Introduction
Seed heteromorphy is defined as the production of different types of seeds by a single plant and is a common strategy to cope with unpredictable climatic conditions (Bhatt and Santo, Reference Bhatt and Santo2016). Seed heteromorphy influences seed dispersal ability, germination, seed dormancy and persistence in the soil (Duràn and Retamal, Reference Duràn and Retamal1989; Puga-Hermida et al., Reference Puga-Hermida, Gallardo, Rodríguez-Gacio and Matilla2003; El-Keblawy and Bhatt, Reference El-Keblawy and Bhatt2015). Seed dimorphism is considered the main type of seed heteromorphy and it is expressed mostly in annual plants (Venable, Reference Venable1985).
The Aegilops genus includes 23 species of annual grasses, occurring in southern Europe, North Africa, the Near East and western and central Asia. Aegilops species occur in various dry, thermophile and low nutrient, often disturbed habitats (e.g. pastures, roadsides, field margins, wastelands; van Slageren, Reference van Slageren1994). Some Aegilops L. species (Triticeae Dumort.) produce caryopses (hereafter referred to as seeds) of different size and shape and different testa colours. Seed dimorphism was also detected and shown to be linked to a complex dispersal strategy (Dyer, Reference Dyer2004; Marañon, Reference Marañon1989; Matilla et al., Reference Matilla, Gallardo and Puga-Hermida2005). In Aegilops geniculata Roth, Ae. kotschyi Boiss., Ae. neglecta Req. ex Bertol. and Ae. triuncialis L. dimorphic pairs of seeds are found in the lower fertile spikelets, with one seed larger and lighter coloured than the other one (Datta et al., Reference Datta, Evenari and Gutterman1970; Wurzburger et al., Reference Wurzburger, Leshem and Koller1976; Dyer, Reference Dyer2004; Marañon, Reference Marañon1989). In Ae. geniculata, only the larger seeds of the dimorphic pair within the spikelets readily germinate, whereas the germination of the smaller seeds is inhibited probably due to the presence of an inhibitor in the glumes (Datta et al., Reference Datta, Evenari and Gutterman1970). A similar behaviour was detected in Ae. cylindrica Host and Ae. triuncialis (Fandrich and Mallory-Smith, Reference Fandrich and Mallory-Smith2005; Dyer, Reference Dyer2017).
Seed heteromorphism in Aegilops is supposed to reflect a bet-hedging strategy to cope with variable environments with unpredictable resource availability (Dyer, Reference Dyer2004). Germination delay for some of the seeds increases the chances that at least some offspring survive, reducing also the competition among sibling seedlings (Nave et al., Reference Nave, Avni, Ben-Zevi, Hale and Distelfeld2016). This strategy could be particularly functional in Aegilops, considering its very limited dispersal capacity (Dyer, Reference Dyer2004; van Slageren, Reference van Slageren1994; Volis, Reference Volis2016). It has been hypothesized that in Aegilops the largest seeds of the dimorphic pair germinate after the first rainfall at the end of summer, or beginning of autumn, whereas the smallest seeds germinate later (Datta et al., Reference Datta, Evenari and Gutterman1970; Marañon, Reference Marañon1989), probably during the following spring (Onnis et al., Reference Onnis, Bertacchi, Lombardi and Stefani1995). Consequently, smaller seeds should be able to maintain a high viability for lingering after dispersal than larger ones. Interestingly, the smaller seeds of Ae. triuncialis remained viable longer during storage under laboratory conditions compared with larger seeds (Dyer, Reference Dyer2017) and similar patterns were found in dimorphic seeds of Dasypyrum villosum (L.) P. (De Pace et al., Reference De Pace, Vaccino, Cionini, Pasquini, Bizzarri, Qualset and Kole2011), suggesting that different germination patterns across seed morphs could reflect different seed longevities, i.e. with small seeds being inherently longer-lived than large seeds, since they have to persist longer in the soil before germination. Despite this, little is known about differences of longevity across seed morphs in Aegilops.
The longevity of seeds is an important plant trait, allowing not only seed persistence in the soil but also long-term ex situ plant conservation in germplasm banks (Walters et al., Reference Walters, Wheeler and Grotenhuis2005; Probert et al., Reference Probert, Daws and Hay2009). In orthodox seeds, longevity is mainly determined by seed moisture content and temperature during storage. By experimentally increasing these two factors, it is possible to accelerate ageing processes in the laboratory, thereby estimating seed longevity from survival curves (Ellis and Roberts, Reference Ellis and Roberts1980) and predicting with caution seed persistence in the field (Long et al., Reference Long, Panetta, Steadman, Probert, Bekker, Brooks and Adkins2008). Understanding seed longevity differences among species (see e.g. Walters et al., Reference Walters, Wheeler and Grotenhuis2005; Probert et al., Reference Probert, Daws and Hay2009; Mondoni et al.; 2011; Bernareggi et al., Reference Bernareggi, Carbognani, Petraglia and Mondoni2015; Davies et al., Reference Davies, Newton, Hay and Probert2016) is crucial for the management of ex situ seed conservation facilities, as it allows to plan re-collection or define regeneration intervals (Walters, Reference Walters, Smith, Dickie, Linington, Pritchard and Probert2003).
Ex situ seed conservation is particularly important for crop wild relatives (CWR) whose collections should be representative of the largest possible genetic variability, in order to safeguard it and provide useful traits for crop improvement (Warschefsky et al., Reference Warschefsky, Penmetsa, Cook and von Wettberg2014). Aegilops forms part of the secondary gene pool of wheat, playing a key role in wheat evolution and domestication (van Slageren, Reference van Slageren1994) and being extensively used in breeding programmes (Maxted et al., Reference Maxted, White, Valkoun, Konopka and Hargreaves2008; Kilian et al., Reference Kilian, Mammen, Millet, Sharma, Graner, Salamini, Hammer, Özkan and Kole2011). Furthermore, some Aegilops species are endangered (e.g. Ae. uniaristata Vis., Ae. biuncialis Vis. and Ae. tauschii Coss.; Bilz et al., Reference Bilz, Kell, Maxted and Lansdown2011; Rossi et al., Reference Rossi, Montagnani, Gargano, Peruzzi, Abeli, Ravera, Cogoni, Fenu, Magrini, Gennai, Foggi, Wagensommer, Venturella, Blasi, Raimondo and Orsenigo2013), underlining the need for an effective ex situ conservation of this genus. Therefore, a better understanding of the ecology of these wheat wild relatives is essential for their exploitation in breeding programmes and for their conservation (McCouch et al., Reference McCouch, Baute, Bradeen, Bramel, Bretting, Buckler, Burke, Charest, Cloutier, Cole, Dempewolf, Dingkuhn, Feuillet, Gepts, Grattapaglia, Guarino, Jackson, Knapp, Langridge, Lawton-Rauh, Lijua, Lusty, Michael, Myles, Naito, Nelson, Pontarollo, Richards, Rieseberg, Ross-Ibarra, Rounsley, Hamilton, Schurr, Stein, Tomooka, van der Knaap, van Tassel, Toll, Valls, Varshney, Ward, Waugh, Wenzl and Zamir2013; Dempewolf et al., Reference Dempewolf, Eastwood, Guarino, Khoury, Müller and Toll2014).
The objective of this study was to characterize germination and longevity of heteromorphic seeds in different Aegilops species, integrating field and laboratory investigations. In particular, we aimed to: (1) estimate the longevity of different seed morphs in six Aegilops species using controlled ageing tests (CAT), and (2) investigate germination phenology in seed morphs of Ae. neglecta under laboratory and field conditions.
Materials and methods
Study material
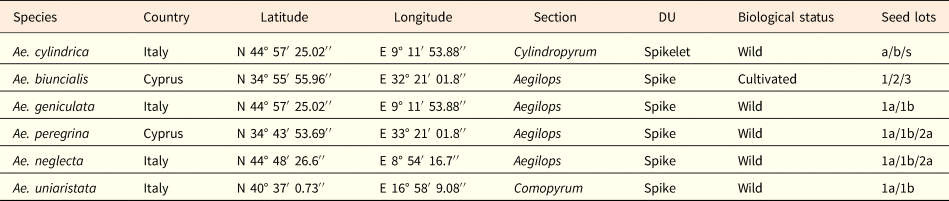
Dispersal units of all Aegilops species were collected in 2015 at the time of natural dispersal (May–June) in nature, except those of Ae. biuncialis that were collected from a first generation population cultivated in Cyprus (Table 1). After collection, seeds were extracted from the dispersal units. For the Aegilops species in which the whole spike is the dispersal unit and therefore spikelets do not disarticulate at maturity (i.e. Ae. geniculata, Ae. neglecta, Ae. peregrina (Hack.) Maire and Weiller and Ae. uniaristata), the basal fertile spikelet was separated from the upper ones and the dimorphic seed pairs extracted from it. After seed cleaning, seeds were kept at 15% relative humidity (RH) and 15°C (ISTA, 2018) until use. Germination phenology of Ae. neglecta both in the laboratory and in the field were studied using fresh seeds, re-collected from the same population in July 2016 and immediately sown. Seed mass was determined by weighing 20 individual seeds, kept at 15% RH, randomly sampled from each seed lot, using an analytical balance (Kern ALJ 220-4 nm).
Table 1. Geographical locations of the sampled populations and type of the six Aegilops species
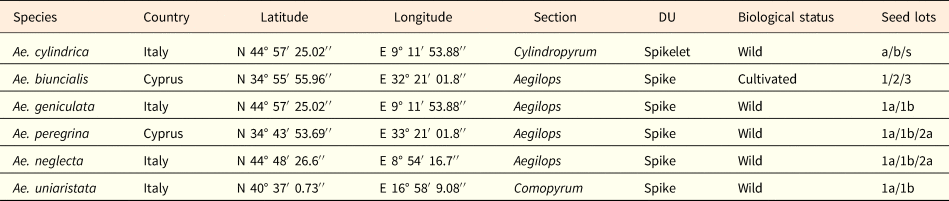
The type of dispersal unit (DU) is indicated. The seed lots extracted in each species by the DU are also provided.
Ageing experiments and longevity assessment
Seed longevity was estimated using a modified protocol for accelerated ageing tests (Newton et al., Reference Newton, Hay and Probert2009). Ageing experiments started in April 2016 and lasted for 6 months. Prior to storage, seeds were first rehydrated for 14 days in open Petri dishes over a non-saturated solution of LiCl in a sealed 300 × 300 × 130 mm sealed electrical enclosure box (Ensto UK Ltd, Southampton, UK) at 47% RH and 20°C. Thereafter, seed equilibrium relative humidity (eRH) was verified with an AW-DI0 water activity probe used in conjunction with a HygroPalm 3 display unit (Rotronic Instruments UK Ltd, Crawley, UK). Once the seed lot was judged to have reached eRH, the initial germination was assessed using triplicates of 20 seeds using the protocol described in the next section. Next, seed lots were stored in sealed box over a non-saturated LiCl solution at 60% RH at 45 ± 2°C in the dark placed in a compact incubator (Binder FD53, Binder GmbH, Tuttlingen, Germany). The RH generated by the LiCl solution in the box was monitored with a data logger inside the enclosure box (Tinytag View 2 Temperature/Relative Humidity Logger, Gemini Data Logger, Chichester, UK). When necessary, the bulk solution was adjusted by adding distilled water, stirring and allowing the solution to equilibrate (Hay et al., Reference Hay, Adams, Manger and Probert2008; Newton et al., Reference Newton, Hay and Probert2009). At three to four intervals during storage, tri-replicates of 20 seeds were removed to assess viability using germination. Different seed lots were exposed to different periods of storage according to their lifespan so that seeds could experience a complete loss of viability.
Seed germination in the laboratory
Triplicates of 20 seeds each per lot were sown on 1% agar in 50 mm diameter Petri dishes that were placed, randomly interspersed, in an alternating temperature (20/10°C) and 12 h daily photoperiod (photosynthetically active radiation 40–50 μmol m–2 s–1 ; LMS 250A, LMS Ltd, Sevenoaks, UK; Guzzon et al., Reference Guzzon, Müller, Abeli, Cauzzi, Ardenghi, Balestrazzi, Rossi and Orsenigo2015). Germination was scored twice a day when the emerging radicle had reached approximately 2 mm. At the completion of each germination test (4 weeks after sowing), ungerminated seeds were cut to confirm whether they were empty. Final germination percentages were adjusted based the number of filled seeds.
Seed germination in the field
A triplicate of 70 spikes from Aegilops neglecta was sown in July 2016 at the original collection site (Table 1). One plot (50 × 60 cm) per each replicate was prepared by removing the vegetation and flattening the soil. The spikes were inserted in the soil surface to simulate natural dispersal (Perrino et al., Reference Perrino, Wagensommer and Medagli2014). Seedling emergence from each spikelet was recorded every 2 weeks for the first 3 months and thereafter, once a month, for the following 11 months until the end of September 2017. Only the emergence of the dimorphic seed pairs of the basal fertile spikelet was recorded. One year after sowing, 20 spikelets per replicate were retrieved and the viability of ungerminated seeds was assessed using a germination test in the laboratory as described above. Data loggers recording temperature and RH were placed at the soil surface (WatchDog data logger, Spectrum Technologies, Inc.) and buried 2 cm deep in the soil (Supplementary Figure 1).
Statistical analysis
Non-parametric Kruskal–Wallis tests were applied to evaluate if the difference in seed masses was significant among different species and if the mass of the different seed lots varied significantly within species.
To estimate p 50, as measure of seed longevity, probit analysis of the seed viability data was carried out using GenStat for Windows, version 9 (VSN International Ltd, Oxford, UK) by fitting the seed viability equation:
where v is the viability in normal equivalent deviates (NED) at time p (days); K i is the initial viability or the intercept on the y-axis (NED), and σ is the standard deviation of the normal distribution of seed deaths in time. Previous studies have highlighted that in some Aegilops species the dimorphic seed pairs extracted from the spikelet show differences in the germination timing, with larger seeds germinating faster than the smaller ones (Datta et al., Reference Datta, Evenari and Gutterman1970; Marañon Reference Marañon1989). To test this observation, differences in germination timing (mean time to germinate, MTG) were recorded of fresh seeds (i.e. not exposed to CAT). The meantime to germination MTG was calculated as:
where n is the number of seeds that germinated within consecutive intervals of time, T is the time between the beginning of the test and the end of a particular interval of measurement, and N is the total number of seeds that germinated. The MTG was calculated using the hour of sowing as initial time.
ANOVA was used to test the significance of the differences, in terms of p 50 and MGT detected among the different seed lots and species. Bonferroni post-hoc tests were used to pairwise compare the differences in p 50 and MTG between seed lots of the species with three seed lots. Data were checked for normality and homogeneity of variance to meet the ANOVA assumption. To this end, MGT data were log-transformed. The F-test was used to determine whether there was a significant increase in residual deviance by constraining seed survival curves for reduced seed number and standard longevity test data to a single slope (σ–1) and intercept (K i). Seed germination was modelled using a binomial probability distribution and a logit link function by means of a generalized linear model (GLM). The Kruskal–Wallis tests, GLMs and ANOVAs were performed using SPSS for Windows, version 21.0.
Results
Seed heteromorphy
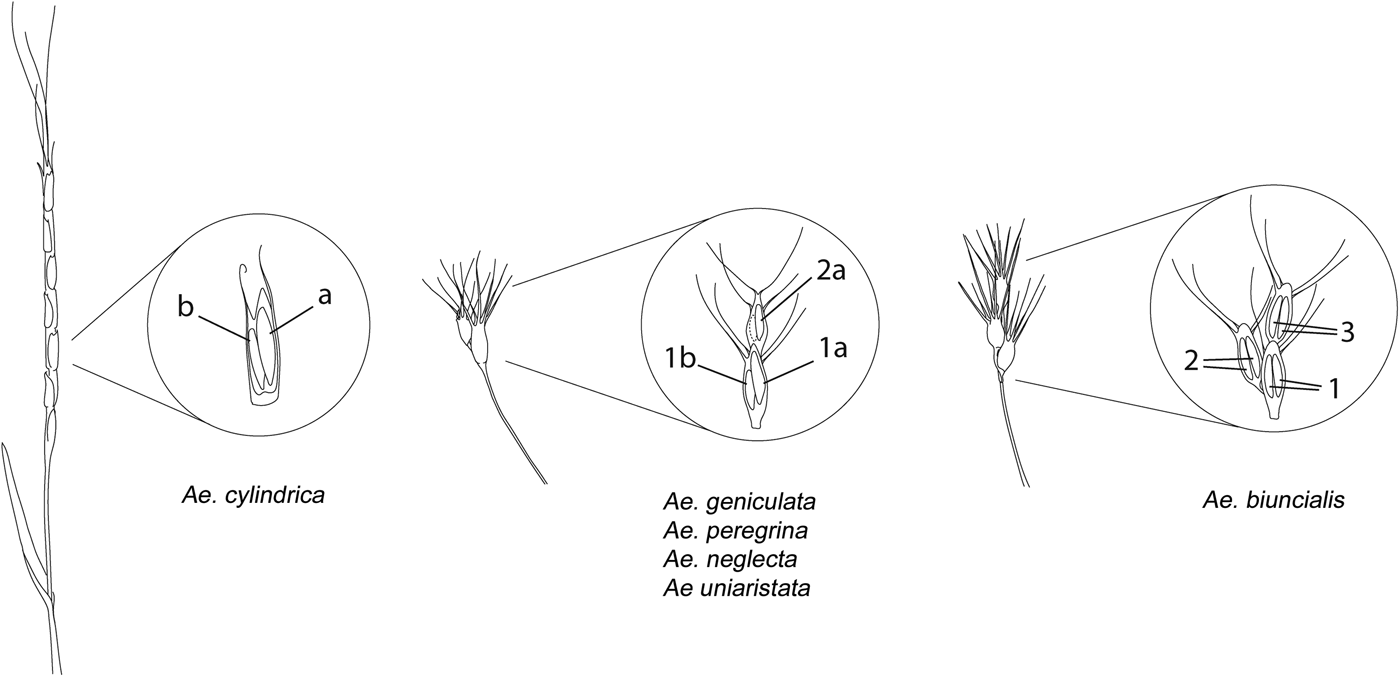
Based on the morphology of the spike and the presence/absence of seed dimorphism within-spikelet, the six species tested in the current experiment were divided into three groups (Fig. 1), and the seed morphs identified were considered as different seed lots during the germination experiment:
(1) In Ae. geniculata, Ae. peregrina, Ae. neglecta and Ae. uniaristata, a clear seed dimorphism was observed in the seed pair of the basal fertile spikelet. The larger and brighter coloured (here after referred as 1a) and the smaller and darker (hereafter referred to as 1b) seeds of the seed pairs, were considered as different seed lots during the ageing experiment (Table 1, Fig. 1). In Ae. peregrina and Ae. neglecta, an additional seed lot was considered (hereafter referred as 2a), consisting of one seed of the second fertile spikelet (only the larger and brighter in case of a dimorphic pair; Table 1, Fig. 1).
(2) Ae. biuncialis shows a similar spike morphology to the aforementioned species, although in our accession no clear size and colour dimorphism could be noticed between seeds from the same spikelet. Therefore, three seed lots were considered for Ae. biuncialis including seeds from the first (basal) fertile spikelet, seeds from the second fertile spikelet and seeds from the third fertile spikelet (hereafter referred as 1, 2 and 3; Table 1, Fig. 1).
(3) In Ae. cylindrica the spike disarticulates at maturity into several (6–8) spikelets, therefore the spikelet serves as the dispersal unit (van Slageren, Reference van Slageren1994). Three seed lots were considered for this latter species, including the larger (a) and smaller (b) seed of the dimorphic pair inside the spikelet and the solitary seed (s) when there was only one seed inside the spikelet (Table 1, Fig. 1). The dimorphic pair in Ae. cylindrica showed only size dimorphism but no colour differences.
Larger seeds from the dimorphic pairs are defined as ‘primary seeds’ (seed lots 1a, a) while the smaller ones are ‘secondary seeds’ (seed lots 1b, b). Example pictures and X-ray scans of the spikes and pictures of the seed morphs are provided in Fig. 2.
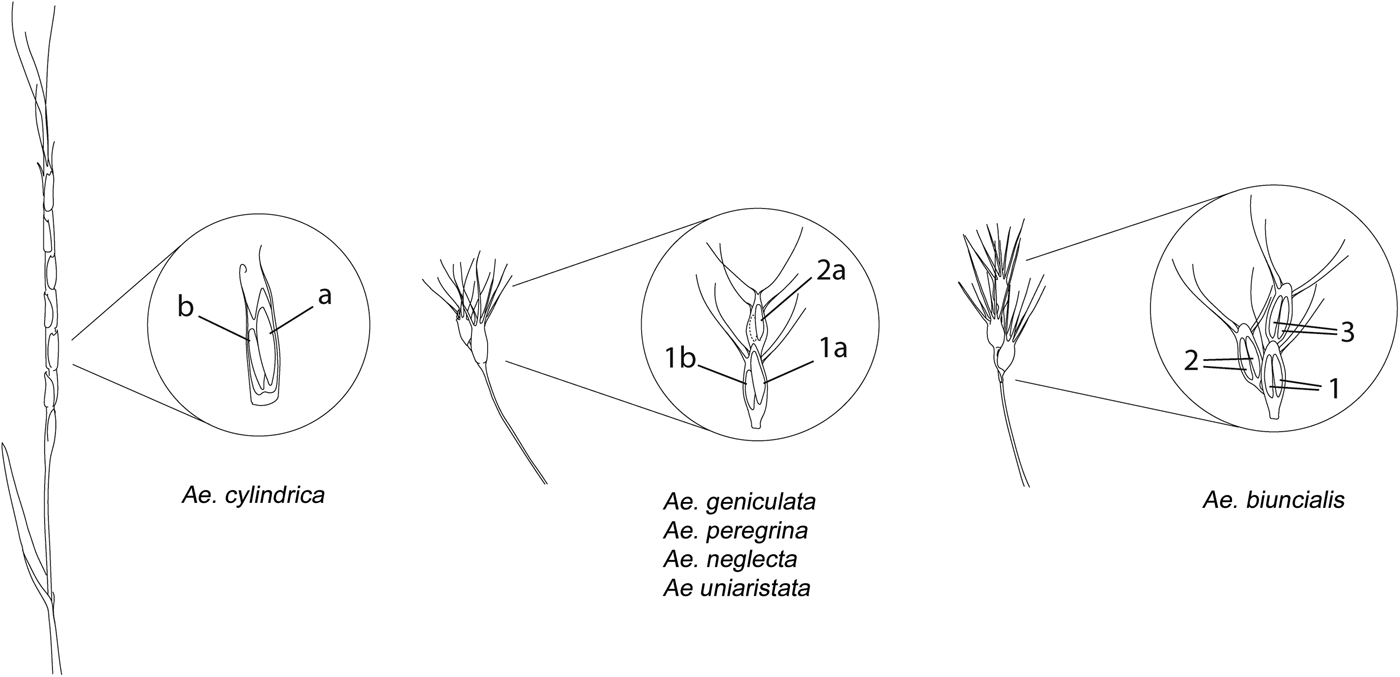
Fig. 1. Schemes of the spikes and internal spikelet morphologies (in the insets) encountered in the tested Aegilops species. On the left, a spike and a spikelet of Ae. cylindrica with the larger (a) and smaller (b) seeds. In the middle, the spike and spikelet morphology of Ae. geniculata, Ae. uniaristata, Ae. peregrina and Ae. neglecta. First fertile spikelet had two dimorphic seeds: one larger (1a) and one smaller (1b). In Ae. peregrina and Ae. neglecta another seed lot was considered (2a) consisting of solitary seeds of the second fertile spikelet or larger seeds in case of the presence of a dimorphic pair also in the second spikelet (smaller seed in dashed line). On the right, spike morphology of Ae. biuncialis, a species that does not show within-spikelet seed dimorphism. Three seed lots were considered, seeds of the basal fertile spikelet (1), seed of the second fertile spikelet (2), and seeds of the third fertile spikelet (3).
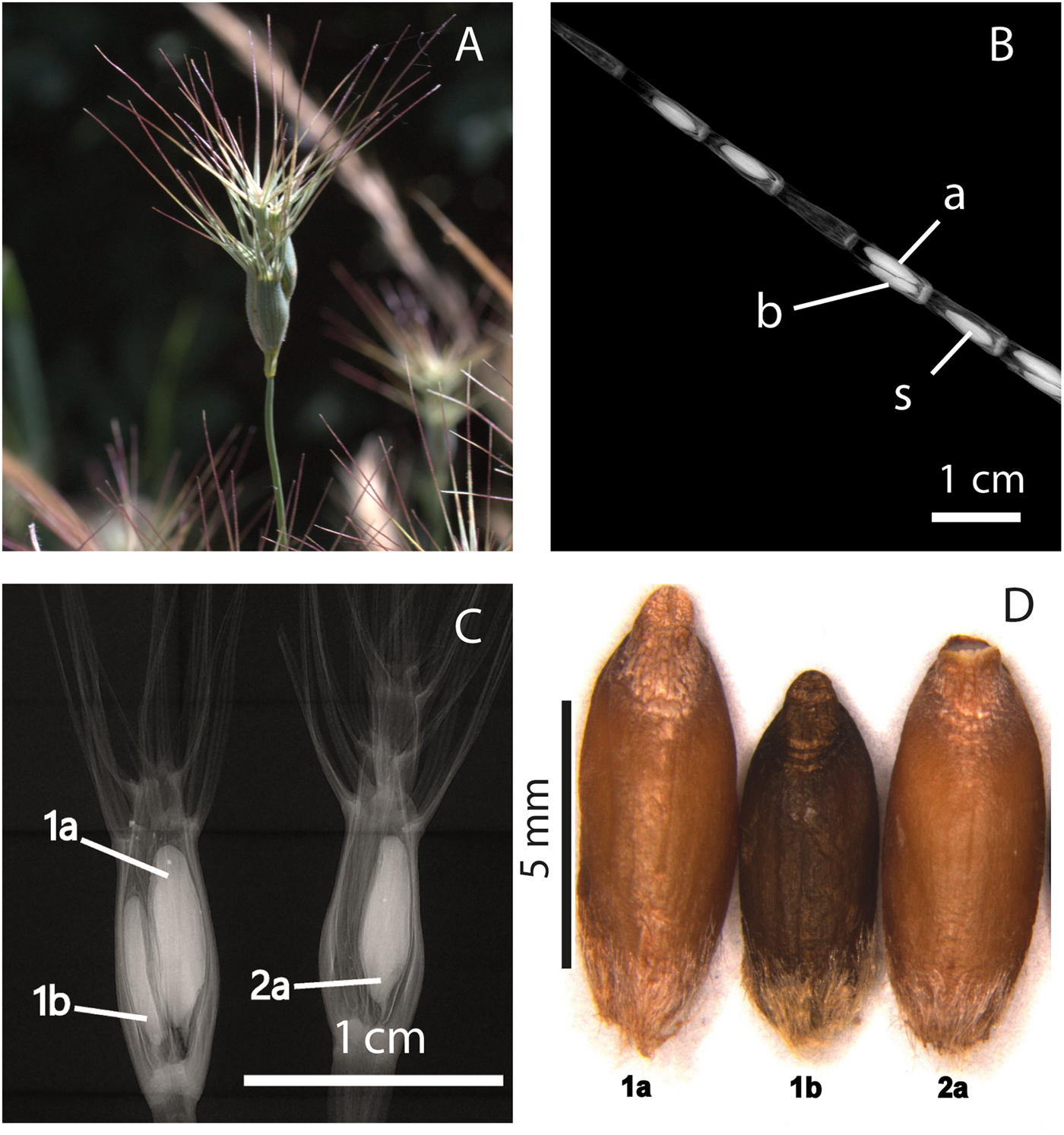
Fig. 2. (A) Spike of Ae. geniculata in the field. (B) X-ray scan of a spike of Ae. cylindrica. Different spikelets can be observed enclosing either one seed (seed lot s) or a dimorphic seed pair with one seed (seed lot a) larger than the other (seed lot b). (C) X-ray scan of a basal fertile spikelet of Ae. geniculata (on the left); the bigger (1a) and the smaller seed (1b) of the dimorphic seed pair can be observed. Moreover, the 2a seed of the second fertile spikelet (on the right) can also be observed. 2a seed lot was not considered for Ae. geniculata in the experiment, but was tested in Ae. neglecta and Ae. peregrina, species with a similar spike morphology to Ae. geniculata. The X-ray scanning was performed with a Faxitron X-ray scanner at the Millennium Seed Bank of the Royal Botanic Gardens, Kew. (D) Seeds extracted from the same spike and belonging to seed lots 1a, 1b and 2a of Ae. neglecta. The picture was taken using a microscope (Zeiss Stemi SV 11) with camera (Zeiss AxioCam). All the pictures were taken by F. Guzzon and edited by M. Canella (University of Padua).
Seed mass
Seed mass varied significantly both among species (Kruskal–Wallis, P < 0.001) and within all the species among seed lots (P < 0.001). Hence in all the species showing dimorphic seed pairs (e.g. Ae. cylindrica, Ae. geniculata, Ae. neglecta, Ae peregrina and Ae. uniaristata; Fig. 1) primary seeds (a, 1a) were significantly heavier than secondary (b, 1b; Table 2; P < 0.05). In Ae. neglecta, seed lot 2a was lighter than both 1a and 1b (P < 0.001), while in Ae. peregrina 2a was lighter than 1a (P = 0.020) and not significantly different from 1b (Table 2; P = 0.159). The seed lot s of Ae. cylindrica was significantly lighter than a (P < 0.001) but not different in seed mass from b (P = 0.779). Finally in Ae. biuncialis, the only tested species not showing within spikelet dimorphism (Fig. 1), seeds 1 were lighter than 2 and 3 (P < 0.001) while seed lots 2 and 3 did not different significantly between them (Table 2; P = 0.799).
Table 2. Values (mean ± standard error) of seed mass, parameters obtained through fitting of the basic seed viability equation (p 50, K i, σ–1) and the initial MTG (before the accelerated ageing treatment) for each seed lot
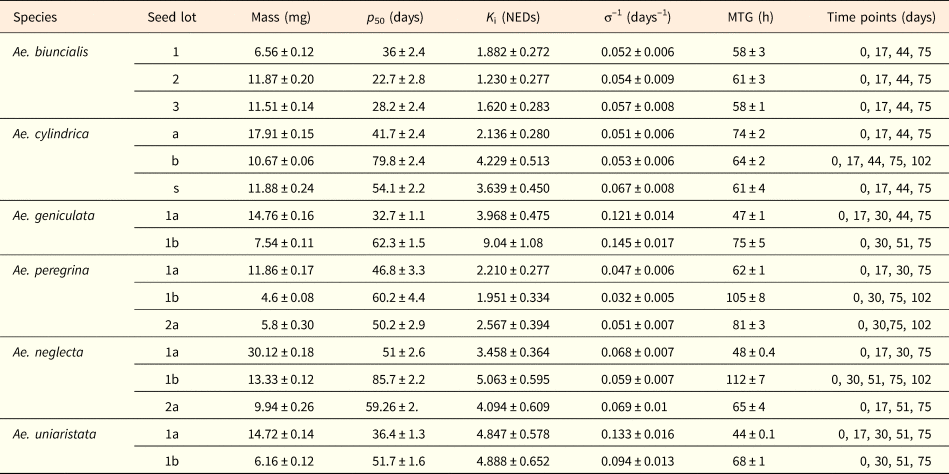
The experimental time points at which seed viability was tested are also indicated.
Seed longevity
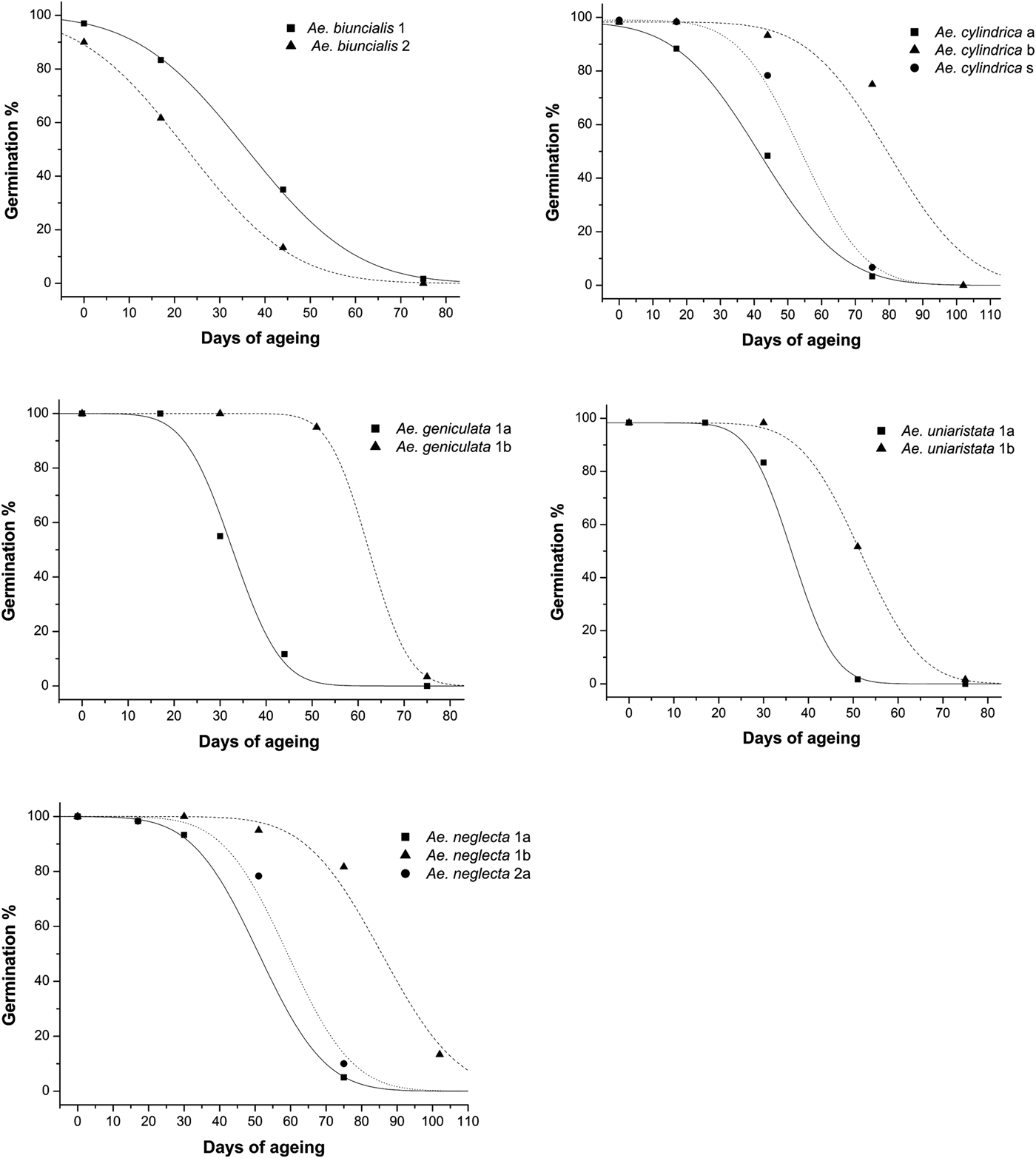
The estimated longevity of seeds (p 50) ranged from 22.74 ± 2.803 days (Ae. biuncialis 2) to 85.72 ± 2.203 days (Ae. neglecta 1b; Table 2). Species and seed lot had a significant effect on the p 50 (d.f. = 5; F = 11.413; P < 0.001 and d.f. = 8; F = 11.078; P < 0.001). Seed longevity was not statistically different among seed lots in Ae. peregrina (P = 0.284), as well as between seed lot 3 and both 1 (P = 0.084) and 2 (P = 0.186) in Ae. biuncialis. In Ae. geniculata, Ae. neglecta and Ae. uniaristata, the secondary seeds (1b) of the second fertile spikelets were always longer lived than the primary ones (1a) (P < 0.05), although to a different extent across species. In particular, estimated secondary seeds (1b) longevity was 1.4- to 1.9-fold greater than primary ones (1a) for Ae. geniculata, Ae. neglecta and Ae. uniaristata. Similarly, in Ae. cylindrica the estimated longevity of the secondary seed lot (b) was significantly (P < 0.001) 1.5-fold greater than the primary seed lot (a). At the same time, the estimated seed longevity of the solitary seeds (s) was intermediate between that of seed lot a (P = 0.026) and b (P = 0.001). Similarly, in Ae. neglecta p 50 of seed lot 2a was intermediate between that of 1a and 1b (P < 0.001) (Fig. 3, Table 2).
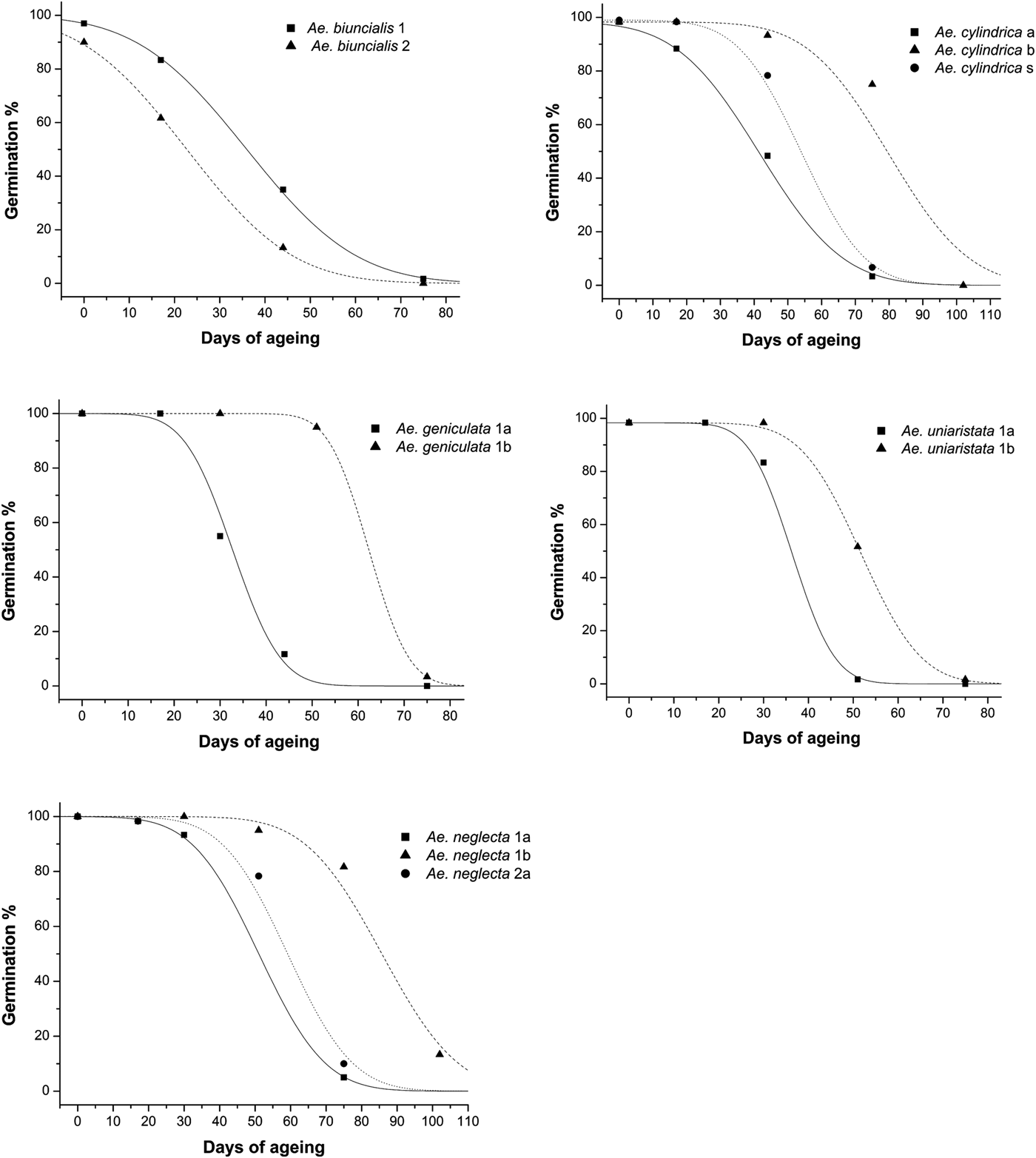
Fig. 3. Survival curves fitted by probit analysis of indicated Aegilops species. In Ae. biuncialis the curve of the seed lot 3 is not presented as the p 50 values of this seed lot was not different from seed lots 1 and 2 of the same species (see text). Survival curves of Ae. peregrina are not presented as the values of p 50 for the three seed lots tested for this species (1a, 1b, 2a) were not significantly different (see text).
Within each species, the slopes (σ–1) of the survival curves across seed lots were not significantly different (P > 0.05). On the other hand, differences in the intercept (K i) of the survival curves were significant (P < 0.05) in all the pairwise comparison, except in Ae neglecta 1a and 2a (F 1,3=3.655), Ae biuncialis 1 and 3 (F 1,3=5.988), Ae biuncialis 2 and 3 (F 1,3=2.422) and in all pairwise comparisons between seed lots in Ae. peregrina (1a and 1b, F 1,3=3.693; 1a and 2a, F 1,3=0.948; 1b and 2a, F 1,3=1.927).
Seed germination in the laboratory
Under laboratory conditions, seed morphs of all species showed high germination percentage (on average >90%), without significant differences across both, species (d.f. = 5, Wald chi-square = 5.342, P = 0.376) and seed morphs (d.f. = 8, Wald chi-square = 12.603, P = 0.126). Mean time to germinate (MTG) was significantly different across species and seed lots (d.f. = 5; F = 2.457; P < 0.05 and d.f. = 8; F = 7.330; P < 0.001).). In Ae. geniculata, Ae. neglecta, Ae. peregrina and Ae. uniaristata, the MGT was significantly greater (1.6, 2.3, 1.7 and 1.5-fold respectively) in the secondary seeds (1b) of the first spikelet then in the primary ones (1a) (P < 0.05). In Ae. peregrina the MTG of seed lot 2a was similar to both those showed by seed lots 1a (P = 0.196) and 1b (P = 0.266), while in Ae. neglecta seed lot 2a germinated significantly faster than seed lot 1b (P < 0.05), but similarly to seed lot 1a (P = 0.195). Finally, no significant differences were observed in the MTG within seed lots in Ae. cylindrica (a,b,s) and Ae. biuncialis (1,2,3) (P > 0.05) (Table 2). Supplementary Figure 2 outlines germination progress curves among the different seed lots.
Seed germination in the field
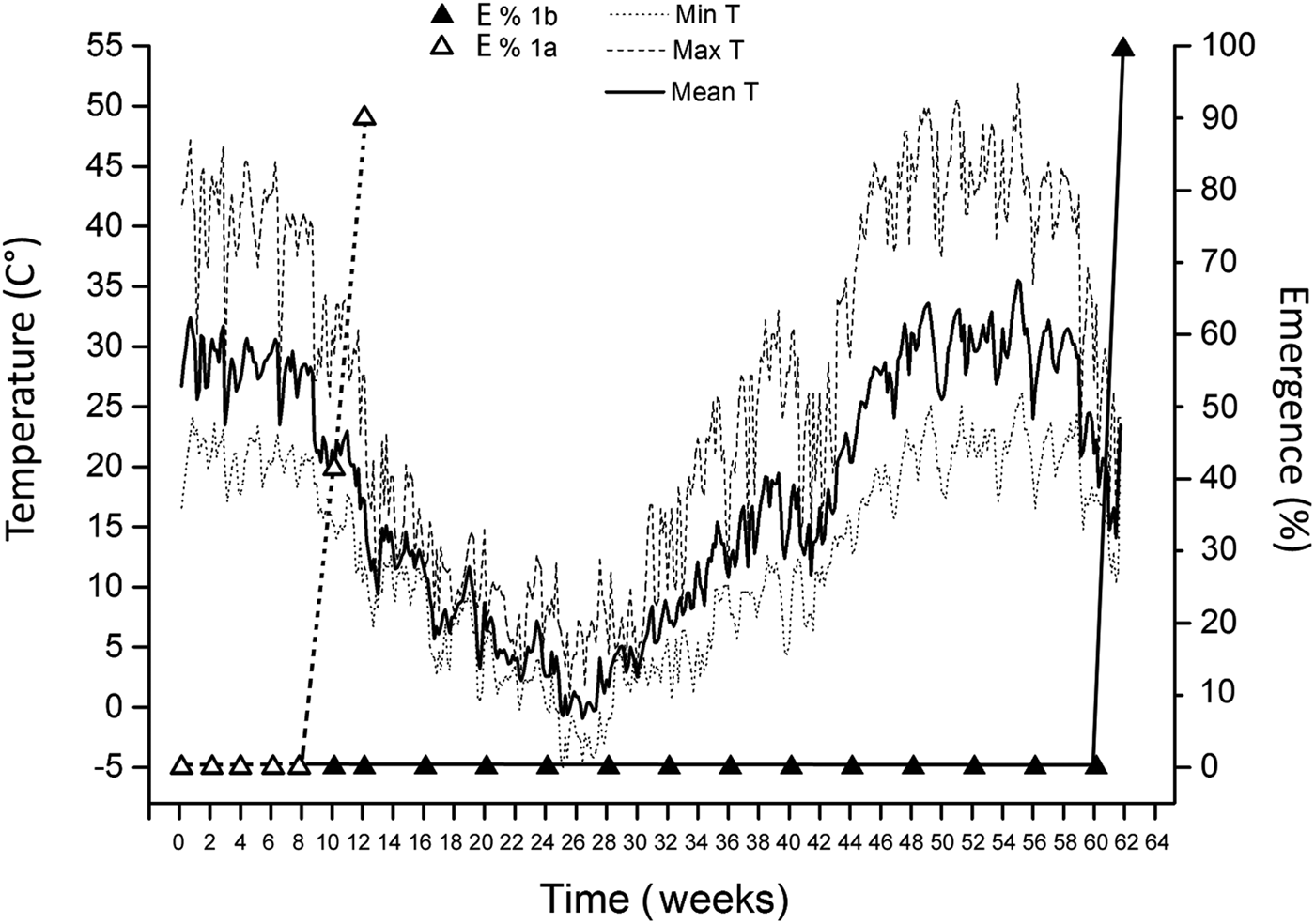
Ten weeks after sowing (and few days after the first rains; end of September 2016) 41.43 ± 14.8% of the primary seeds of the first basal fertile spikelets (seed lot 1a) of Ae. neglecta had emerged (Fig. 4). Two weeks later, 90 ± 8.5% of the primary seeds emerged. Moreover, 1 year after sowing (July 2017) the secondary seeds of the first basal fertile spikelets (seed lot 1b) were all still ungerminated. However, by that time 100% of a subsample of them, retrieved from the soil and extracted from the spikes, could germinate in 3 days under laboratory conditions. Finally, germination of seeds left in the field within the spikes was complete (100%) in September 2017, 14 months after sowing and 1 year after the emergence of primary seeds (Fig. 4).
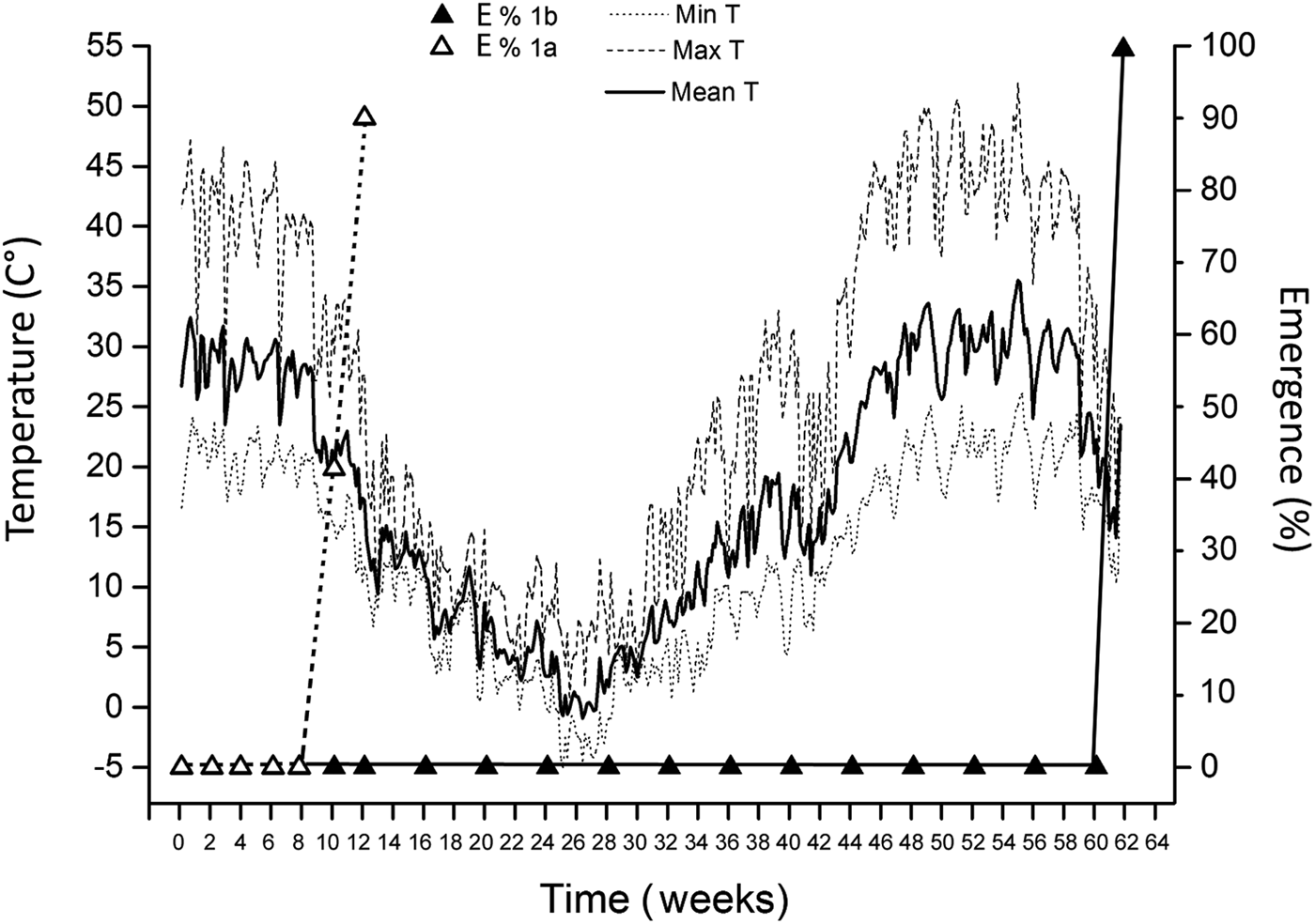
Fig. 4. Percentages of the emergence from primary (1a; white triangles) and secondary (1b; black triangles) seeds of Ae. neglecta in the field. Also shown is the mean daily soil temperature (continuous black line), mean minimum soil temperature (dotted line) and mean maximum soil temperature (dashed line).
Discussion
In this study we estimated the longevity of heteromorphic seeds in six different species of Aegilops. Our aim was to clarify longevity across species and seed morphs of the same dispersal units, thereby providing novel insights into the germination patterns of Aegilops. Results from controlled ageing tests (CAT) were implemented with a field germination experiment on Ae. neglecta to better understand emergence phenology and its relation to seed lifespan.
All species studied here, except Ae. biuncialis, showed within-spikelet seed dimorphism, with primary seeds being always significantly heavier than secondary seeds. At the same time, seeds from different spikelets also differed in their mass. The seed heteromorphy detected in the study accessions is consistent with the findings of Datta et al. (Reference Datta, Evenari and Gutterman1970), Marañon (Reference Marañon1989) and Dyer (Reference Dyer2017) in Ae. geniculata, Ae. neglecta and Ae. triuncialis, suggesting that a significant difference in the mass of the different seed morphs produced by the same spike and especially the presence of dimorphic seed pairs is a widespread characteristic in the genus Aegilops.
Dimorphic seeds studied here reflected differences in both germination and longevity. In particular, the germination of seeds in the laboratory was high (on average >90%) and quick (i.e. with MGT ranging from 44 to 112 hours; Table 2) indicating the absence of dormancy mechanisms when seeds are extracted from their dispersal units. At the same time, the secondary seeds (1b) of the dimorphic pair showed a delayed germination compared with the primary seeds (1a), i.e. in Ae. geniculata, Ae. neglecta, Ae. peregrina and Ae. uniaristata, confirming previous observations (Datta et al., Reference Datta, Evenari and Gutterman1970; Marañon, Reference Marañon1989). However, in Ae. cylindrica the MTG was similar between the dimorphic seed pair, underlining that different germination patterns may exist across species in Aegilops. A delayed germination in the smaller seeds of several Aegilops has also been observed in natural conditions (i.e. with seeds enclosed in the dispersal unit) and explained by the influences of both maternal and sibling factors, through the presence of an inhibitor in the glumes, internal levels of gibbellerins or competition for essential metabolites among sibling seeds (Lavie et al., Reference Lavie, Levy, Cohen, Evenari and Guttermann1974; Wurzburger and Leshem, Reference Wurzburger and Leshem1967; Wurzburger and Leshem, Reference Wurzburger and Leshem1969; Wurzburger and Koller, Reference Wurzburger and Koller1973; Dyer, Reference Dyer2004). However, the implications of such constrains on the emergence phenology in nature were not well understood. Here we have shown that differences in MTG between dimorphic pairs of Ae. neglecta were exacerbated under field experiments, with primary seeds (1a) emerging soon after dispersal (i.e. 3 months later, in autumn, after the first rains) and secondary seeds (1b) germinating 14 months after dispersal (Fig. 1), indicating the possibility of dormancy mechanisms in the secondary seeds imposed by the spikelet (Datta et al., Reference Datta, Evenari and Gutterman1970). The dormancy of the secondary seed may be released when the primary, larger seed is not present any more in the dispersal unit and the effects of the water-soluble inhibitor have been weakened by several wet and dry periods (Dyer, Reference Dyer2004). Nevertheless, further studies are needed to clarify the dormancy of Aegilops secondary seeds when enclosed in the dispersal units, since other explanations of this phenology (e.g. different thermal requirement for the germination of the different seed morphs) cannot be completely ruled out. The delay in the germination of the secondary seeds in the field is an interesting and confirmatory observation, outlining that the shift in the germination timing between seed morphs in nature spreads the seedling emergence over two consecutive years.
Supporting a previous observation that CAT holds a great potential to predict seed persistence in soil seed banks (Long et al., Reference Long, Panetta, Steadman, Probert, Bekker, Brooks and Adkins2008), our results show that seed longevity of the different seed morphs reflected their soil seed bank persistence in Ae. neglecta. In this species, primary seeds (1a) were significantly shorter lived than secondary seeds (1b) from the same spikelets and, consistently, the former germinated much sooner after dispersal than the latter (see above). Consequently, it appears that the higher resilience to ageing of the secondary seeds detected in Ae. neglecta may be needed to maintain high viability after dispersal to fulfil germination phenology. Additionally, differences in p 50 across seed morphs could be linked to differences in their initial seed viabilities (K i) and not to differences in the deterioration rate (σ–1) (Fig. 3). This strategy allows secondary seeds to retain full viability during the initial stages of ageing for a longer time compared with primary ones, both under CAT and in the field. Indeed, results from field experiments in Ae. neglecta showed that longer-lived secondary seeds retained the full viability for over 1 year after sowing, with 100% of them emerging 14 months later. The same dimorphism in seed longevity was shown in all other species with within-spikelet dimorphism (except Ae. peregrina), indicating that a similar germination phenology should be expected also in these taxa. The delayed germination and high resilience to ageing showed by secondary seeds reinforce the hypothesis of a bet-hedging strategy in the germination ecology of Aegilops (Dyer, Reference Dyer2004). Supporting this view, seed heteromorphy has been associated with a bet-hedging germination strategy in several plant genera (Silvertown, Reference Silvertown1984; Imbert, Reference Imbert2002).
A major question is whether the mechanisms of seed ageing under CAT resemble those of the cool dry conditions of seed banks (i.e. 15% RH, –20°C), rather than those occurring in the wild (Long et al., Reference Long, Panetta, Steadman, Probert, Bekker, Brooks and Adkins2008). Despite the fact that we could not investigate the biochemical process of ageing and the exact seed water content, the air humidity the spikes of Ae. neglecta experienced at the soil surface after dispersal was (on average) similar to that used in CAT (60% RH) for most of the time (i.e. 50–70% for 10 months, December to September), although temperatures were much lower (i.e. <15°C for 6 months, then between 20 and 27°C) (Supplementary Figure 1). In this regard, longevity of seeds at RH <70% is increased at lower temperatures, explaining the high survival 1 year after sowing of the secondary seeds. Temperature enhances the rate of deteriorative reactions occurring in seeds. In particular, above a critical temperature (e.g. estimated in approximately 40°C in Vigna radiate; Murthy et al., Reference Murthy, Kumar and Sun2003) the solid-like glassy state may soften into the rubbery or even liquid state (i.e. glass transition temperature), enhancing molecular mobility, thereby the deteriorative reactions proceed rapidly (Walters, Reference Walters1998; Buitink and Leprince, Reference Buitink and Leprince2008). Consequently, our results indicate that differences in the deterioration process that seeds experience in the wild and under CAT might exist mostly due to temperature differences.
Despite our data reinforcing the hypothesis that in the dimorphic seed pairs of Aegilops, the primary and shorter-lived seeds germinate soon after the dispersal, while the secondary and longer-lived seeds create a more persistent soil seed bank (Datta et al., Reference Datta, Evenari and Gutterman1970; Marañon, Reference Marañon1989; Onnis et al., Reference Onnis, Bertacchi, Lombardi and Stefani1995), the similar longevity found among seed lots in Ae. peregrina indicates that the regeneration strategy may follow different patterns in this species. Additionally, in seed lot 2a (consisting of seeds of the second fertile spikelet; Fig. 1) of Ae. neglecta the p 50 was intermediate to that of primary and secondary seed lots (1a and 1b). Similarly, in Ae. cylindrica, seed lots from single-seeded spikelet (s) had an intermediate p 50 comparable to that of the primary (a) and the secondary (b) seeds from the double-seeded spikelet. Moreover, in Ae. biuncialis, even if seed dimorphism was not detected, seeds from different spikelets on the same spikes significantly differed in their p 50. Therefore, further studies are needed to understand whether longevity differences across these latter seed morphs may correlate with their germination phenology (and/or vice versa), as shown here for dimorphic pairs of Ae. neglecta. Supporting this view, previous studies highlighted some germination differences across a range of seed morphs from different spikelets in Aegilops (Datta et al., Reference Datta, Evenari and Gutterman1970; Fandrich and Mallory-Smith, Reference Fandrich and Mallory-Smith2006; Orsenigo et al., Reference Orsenigo, Guzzon, Abeli, Rossi, Vagge, Balestrazzi, Mondoni and Müller2017).
Seed longevity guarantees the long-term ex situ conservation of plant genetic resources (PGR) within gene banks (Walters et al., Reference Walters, Wheeler and Grotenhuis2005). The longevity ranking of seed lots based on data from controlled ageing tests is expected to be similar to that for seed lots in long-term storage, i.e. seed lots that are identified as losing viability quickly in the controlled ageing test will also lose viability quickly during long-term conservation storage (Probert et al., Reference Probert, Daws and Hay2009; Hay and Whitehouse, Reference Hay and Whitehouse2017). The significant differences in seed longevity among heteromorphic seeds in most of the Aegilops species tested here, suggest that different seed morphs are also likely to have different longevities under ex situ seed bank storage, with secondary seeds being longer lived than primary seeds. The fundamental aim of ex situ seed collections of CWR is to capture all the possible adaptive traits in the seed samples (Warschefsky et al., Reference Warschefsky, Penmetsa, Cook and von Wettberg2014). Several differences have been detected at the biochemical, genetic and morphological levels, as well as in abiotic stress resistance among different seed morphs in species of Poaceae (Datta, Reference Datta, Evenari and Gutterman1970; De Gara et al., Reference De Gara, Paciolla, Liso, Stefani and Arrigoni1991; Cremonini et al., Reference Cremonini, Colonna, Stefani, Galasso and Pignone1994; Frediani et al., Reference Frediani, Colonna, Cremonini, De Pace, Delre, Caccia and Cionini1994; Orsenigo et al., Reference Orsenigo, Guzzon, Abeli, Rossi, Vagge, Balestrazzi, Mondoni and Müller2017). It follows that different seed morphs of Aegilops should be held separately in storage and their viability, regeneration or recollection intervals (Hay and Probert, Reference Hay and Probert2013) should follow different timings, in order to avoid important loss of genetic resources.
Finally, seed longevity in the Aegilops species tested here was, on average, longer compared with that of other grasses. For example, across 722 seed collections of Poaceae exposed to CAT, Probert et al. (Reference Probert, Daws and Hay2009) found an average p 50 value of 26.7 days. Similarly, Mondoni et al. (Reference Mondoni, Probert, Rossi, Vegini and Hay2011) could not find any grasses with a p 50 higher than 38 days. However, in our study overall p 50 in Aegilops was on average higher (i.e. about 50 days), with some seed lots showing a p 50 of about 80 days (Ae. cylindrica b, Ae. neglecta 1b). Thus, the seed lots in our study can be described as having a medium longevity in air-dry storage, based on a logarithmic scale to categorize species according to their relative longevity (Probert et al., Reference Probert, Daws and Hay2009; i.e. p 50 ≤ 1 day indicates ‘very short’ seed longevity; p 50 >1 to ≤10, ‘short’; p 50 >10 to ≤100, ‘medium’; p 50 >100 to ≤1000, ‘long’; p 50 >1000, ‘very long’). Such higher seed longevity of Aegilops compared with that shown by other Poaceae may be due to specific adaptations of seeds of this genus to warm and dry environments, where seeds need to persist, in a dry state, for long periods between bouts of rainfall sufficient to allow successful establishment (Probert et al., Reference Probert, Daws and Hay2009). Supporting this view, seeds produced by species growing in Mediterranean habitats, as the ones in which Aegilops thrives, are known to be among the longest lived found worldwide (Merrit et al., Reference Merritt, Martyn, Ainsley, Young, Seed, Thorpe, Hay, Commander, Shackelford, Offord, Dixon and Probert2014).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S096025851800034X
Author ORCIDs
Filippo Guzzon http://orcid.org/0000-0001-5305-2259.
Acknowledgements
The authors are grateful to J. Terry and R. Eastwood (Millennium Seed Bank, Royal Botanic Gardens Kew) for providing the Cypriot seed accessions. We are also indebted to Silvia Bodino (University of Pavia, Plant Germplasm Bank of the University of Pavia) for the help with seed cleaning. We also thank Marco Canella (University of Padua) for drawing Fig. 1 and for the digital editing of all the figures. Finally, we need to thank Professor O. Leprince and five anonymous reviewers for their comments on earlier versions of the manuscript. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.