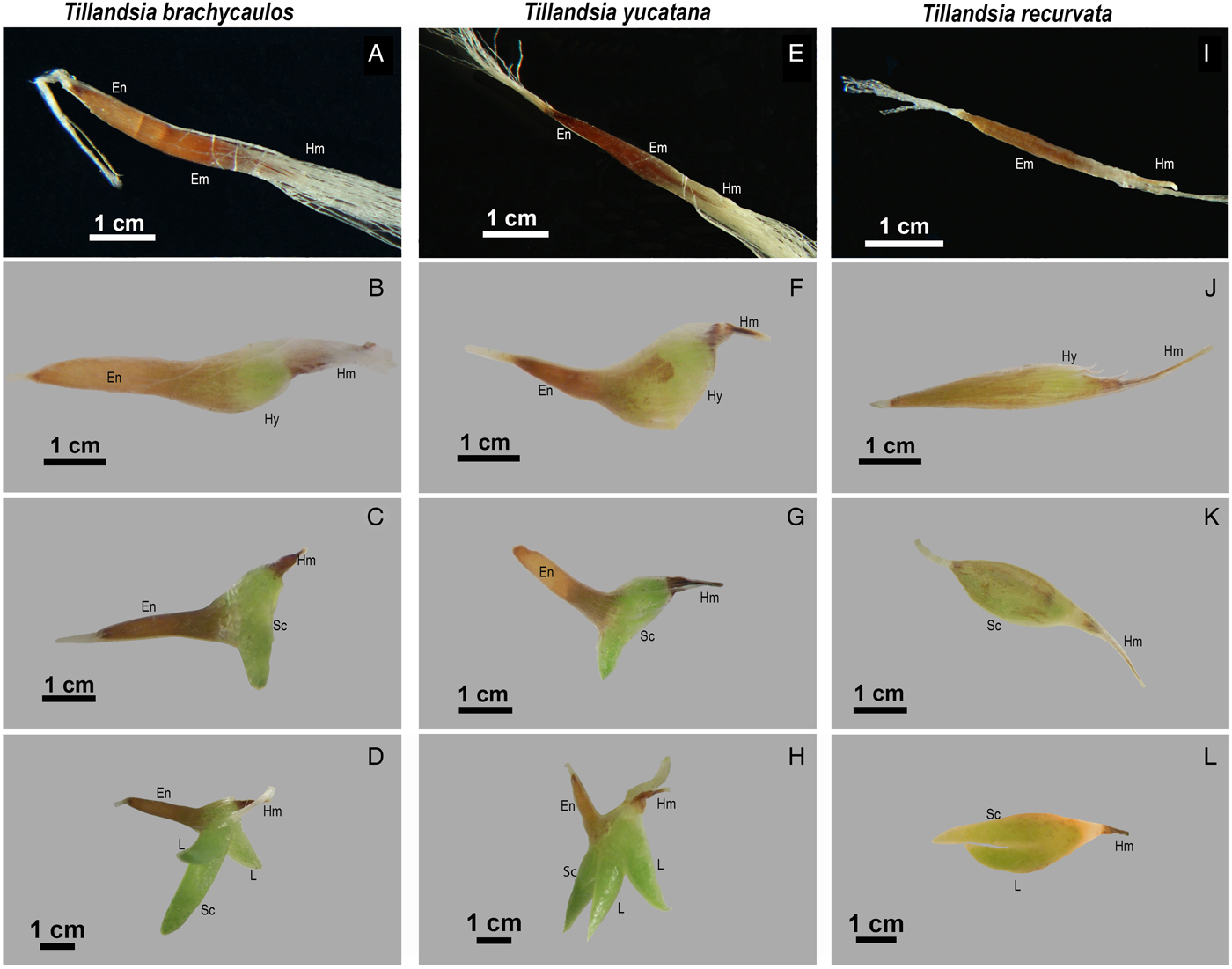
Figure 5. Differences in the visible germination process of three Tillandsia species. (A–D) T. brachycaulos. (E–H) T. yucatana. (I–L) T. recurvata. (A) Dry seed T. brachycaulos. (E) Dry seed T. yucatana. (B,F) Protrusion of the hypocotyl, seed coat is ruptured transversally (8 days). (C,G) Development of scutellum (18 days). (D,H) Leaf growth (35 days). (I) Dry seed T. recurvata. (J) Protrusion of the hypocotyl, seed coat is ruptured longitudinally while the scutellum grows. (K) Developed scutellum (18 days). (L) First leaf appears after ca 80 days. Em, embryo; En, endosperm; Hm, haustorium; Hy, hypocotyl; L, leaf; Sc, scutellum.
In the five species with an endosperm, the seed coat protected the reserves, which decreased progressively in size after germination (Fig. 5A–D and E–H). During the first 2 months, the endosperm remained visibly turgid and apparently served as a source of reserves. At that point the seedling had produced up to five leaves and seemed less dependent on the shrinking endosperm. Under controlled experimental conditions the seed coat appeared completely dry after approximately 6 months, and in the second or third month an adventitious root system developed. For T. recurvata, which has no endosperm, the scutellum had a predominant role for a longer time.
Histochemical components in seeds
The photomicrographs of the stained cross-sections showed that all six species stored similar biomolecules, either in the endosperm or, for T. recurvata, within the embryo. Starch granules were observed in all species stained in purple (Fig. 6A,B) or using polarized light, as the typical Maltese cross (Fig. 6D). Crystals were ubiquitous within the endosperm, most likely of calcium oxalate (Fig. 6F). Similarly ubiquitous were proteins stained in blue localized in the embryo (Fig. 6C) and endosperm (Fig. 6G). Insoluble polysaccharides (stained in magenta) were found in the endosperm (except for T. recurvata) and seed coat (Fig. 6G). Lipids (stained in red) were observed in the hypocotyl–radicle axis that is differentiated as a zone of constriction separating the radicle and the embryo (Fig. 6E); lipids were also present in the aleurone layer (Fig. 6H), which was found between the embryo and seed coat.
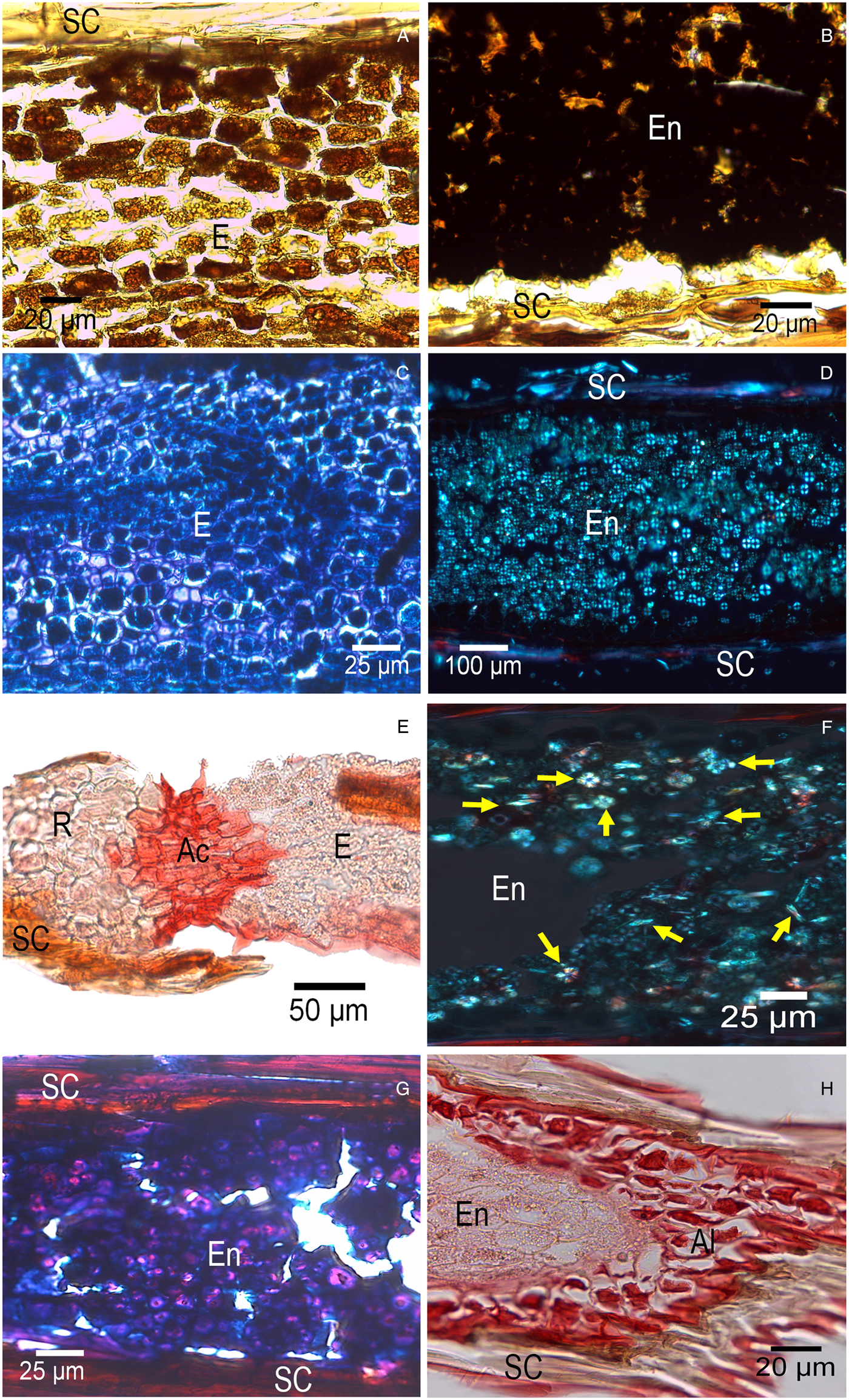
Figure 6. Photomicrographs of seeds of six Tillandsia species. (A) Starch granules in the embryo of Tillandsia recurvata. (B) Starch granules in endosperm of T. schiedeana. (C) Proteins in the embryo of T. yucatana. (D) Starch granules under polarized light in endosperm of T. brachycaulos. (E) Lipid reserves (red) in the constriction area in the radicle-hypocotyl axis of T. recurvata. (F) Starch granules and crystals (yellow arrows) in endosperm of T. juncea. (G) Structures containing proteins and polysaccharide (proteins in blue and polysaccharides in magenta) in endosperm of T. polystachia. (H) Lipid reserves in the aleurone layer of T. yucatana. Al, aleurone layer; Ac, constriction area; E, embryo; En, endosperm; R, root. Panels A, B, C, E, G and H are with light microscopy; polarized light was used in panels D and F.
Discussion
In recent years, the genera and subgenera within the subfamily Tillandsioideae have undergone controversial redefinitions (Spencer and Smith, Reference Spencer and Smith1993; Grant, Reference Grant1995; Espejo-Serna, Reference Espejo-Serna2002; Barfuss et al., Reference Barfuss, Till, Leme, Pinzón, Manzanares, Halbritter, Samuel and Brown2016). The definition of this clade remains a work in progress, partly due to the large number of species it encompasses. In the present study we found relevant seed traits which may be useful for cladistics analyses, such as the imbrications in the hairs that make up the coma. We also found a highly divergent seed anatomy and coma structure in Tillandsia recurvata, compared with the other Tillandsia species. These differences may reflect the phylogenetic distance between the subgenera.
The structure of the coma imbrications (Fig. 1B,E,H,K,N,P) differed between the studied Tillandsia species, and further, unpublished observations suggest that this variation may extend to other species. Unfortunately, no other study has analysed this particular structure. Hence, the function of the imbrications and their usefulness for taxonomic studies (Benzing, Reference Benzing1982) are unresolved and await further study.
A significant divergence in seed traits was observed between T. recurvata and the other five species studied. In T. recurvata the coma forms a double umbrella (Fig. 1J,L), unlike the single umbrella, which is observed in the other species (Fig. 1A,F,G,M,O,Q). There is a wide taxonomic distance between T. recurvata and the other species, the former being in the more derived subgenus Diaphoranthema while the latter belong to the subgenus Tillandsia (Barfuss et al., Reference Barfuss, Till, Leme, Pinzón, Manzanares, Halbritter, Samuel and Brown2016). Magalhães and Mariath (Reference Magalhães and Mariath2012) compared the coma structure of eight Vriesea species and six Tillandsia species, which belonged to the subgenus Diaphoranthema (two species) and to the sister subgenus Anoplophytum (four species). They describe the double umbrella in all the Tillandsia species in their study and infer that the double umbrella can be used to discern Tillandsia from Vriesea which has a single umbrella. Our study suggests that the single umbrella can be a shared character among Vriesea and some Tillandsia species, and the double umbrella seems to be a derived character present in species such as T. recurvata and T. stricta. Furthermore, the character (one or two umbrellas) seems to be more correlated to the phylogeny (Barfuss et al., Reference Barfuss, Till, Leme, Pinzón, Manzanares, Halbritter, Samuel and Brown2016) than to the environment, as those species included in the Magalhães and Mariath (Reference Magalhães and Mariath2012) study are located in more temperate and humid environments compared with the species sampled in the present study, which are found in seasonally dry forests. While many other studies may mention the coma, and may provide illustrations or photographs (Palací et al., Reference Palací, Brown and Tuthill2004; Scatena et al., Reference Scatena, Segecin and Coan2006; Magalhães and Mariath, Reference Magalhães and Mariath2012; Corredor-Prado et al., Reference Corredor-Prado, Schmidt, Steinmacher, Guerra, Bouzon, Dal Vesco and Pescador2014), the degree of detail is usually not enough to analyse the differences mentioned here, and thus more species surveys are needed to define the extent of the double or single umbrella within both Vriesea and Tillandsia.
Anatomically, the seed of T. recurvata is the most divergent of the species studied. As previously reported by Montes-Recinas et al. (Reference Montes-Recinas, Márquez-Guzmán and Orozco-Segovia2012), the species lacks an endosperm (Table 2), which diverges from all other congenerics studied to date (Cecchi-Fiordi et al. Reference Cecchi-Fiordi, Palandri, Turicchia, Tani and Di Falco2001; Scatena et al., Reference Scatena, Segecin and Coan2006; Magalhães and Mariath, Reference Magalhães and Mariath2012; Montes-Recinas et al.,Reference Montes-Recinas, Márquez-Guzmán and Orozco-Segovia2012; Sosa-Luría et al., Reference Sosa-Luría, Chávez-Servia, Mondragón-Chaparro, Estrada-Gómez and Ramírez-Vallejo2012). Evolutionarily, there seems to be a progression from seeds with a large endosperm in Vriesea species (73–77 %), with a reduced endosperm in the subgenus Tillandsia (33–55 %) and a further reduction up to a complete loss in the subgenus Diaphoranthema, even though this lack of endosperm was not found in another Diaphoranthema species, T. usneoides (35%; Magalhães and Mariath, Reference Magalhães and Mariath2012). Noteworthy, an evolutionary trend towards a reduced endosperm for the sake of larger embryos has been observed in the angiosperms in general (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006).
The anatomical differences in T. recurvata are accompanied by differences in germination and early seedling development. These developmental differences may also relate to the lack of endosperm. In the species with an endosperm, the endosperm stays turgid for at least 6 months (Fig. 5B,C,D,F,G,H). This suggests that the endosperm is still supporting seedling growth during this time and may explain the faster leaf development compared with T. recurvata (Fig. 5D,H,L).
Vandelook et al. (Reference Vandelook, Janssens and Probert2012) found that the species lacking an endosperm in the family Apiaceae exhibited faster germination, compared with those with an endosperm. This same pattern was found in the species in this study, where T. recurvata had the highest mean germination rate (Fig. 4). Vandelook et al. (Reference Vandelook, Janssens and Probert2012) suggested that endosperm reduction may have evolved as an adaptation to dry sites where the period of favourable conditions for growth and establishment is shorter than in wetter environments.
There was also a non-significant trend for T. yucatana, the other species from the drier part of the peninsula, to show high germination rates and a small endosperm. These patterns may be worth studying with a larger number of Tillandsia species and a wider range of habitats in order to discern a trend between the seed traits and the environment.
Seed terminal velocity was lower in the two species from dry sites, compared with the wet forest species, T. polystachia (Fig. 3). A reduced velocity may increase the possibility for dispersal in patchy drier sites, where distances between suitable hosts may be larger than in closed moister forests. The higher buoyancy was achieved by a larger coma in T. yucatana, and a short but more ornamented coma and a lower seed mass in T. recurvata (Table 2). Overall, terminal velocity was not related to either coma length or seed mass (Supplementary Fig. S3).
The terminal velocity reached by seeds is highly relevant to the distances at which the seed can disperse. Matlack (Reference Matlack1987) studied seed terminal velocity under still air for 38 anemochorous species and found that plumed seeds were in general faster than winged or samara seeds, although value ranges sometimes overlapped. Plumed seeds had velocities ranging from 1.66 to 0.07 m s–1, which places the Tillandsia seeds in the lower range of the spectrum, with values of 0.36 to 0.29 m s–1 (Fig. 3). In Tillandsia, besides small seed sizes and large comas, the seed coat is covered by dead cells filled with air (Fig. 1C,F) that aid in their flotation (Madison, Reference Madison1977). It is worth noting that none of the 38 species included in the Matlack study was an epiphyte, thus it would be interesting to test the range shown by epiphytic species, for which dispersal to the top canopy may be crucial for establishment. Field studies have found that even though Tillandsia species produced many seeds, very few seeds can reach distances greater than 15 m from the source (García-Franco and Rico-Gray, Reference García-Franco and Rico-Gray1988; Mondragón and Calvo-Irabien, Reference Mazer2006).
On some of the attributes measured, the two species from the dry sites seemed to have opposite strategies, as T. yucatana had longer comas, heavier seeds and higher germinability, in contrast to the shorter comas, lighter seeds and lower germinability in T. recurvata, while the rest of the species clumped at intermediate values (Table 2, Fig. 4). The positive correlations we found between both coma length and germinability in relation to seed mass are highly driven by the differences between T. yucatana and T. recurvata (Fig. 2). In general, heavier seeds increase germination and establishment success (Mazer, Reference Mazer1989; Westoby et al., Reference Westoby, Falster, Moles, Vesk and Wright2002; Paz and Martínez-Ramos, Reference Paz and Martínez-Ramos2003; Peco et al., Reference Peco, Rico and Azcárate2009), yet the applicability of this pattern for Tillandsia would need further study, including more species. Heavier seeds in Tillandsia may implicate reduced dispersal ability, and thus variation in this trait was very small. Seed size varied by only 0.2 mm (4%) among the species, while mass varied by only 0.2 mg (33%, Table 2).
There were large differences in germinability among species, from germination close to 90% in T. brachycaulos and T. yucatana, to values <50% in T. polystachia and T. recurvata. The causes behind these differences in germinability are unknown, as seed viability and dormancy would have to be further evaluated. Dormancy has not been reported in Tillandsia; generally, seeds show rapid germination, often with high germinability under controlled conditions in an interval of 5–15 days after watering (Bader et al., Reference Bader, Menke and Zotz2009; Cascante-Marin et al., Reference Cascante-Marín, von Meijenfeldt, de Leeuw, Wolf, Oostermeijer and den Nijs2009; Montes-Recinas et al., Reference Montes-Recinas, Márquez-Guzmán and Orozco-Segovia2012; Valencia-Díaz et al., Reference Valencia-Díaz, Flores-Palacios, Rodríguez-López, Ventura-Zapata and Jiménez-Aparicio2010), unless they are severely stressed (Bader et al., Reference Bader, Menke and Zotz2009). In general, epiphyte seeds do not seem to form seed banks, but germinate immediately after dispersal at the end of the unfavourable season (reviewed in Mondragón et al., Reference Mondragón, Valverde and Hernández-Apolinar2015). A study performed on six Tillandsia species from Oaxaca, Mexico, found that germinability differences were largely explained by seed viability, determined through seed X-rays (Sosa-Luría et al. Reference Sosa-Luría, Chávez-Servia, Mondragón-Chaparro, Estrada-Gómez and Ramírez-Vallejo2012). Loss of viability was explained by incomplete embryo formation, which was found in 11–39% of the seeds, depending on species. Environmental stress, such as limited water availability, may be a cause for the incomplete seed formation (Zotz and Asshoff, Reference Zotz and Asshoff2010; Zotz et al., Reference Zotz, Bogusch, Hietz and Ketteler2010); alternatively, morphological dormancy could also explain this observation.
Conclusions
We report differences in the coma structure between the species in our study with a double umbrella in T. recurvata and a single umbrella in the other five Tillandsia species. We confirmed that T. recurvata lacks an endosperm, a trait so far only described for this species within the genus. These morphological differences are concomitant with functional differences such as fast germination, but slower initial growth of T. recurvata and an enhanced dispersal capacity (low seed terminal velocity with a small coma). In general, increases in seed mass were associated with increases in coma size, thus maintaining seed low terminal velocity compared with other anemochorous species. Variation in seed size and mass were very small. Nevertheless, seed mass was also positively related to germinability. Our results highlight the need to characterize the seed structure and function of more Tillandsia species in order quantify the range of trait variation as well as their importance for taxonomical studies and/or ecological relationships.
Acknowledgements
We are grateful to C.A. Thanos and three anonymous reviewers who provided useful comments to improve the manuscript. We thank the authorities of the study sites: National Park Dzibilchaltún, Biocultural Reserve Kaxil-Kiuic and Calakmul Biosphere Reserve. We thank Maria Goreti Campos, Lilia Lorena Can Itza and Alejandro Martínez Mena for technical help processing, observing and photographing seeds at the microscopes at both CICY and UNAM; Ricardo Wong and Mónica Karina Pérez Pacheco for technical support while processing seeds for anatomical and histochemical analyses; and Manuela Tamayo and Harry A. Moreno Torres for help in the field and with statistical analyses.
Financial support
N.C.-G. received PhD and postdoctoral scholarships from CONACYT (224268 and 14099), as well as CICY and CONACYT travel grants (2014-MZO2015, 290842). The work was supported by SEP-CONACYT projects 80181 and 221490.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258518000247










