Introduction
Schinus molle L. (Anacardiaceae), known as the pepper tree, occurs in the subtropical regions of South America, including areas of Argentina, Bolivia, Chile and Brazil, but is cultivated in many countries (Lim, Reference Lim2012; Silva-Luz and Pirani, Reference Silva-Luz and Pirani2013). The fruit of Anacardiaceae is a drupe and the mesocarp is usually resinous (Simpson, Reference Simpson2006; Pell et al., Reference Pell, Mitchell, Miller, Lobova and Kubitzki2011). In the genus Schinus the endocarp of the drupe is comprised of three layers of sclereids, classified as the Anacardium-type endocarp, that remains attached to seeds (Wannan, Reference Wannan2006; Pell et al., Reference Pell, Mitchell, Miller, Lobova and Kubitzki2011; Oliveira and Mariath, Reference Oliveira and Mariath2015). This Anacardium-type structure of endocarps is referred to as the cause of seed physical dormancy (Li et al., Reference Li, Baskin and Baskin1999a). However, the dormancy of S. molle seeds was suggested by preliminary evidence as physiological (Morfin, Reference Morfin1985; Jøker et al., Reference Jøker, Cruz, Morales and Rojas2002). Therefore, the causes of dormancy of S. molle seeds are still unclear and must be investigated to improve plant production.
Seed dormancy has been defined as the incapacity of viable seeds to germinate under favourable conditions (Bewley, Reference Bewley1997; Bentsink and Koornneef, Reference Bentsink and Koornneef2008). The dormancy response is controlled by environmental factors such as light, temperature and seed storage conditions (Bentsink and Koornneef, Reference Bentsink and Koornneef2008). Dormant seeds cause problems in crop plant production and must be treated to improve the germination parameters.
Schinus molle is cultivated worldwide as trees for urban areas, and is also important as a medical plant and for the restoration of areas polluted by heavy metals (Iponga et al., Reference Iponga, Milton and Richardson2008; Lim, Reference Lim2012; Pereira et al., Reference Pereira, Rodrigues, Correa, Castro, Ribeiro and Pereira2016). Despite its widespread use, seed germination of this species is usually very low (Demelash et al., Reference Demelash, Tigabu and Odén2003). However, the reasons for seed dormancy in this species are still unclear, and the effects of seed scarification and storage on germination have never been tested. Since S. molle has high potential for use for crop and medical proposes, enhancing seed germination would greatly benefit plant production. Thus, the objective of our work was to evaluate the germination of S. molle seeds as related to environmental conditions, scarification, storage time and seed anatomy.
Materials and methods
Seed sampling
Fruits from a population of 25 plants of S. molle were collected in the southern region of Minas Gerais state, Brazil (21°25′45″S and 45°56′50″W) in October 2014. Samples were taken to the laboratory and dried at room temperature. The exocarps were removed manually from dried fruits. Seeds (here we call ‘seeds’ the seed plus parts of mesocarp and endocarp) were surface sterilized with 50% sodium hypochlorite for 10 min, washed twice with distilled water and then dried at 35°C for 72 h.
Dormancy alleviation tests
Newly collected samples were divided into the following groups: complete drupes (T1), seeds (T2), seeds mechanically scarified by using 25 g of sand in a mortar and pestle for 10 min (T3), seeds heat scarified in water at 80°C for 10 min (T4) and seeds acid scarified using 20 ml of sulphuric acid (H2SO4) for three different time intervals: 1 (T5), 3 (T6) and 5 min (T7) (Fig. 1). After acid scarification, the acid was neutralized with 40 ml of sodium bicarbonate and then seeds were washed twice with distilled water. All samples (treatments T1–T7) were placed under three photoperiods: 0 (dark), 12 or 24 h of light. The experiment was designed as a factorial 7 × 3 and two replicates of 25 seeds were used. For all scarification treatments, sets of 25 seeds were submitted separately to each method. Seeds were germinated in uncovered black plastic boxes (Gerbox; J. Prolab, São José dos Pinhais, Brazil) containing filter-paper sheets moistened with 3.5 ml of distilled water and placed at a controlled temperature of 25 ± 2°C in a BOD-type chamber (Novatecnica, Piracicaba, Brazil). The experiment was carried out for 38 d, germination (%) was monitored daily and the speed index (GSI) was calculated. Germination percentage was calculated from the equation: G% = (n/N) × 100, where n = the number of germinated seeds and N = the total number of seeds (25). The GSI was calculated from the equation: GSI = n/T 1 + n/T 2 + n/T 3 + … + n/T 38, where n = the number of germinated seeds and T = time in days (T 1 = day 1, T 2 = day 2 … T 38 = day 38).

Figure 1. Dormancy alleviation methods using newly collected seeds. T1 = drupes, T2 = seeds, T3 = mechanically scarified, T4 = thermally scarified, T5 = acid scarified for 1 min, T6 = acid scarified for 3 min, T7 = acid scarified for 5 min.
Electrical conductivity tests were performed to evaluate seed vigour, with four replicates of 25 seeds subjected to the same scarification treatments and further placed in 75 ml of distilled water at 25°C in the dark for 24 h, and then the electrical conductivity was measured with a conductivity meter (W12D; Bel Engineering, Monza, Italy).
Separation of seeds by specific gravity
After the previous experiment, the best scarification and light periods were selected for further testing. Floating seeds when separated in water may be non-viable. Thus, for the separation by specific gravity, sets of 3000 seeds were placed in a beaker containing 500 ml of distilled water and then separated into three groups after 15 min: non-treated (not placed in water), floating and sunken seeds. After separation, one seed set was subjected to scarification with H2SO4 for 1 min and one other was not scarified. The seeds were placed in uncovered black plastic boxes (Gerbox) containing filter-paper sheets moistened with 3.5 ml of distilled water and maintained at 25 ± 2°C and 24-h photoperiod for 38 d. The experiment was conducted in a factorial 3 × 2 design with five replicates of 25 seeds. The germination percentage and the GSI were evaluated.
Dry-storage test
The dry-storage test was performed on seeds stored at two different temperatures [4°C (in a refrigerator) and 22°C (room temperature)] in paper bags. Seeds were sampled every 3 months for 1 year, starting with newly collected seeds (storage time zero). Stored seeds were sampled and then subjected to scarification with H2SO4 for 1 min. The seeds were germinated in uncovered black plastic boxes (Gerbox) containing filter-paper sheets moistened with 3.5 ml of distilled water, at a controlled temperature of 25 ± 2°C in a BOD-type chamber with 24-h photoperiod. The experiment was conducted in a factorial 4 × 2 design with six replicates of 25 seeds. The germination percentage and the GSI were evaluated.
Anatomy of seeds
Some newly collected seeds were acid scarified for 1 min and others were non-scarified. Both sets of seeds were fixed in FAA solution (formaldehyde, acetic acid and 70% ethanol) for 72 h and stored in 70% ethanol. Seeds were dried in increasing ethanol concentrations (70%, 90% and 100%) at 2-h intervals. The samples were embedded in Historesin according to the manufacturer's instructions (Leica Microsystems, Wetzlar, Germany). Sections were obtained using a semi-automated rotary microtome (Yidi YD-335; Jinhua Yidi Medical Appliance Co., Ltd, Zhejiang, China). The sections were stained with 1% toluidine blue and mounted on slides with Canada balsam (O`Brien et al., Reference O'Brien, Feder and McCully1964). The slides were photographed using a microscope attached to an image capture system (CX31, Olympus, Tokyo, Japan).
Histochemical studies
All histochemical studies were performed on seeds imbibed for 48 h in distilled water. Free-hand sections were performed with the aid of a steel blade, and further semi-permanent slides were prepared according to specific methodologies proposed by Johansen (Reference Johansen1940). Detection of tannins was performed with a solution containing 2% ferrous sulphate, 10% formalin and 90% distilled water. After 24 h of immersion in ferrous sulphate solution, the sections were washed in distilled water and mounted on the slides using 50% glycerol (v/v). For the detection of lipids, the 0.5% Sudan III (w/v) solution was used, placing sections in this solution for 10 min and then washing in distilled water. Sections were mounted using 50% glycerol (v/v). The slides were photographed using a light microscope attached to an image capture system (CX31, Olympus, Tokyo, Japan).
Lignin was detected using fluorescence microscopy analysis. Cross-sections of seeds were placed in a solution containing distilled water and 0.1% berberine hemi-sulphate (w/v) for 1 h and then washed in distilled water. Further, sections were kept in 0.5% aniline blue (w/v) solution for 30 min and then washed twice with distilled water. Sections were mounted in a solution of 0.1% FeCl3 (w/v) in 50% glycerol (v/v) (Brundrett et al., Reference Brundrett, Enstone and Peterson1988). The observations were made with a fluorescence microscope (BX60, Olympus) equipped with a cooled monochrome camera (Olympus). Images were captured with ultraviolet excitation/emission wavelengths of 358–461 nm (Brundrett et al., Reference Brundrett, Enstone and Peterson1988).
Dye-tracking test
A dye-tracking experiment was performed to determine the water-uptake pathway throughout the mesocarp and endocarp and how water arrived at the inner parts of the seed. Seeds were incubated in 0.5% toluidine blue solution (w/v) at room temperature for 48 h. Seeds were sampled and subjected to the same plant microtechniques described above in the ‘Anatomy of seeds’ section. The slides were photographed using a microscope attached to an image capture system (CX31, Olympus).
Seed imbibition curve
Imbibition curves were generated for non-scarified seeds and for those scarified with H2SO4 for 1 min. Sets of 25 seeds were placed in uncovered black plastic boxes (Gerbox) containing filter paper moistened with 3.5 ml of distilled water and incubated at 25 ± 2°C. The seed fresh mass was measured at 30-min intervals until constant fresh mass was obtained.
Statistical analysis
Statistical analyses were performed using the SISVAR 5.0 software (Ferreira, Reference Ferreira2011). Before parametric analysis, data were submitted for a normal distribution test (Shapiro–Wilk). In addition, data were subjected to analysis of variance, and means were compared by the Scott–Knott test at 5% probability or subjected to regression analysis.
Results
Dormancy alleviation
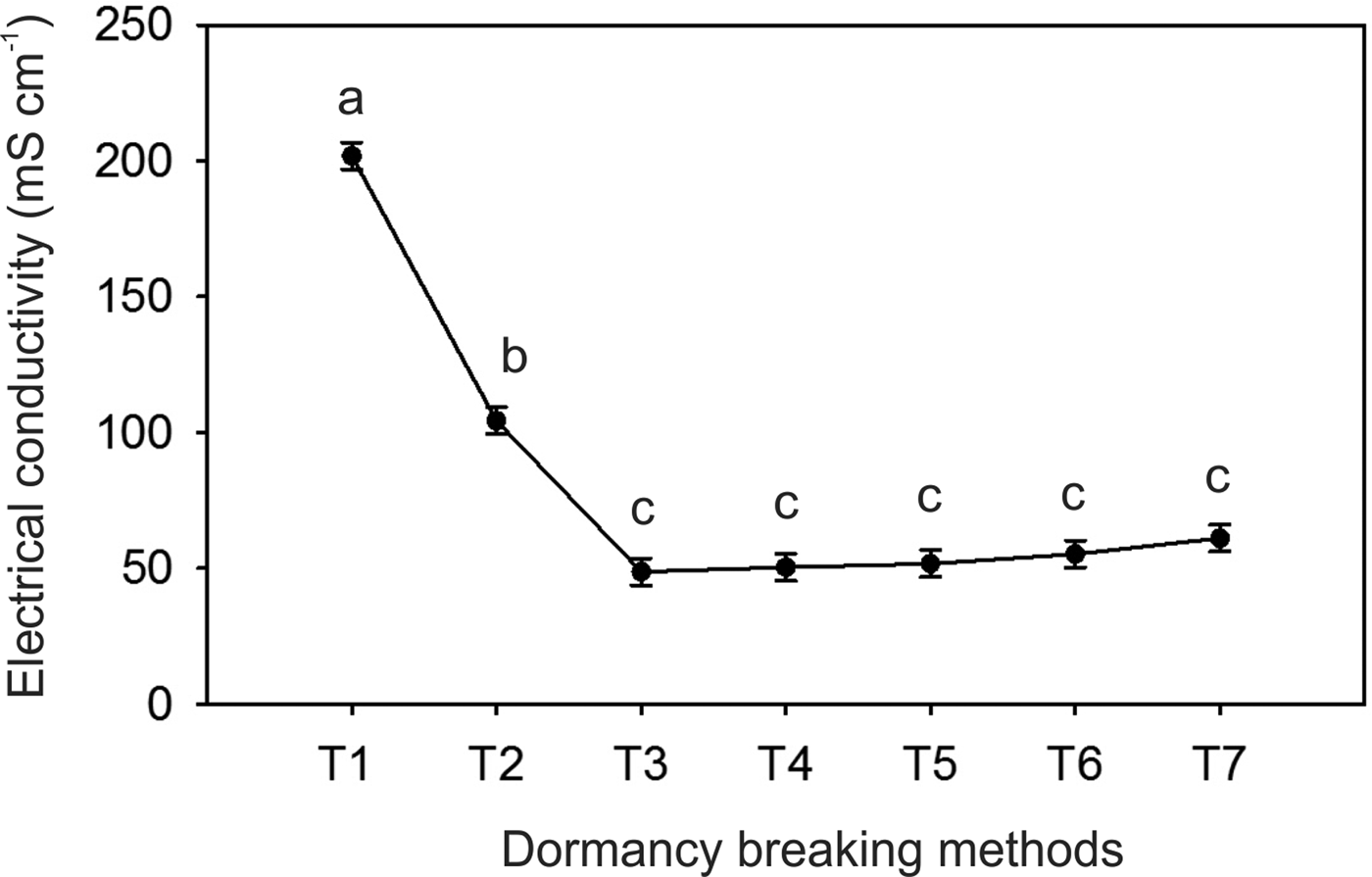
There was no significant interaction between the scarification and photoperiod (P > 0.05). However, all acid scarification treatments showed higher germination percentages compared to other scarification methods (Fig. 2A). Regarding the photoperiod, the highest germination percentage was found at constant light (Fig. 2B). No differences in the GSI were promoted by the scarification treatments (P = 0.24); however, the 24-h photoperiod increased this parameter (Fig. 2C). In addition, all the scarification treatments reduced the electrical conductivity of the seeds (Fig. 3).

Figure 2. Germination and germination speed index (GSI) of newly collected seeds of S. molle. Data shown in (B) and (C) are combined from all treatments. Means followed by the same letter do not differ by the Scott–Knott test at the 5% significance level. T1, Drupes; T2, seeds; T3, mechanically scarified; T4, thermally scarified; T5, acid scarified for 1 min; T6, acid scarified for 3 min; T7, acid scarified for 5 min.
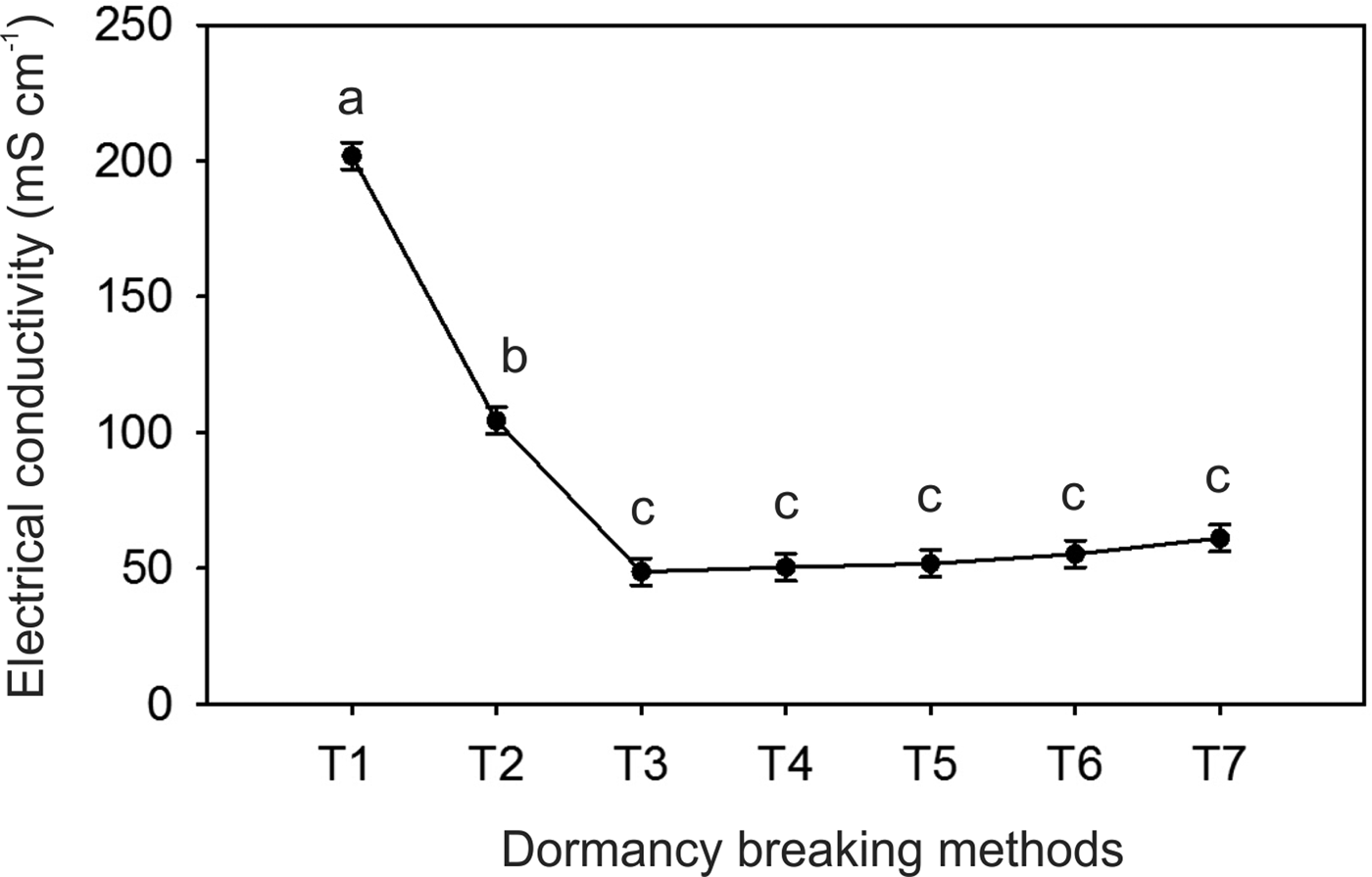
Figure 3. Electrical conductivity (mS cm−1) of newly collected seeds subjected to different dormancy breaking methods. Means followed by the same letter do not differ by the Scott–Knott test at the 5% significance level. T1, drupes; T2, seeds; T3, mechanically scarified; T4, thermally scarified; T5, acid scarified for 1 min; T6, acid scarified for 3 min; T7, acid scarified for 5 min.
Separation of seeds by specific gravity
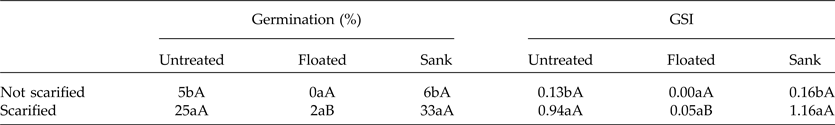
The seeds that sank showed both higher germination percentage and GSI as compared to those that floated (Table 1). In addition, acid scarification significantly enhanced the germination percentage and GSI of the seeds that sank but not of the floating seeds (Table 1). The seeds that sank were viable, showing the mesocarp, endocarp and the fully developed embryo closely attached to endocarp (Fig. 4A). However, floating seeds showed large empty spaces and non-viable embryos (Fig. 4B).

Figure 4. Morphology of sunken (A) and floating (B) Schinus molle seeds. In (B) the floating seed shows empty spaces and a non-viable embryo. ms, mesocarp; en, endocarp; em, embryo.
Table 1. Germination and GSI of S. molle seeds separated by specific gravity, then scarified with H2SO4 for 1 min or not scarified, and maintained at a 24-h photoperiod
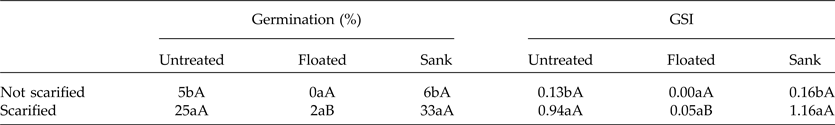
The lower-case letters compare the scarified seeds (columns) and the uppercase the seeds which were separated by specific gravity (lines). Means followed by the same letter in columns or lines do not differ by the Scott–Knott test at the 5% significance level.
Dry storage
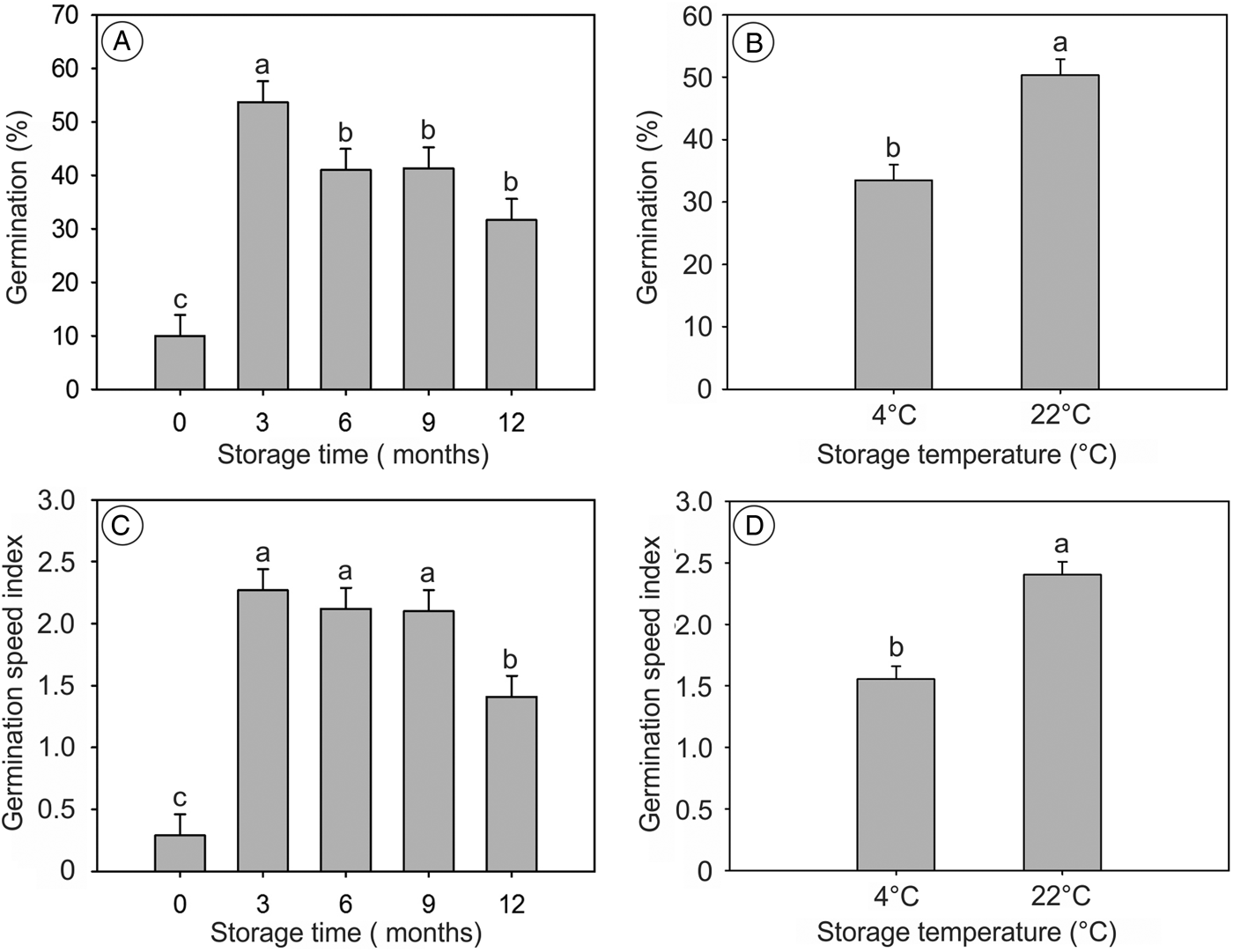
Storage time and temperature promoted differences in germination parameters; however, no significant interaction between these factors was found (P > 0.05). Germination percentage and GSI showed higher means for all storage times compared to newly collected seeds; however, both variables decreased after longer storage times (Fig. 5A and C). In addition, seeds stored at 22°C showed higher germination percentage and GSI (Fig. 5B and D).
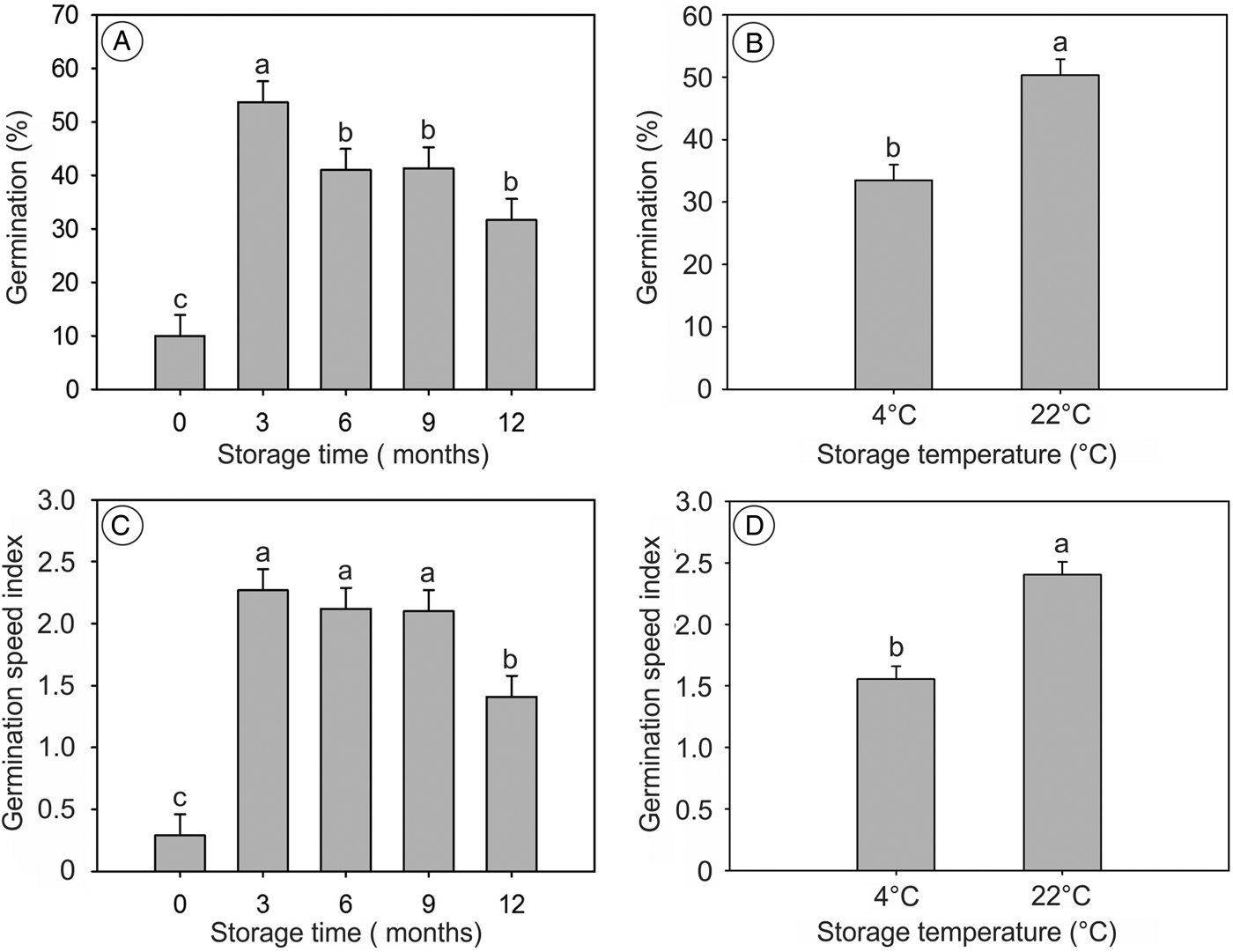
Figure 5. Germination and GSI of S. molle seeds stored at 4 and 22°C, for 12 months, with a 24-h photoperiod and scarified with H2SO4 for 1 min. In (A) and (C) the storage time zero refers to newly collected seeds. (B) and (D) show data combined for all storage times. Means followed by the same letter do not differ by the Scott–Knott test at the 5% significance level.
Seed anatomy
S. molle seeds displayed an external part containing several layers of parenchyma cells (pc) and secretory cavities (sc) surrounded by sclerenchyma. These tissues were comprised of attached parts from the mesocarp (Fig. 6). Acid scarification killed all cells in the mesocarp, and cytoplasm and cell content were lost (Fig. 6). The acid scarification also changed the seed surface, darkening and revealing open secretory cavities (Fig. 6A and B). Untreated cells were turgid and round, while acid-scarified cells showed irregular cell walls and had lost content (Fig. 6). Likewise, the secretory cavities in acid-scarified seeds were empty and surrounding cells appeared dead (Fig. 6). The endocarp consisted of three layers of sclereids surrounding the embryo (Fig. 6) plus the one-layered vestigial seed coat, as reported by Carmello–Guerreiro and Paoli (Reference Carmello-Guerreiro and Paoli2005) for Schinus species.

Figure 6. Morphological and anatomical structure of S. molle seeds. (A, C, E and G) Non-scarified seeds, and in (B, D, F and H) seeds were scarified with H2SO4 for 1 min. In (B) the endocarp and the embryo remained intact after scarification. The acid scarification removed part of the parenchyma layers in the mesocarp (arrow) compared to intact seeds (C and D). The scarification method killed all cells that still remained in the mesocarp. In these cells the cytoplasm and cell content were lost and only cell walls remained. The endocarp features three layers of palisade cells, composed of osteosclereids (ost) and macrosclereids (mac). In (F) the arrow indicates the outermost layer of the mesocarp. pc, parenchyma cells; ms, mesocarp; en, endocarp; em, embryo; sc, secretory cavity.
Histochemical studies
Tannin histochemical tests showed negative results, as positive tannin-containing vacuoles were expected to be of blue-green colour (Fig. 7A and B). However, lipids were detected within secretory cavities (as red-stained content) and although some remained present, most lipid content was removed by acid scarification (Fig. 7C and D). Lignin detection by fluorescence showed an intense response from endocarp and external cell layers of the mesocarp (Fig. 7E). The lignin content of the endocarp was not affected by scarification; however, the external layers of mesocarp showed lignin content in untreated seeds but not in the scarified ones (Fig. 7F).

Figure 7. Histochemical tests of non-scarified (A, C and E) and acid-scarified (B, D and F) Schinus molle seeds. (A) and (B) show the ferrous sulphate test for tannin (not detected). (C) and (D) show the Sudan III test for lipids; arrows indicate lipids within secretory cavities. (E) and (F) show fluorescence detection of lignin; arrows indicate the outermost layer of the mesocarp, which was removed by acid scarification (F). ms, mesocarp; en, endocarp; em, embryo; sc, secretory cavity; pc, parenchymal cells.
Dye-tracking test
The surface of S. molle seeds showed a region related to radicle protusion during germination (Fig. 8). This region was closed in untreated seeds but acid scarification promoted its opening and, hence, exposed the embryo (Fig. 8B). Figure 8C shows that only mesocarp parenchymal cells were stained. Toluidine blue cannot reach the inner parts of the seed unless it goes through the carpellary slit, were no secretory cavities were found and a suture line was visible (Fig. 8D). Therefore, seed imbibition occurs mainly in the region of the carpellary slit were mesocarp parenchyma is found.

Figure 8. The water path during imbibition of Schinus molle seeds, tracked by toluidine blue infiltration. (A, B) The seed surface at the region of radicle protrusion in untreated (A) and scarified seeds (B) after 48 h of imbibition. In the acid-scarified seeds (B) the radicle-protrusion region is open, while it is closed in untreated seeds. (C, D) The path of the toluidine blue dye throughout the mesocarp. In (D) a carpellary hilar slit (arrow) is shown and the asterisk indicates a partial break. ms, mesocarp; en, endocarp; em, embryo; sc, secretory cavity; pc, parenchymal cells.
Seed imbibition curve
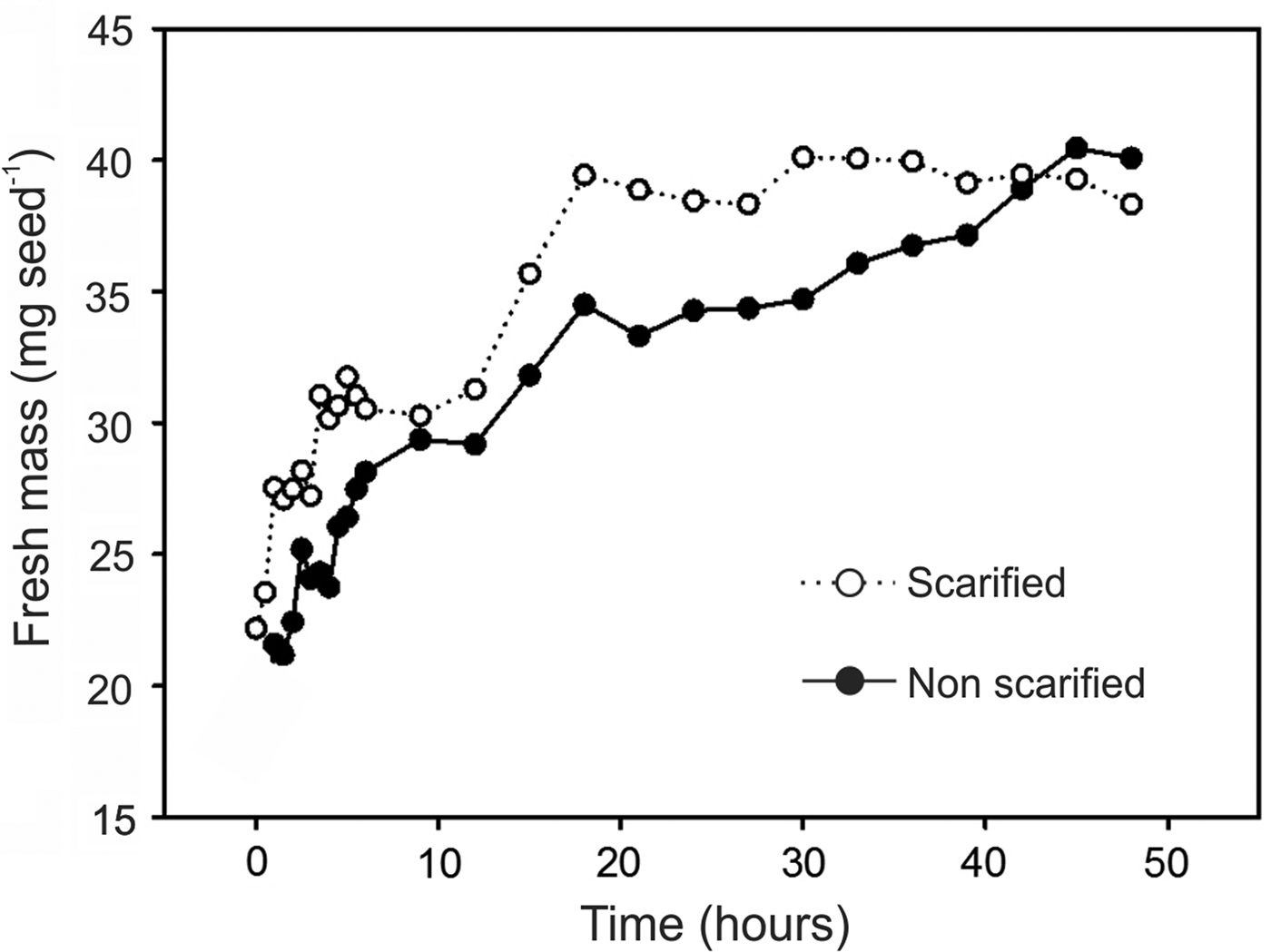
Imbibition curves showed constant increasing mass for both scarified and non-scarified seeds (Fig. 9). However, scarified seeds completed imbibition after 18 h while non-scarified ones achieved this point later, after 45 h. Soaked S. molle seeds showed an average of 40 mg fresh mass in both treatments, corresponding to twice the initial mass (Fig. 9).
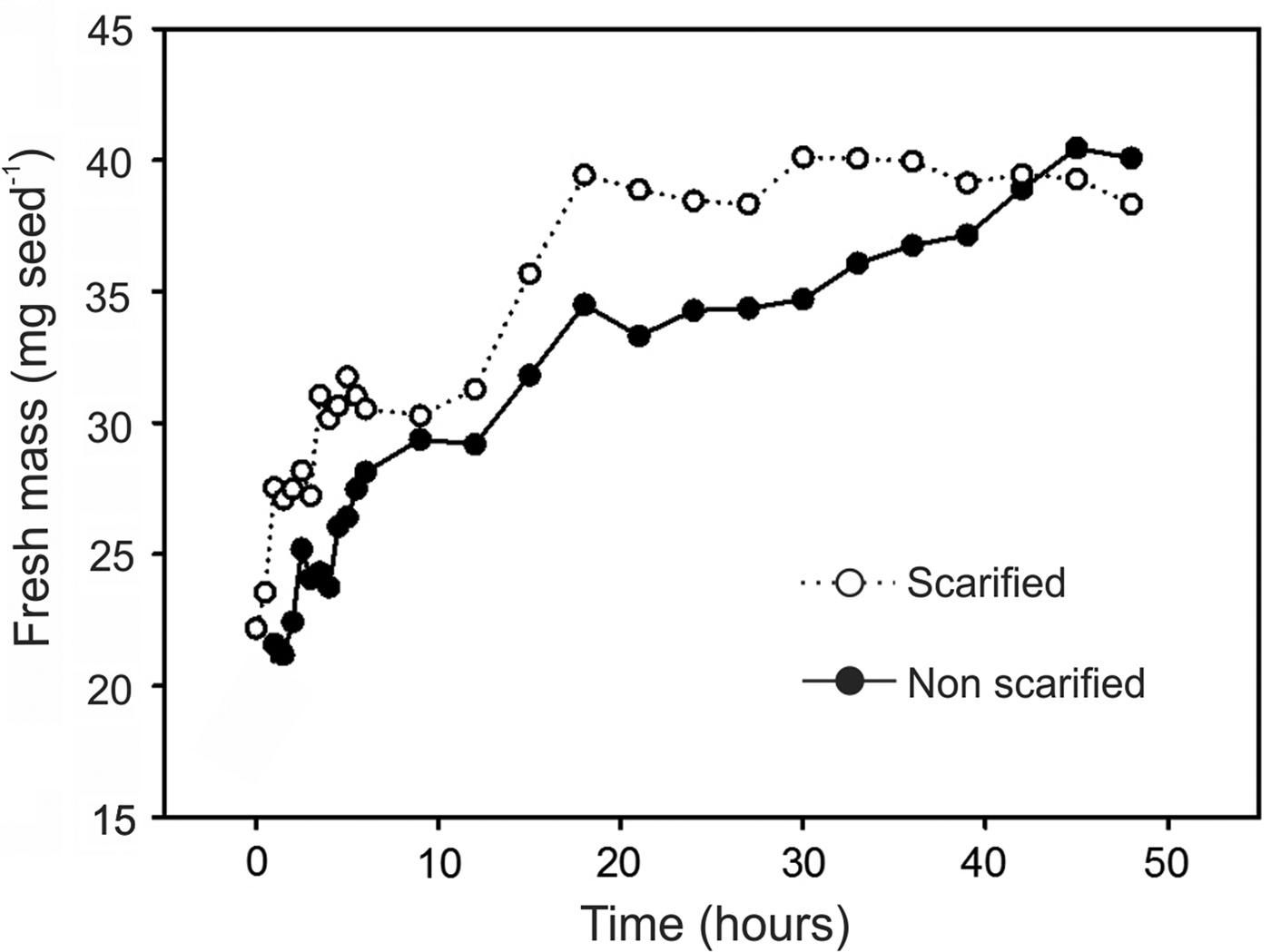
Figure 9. Imbibition curves of acid scarified and non-scarified Schinus molle seeds.
Discussion
Seeds of Anacardiaceae species, such as Rhus L., usually show physical dormancy, which is promoted by an impermeable endocarp (Li et al., Reference Li, Baskin and Baskin1999b). The three-layered endocarp containing sclereids has been reported as the main cause of seed dormancy for Schinus species (Carmello-Guerreiro and Paoli, Reference Carmello-Guerreiro and Paoli2005). However, we found that parts of the mesocarp still attached to seeds may only reduce water uptake but not really cause seed dormancy. As shown in Fig. 9, seed imbibition still occurred in non-scarified seeds, albeit at a lower rate. However, histochemical studies showed that acid scarification removed part of the lipids and the lignin of mesocarp cells. These results corroborate the work of Morfin (Reference Morfin1985) who reported the inhibition of S. molle seed germination by the mesocarp. In addition, Jøker et al. (Reference Jøker, Cruz, Morales and Rojas2002) suggested that the oil in the pericarp may inhibit germination. Likewise, according to Zahed et al. (Reference Zahed, Hosni, Brahim, Kallel and Sebei2010) the essential oil of S. molle shows allelopathic effects by inhibiting the germination of wheat seeds. Thus, removing these compounds by scarification improved imbibition speed, favouring seed germination in S. molle. Acid scarification is often reported to enhance germination in seeds with hard coats (Varela and Lizardo, Reference Varela and Lizardo2010). Fruits showing hard parts attached to seeds had improved germination after acid scarification (Li et al., Reference Li, Baskin and Baskin1999a). According to Puglia et al. (Reference Puglia, Grimaldia, Cartaa, Pavonea and Toorop2015) the pericarp of Glebionis coronaria L. is a determinant of seed dormancy and its removal by scarification enhances germination. According to Hosni et al. (Reference Hosni, Jemli, Dziri, M'rabet, Ennigrou, Sghaier, Casabianca, Vulliet, Brahimd and Sebei2011), 5.35% of the dry weight of mature S. molle fruits is composed of fatty acids and 1.15% of essential oils. All these compounds are hydrophobic and can reduce water uptake during seed imbibition. Apparently, removing the cytoplasm of parenchymal cells and contents of the secretory cavities improved imbibition speed and germination of S. molle seeds. In addition, the cell wall is comprised mainly of cellulose which is strongly hydrophilic and this may have favoured seed imbibition. As all acid-scarification treatments had the same effect, treatment for only 1 min can be used with satisfactory results for S. molle seeds. The seed imbibition curves show that acid-scarified seeds achieve quicker imbibition than non-scarified ones, which take almost three times as long to complete the process. According to Rosental et al. (Reference Rosental, Nonogaki and Fait2014), the starting point of seed germination is the imbibition process that reactivates the metabolism of dry seeds, preparing for germination. Therefore, a quicker imbibition promoted by acid scarification in S. molle seeds may have enhanced seed metabolism, improving both overall germination and seed vigour. The water path during imbibition was located only at the carpellary hilar slit, which is a common trait in seeds containing palisade layers. As the carpellary hilar slit is comprised mainly of mesocarp parenchymal cells, the removal of its lipid and lignin content may have enhanced imbibition speed. Thus, despite previous reports of physical dormancy in Anacardiaceae seeds, our results show that in S. molle no real physical dormancy is present and acid scarification only increases imbibition speed.
Methods other than acid scarification had no effect on seed germination, although decreased conductivity was found. Increased conductivity is related to the leakage of sugars, ions and other metabolites (Fessel et al., Reference Fessel, Vieira, Cruz, Paula and Panobianco2006) and this method is proposed as a test for the physiological potential of seeds (Panobianco et al., Reference Panobianco, Vieira and Perecin2007). However, in this work, scarification methods probably reduced electrical conductivity by removing parts of the mesocarp attached to seeds. According to Carmello-Guerreiro and Paoli (Reference Carmello-Guerreiro and Paoli2002) the mesocarp of Schinus terebinthifolius contains parenchyma, secretory cavities and calcium oxalate crystals. This structure is very similar to that of the S. molle seeds, and these tissues can retain sugars, ions and oils that may leak during seed imbibition, increasing electrical conductivity. Therefore, scarification removed these tissues and electrical conductivity cannot be related to seed vigour for this specific situation.
Photoperiod modulates the germination of photoblastic seeds (Oh et al., Reference Oh, Yamaguchi, Kamiya, Bae, Chung and Choi2006). Light is critical for the regulation of seed germination due to changes in the abscisic acid/gibberellin balance (Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). According to Chen et al. (Reference Chen, Cao, Baskin and Baskin2013) seeds from tropical tree species only germinate in open areas, while light availability is commonly required particularly by small seeds. Therefore, S. molle shows positive photoblastic seeds, as constant light enhanced its germination.
According to Demelash et al. (Reference Demelash, Tigabu and Odén2003), separating the seeds of S. molle using specific gravity led to a 22% improvement of germination in those that sank. We observed similar results, with 32% increased germination in seeds that sank. The separation of seeds by specific gravity is a common method to separate viable seeds (which sink) from dead or floating insect-damaged ones (Demelash et al., Reference Demelash, Tigabu and Odén2003). Likewise, we found that floating seeds consisted of non-viable embryos and large empty spaces which made them float. Thus, the separation by specific gravity together with acid scarification can effectively improve seed germination in S. molle by removing the non-filled, non-viable seeds.
Several studies reported the increase in germination following dry storage, due to the reduction of levels of inhibitors in the embryo, mainly abscisic acid (Deno, Reference Deno1993; Hidayati et al., Reference Hidayati, Baskin and Baskin2002; El-Keblawy and Al-Rawai, Reference El-Keblawy and Al-Rawai2006; Bentsink and Koornneef, Reference Bentsink and Koornneef2008). This can also be related to the increased receptiveness of signals given by gibberellins (Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). The optimal period for breaking dormancy of S. molle seeds seems to be up to 3 months, since germination decreased at longer intervals. However, even after 12 months under dry storage, the means of the germination parameters were higher than for newly collected seeds. Therefore, the seeds of S. molle have physiological dormancy that can be alleviated during dry storage. Likewise, even seeds stored for 12 months were viable. This is an important trait for reforestation, since seeds can be stored and new plants can be produced for prolonged periods of time.
S. molle seeds stored at room temperature showed a higher germination percentage and vigour as compared to those stored at 4°C. This was also reported in Arabidopsis, where higher seed storage temperatures improved germination (Basbouss-Serhal et al., Reference Basbouss-Serhal, Leymarie and Bailly2016). However, this depends on species, since Escobar and Cardoso (Reference Escobar and Cardoso2015) showed that the seeds of tropical trees show better performance when stored under low temperatures. Likewise, the physiological basis of improved germination under higher temperature is still unclear, since it depends on several traits which are specific for one particular species.
Conclusion
S. molle seeds are positively photoblastic and show physiological dormancy, which can be alleviated by acid scarification and dry storage. Seeds can be stored for over 1 year without significant decreases in germination parameters compared to newly collected seeds. Scarification enhances seed germination by the removal of lipid and lignin from mesocarp cells.
Financial support
The authors thank CNPq [Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Counsel of Technological and Scientific Development)], CAPES [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Coordination for the Improvement of Higher Education Personnel)], and FAPEMIG [Fundação de Amparo à Pesquisa do estado de Minas Gerais (Minas Gerais State Research Foundation)] for scholarships given to M.P.P., F.F.C. and A.A.C.
Conflicts of interest
None.