Introduction
Despite their sessile habit, plants select their habitats indirectly through the precise mechanisms of germination cueing to environmental conditions (Donohue, Reference Donohue2003). Undoubtedly, seed dormancy is one of the mechanisms that allow plant species to survive in temporally variable and unpredictable environments (Bulmer, Reference Bulmer1984; Rees, Reference Rees1994; Cohen, Reference Cohen1996; Tielbörger and Valleriani, Reference Tielbörger and Valleriani2005). To a great extent, germination cueing determines precisely a particular combination of environmental conditions that a germinant will encounter. Predictive germination is the optimal strategy when germination percentages increase with increasing favourability of the environment (Pake and Venable, Reference Pake and Venable1996). As an adaptive mechanism, dormancy increases the reproductive success of the mother plant.
The seed germination response to ecological factors directly impacts establishment, distribution and abundance of species, since it is a key element affecting population dynamics, especially in semi-arid and arid environments (Valverde et al., Reference Valverde, Quijas, López-Villavicencio and Castillo2004). In particular, temperature is regarded as a key factor regulating seed dormancy and germination (Baskin and Baskin, Reference Baskin and Baskin1988; Gutterman, Reference Gutterman1993; Moot et al., Reference Moot, Scott, Roy and Nicholls2000; Probert, Reference Probert and Fenner2000). Soil moisture is the most important factor controlling seed germination and plant establishment in arid environments (Koller, Reference Koller1969; Gutterman, Reference Gutterman1990). However, soil moisture must be adequate for germination when temperatures are favourable for germination of non-dormant seeds, and this is especially critical in arid environments (Beatley, Reference Beatley1974; Ghazanfar, Reference Ghazanfar1997; Pías and Guitián, Reference Pías and Guitián2001; Ma and Tan, Reference Ma and Tan2007). Further, light may play a role in regulating germination after dormancy is broken (Koller et al., Reference Koller, Sachs and Negbi1964; Rojas-Aréchiga et al., Reference Rojas-Aréchiga, Orozco-Segovia and Vázquez-Yanes1997; Huang and Gutterman, Reference Huang and Gutterman1999, Reference Huang and Gutterman2000; Pons, Reference Pons and Fenner2000; Flores et al., Reference Flores, Jurado and Arredono2006).
Since germination cueing to time(s) when the environment is favourable for seedling establishment and subsequent growth and development of plants is an important adaptation of species to their habitat, there has been much interest in germination of desert species. However, we know little about timing of germination in seeds of desert ephemerals. Ephemeral (perennial) species are a particular component of desert floras that can take advantage of water resources and temperature conditions in spring to complete their life cycle rapidly in about 2 months. They mainly occur in Central Asia, the Junggar Basin of China, the Mediterranean coast, West Asia and North Africa (Mao and Zhang, Reference Mao and Zhang1994). In China, desert spring ephemerals occur only in northern Xinjiang Province, with the eastern edge of the Junggar Basin as the easternmost limit of distribution (Mao and Zhang, Reference Mao and Zhang1994). In the Gurbantunggut Desert, which is located in the hinterland of the Junggar Basin, Wang et al. (Reference Wang, Jiang, Lei, Zhang and Qian2003) found 45 ephemeral species, including ephemeretum species and ephemeroid species, acting as the major contributors to dune surface stabilization and being components of the pioneer vegetation. As important and unique components of the Chinese desert flora, ephemeral species have attracted the attention of botanists, but their work has focused mostly on the flora (Liu, Reference Liu1982), plant geography (Mao and Zhang, Reference Mao and Zhang1994), phenology (Wang, Reference Wang1993; Ma et al., Reference Ma, Liu and Tan2006; Ma and Tan, Reference Ma and Tan2007), and characteristics of fecundity (Liu et al., Reference Liu, Zhang, Qin, Jiang, Lan and Zhang2007). Recently, ecological investigations on seed dormancy and germination of desert species have increased (Tobe et al., Reference Tobe, Zhang and Omasa2006; Wesche et al., Reference Wesche, Pietsch, Ronnenberg, Undrakh and Hensen2006; Tang et al., Reference Tang, Tian and Long2009), but we still know little about ephemeral herbaceous perennial species. Thus, the purpose of our work was to investigate seed dormancy and germination in three ephemeral species in the deserts of the Junggar Basin, China.
In our study, we used Ixiolirion tataricum (Pall.) Herb. (Amaryllidaceae), Tulipa iliensis Rgl. (Liliaceae) and Scorzonera pusilla Pall. (Compositae) as target species. I. tataricum and T. iliensis are both important wild flower resources (Yin et al., Reference Yin, Zhang, Wei and Yan2004). I. tataricum and T. iliensis have bulbs, and S. pusilla has a tuber. When aerial parts wither in early June, the underground organs remain alive, and new shoots from underground buds emerge next spring. Seeds are produced in May and are dispersed as the shoots wither. Our purpose was to investigate seed dormancy and germination. Since phenological data for more than 60 ephemerals indicated that germination begins in late March in the Gurbantunggut Desert (Wang et al., Reference Wang, Jiang, Lei, Zhang and Qian2003; Ma et al., Reference Ma, Liu and Tan2006; Zhang and Tan, Reference Zhang and Tan2007), we hypothesized that seeds of the three study species would germinate in spring and that temperature and soil moisture conditions of spring would be important aspects of the cueing for germination to occur in early spring.
Materials and methods
Habitat description
Our three chosen ephemeral species grow in sandy, stable dunes along with shrubs such as Tamarix ramosissima Ledeb. at the foot of the Jiangjun Mountain in the southern part of the Junggar Basin, about 10 km north of Shihezi City, Xinjiang, China (44°11.77′N, 86°5.16′E, altitude 630–660 m; annual precipitation, c. 202 mm; annual mean temperature, 6.8°C; the hottest month is July with a mean temperature between 25.1 and 26.1°C). In the habitat, 71.5% of the annual precipitation falls between April and October and 39% between April and June (1990–2001), with usually < 17 mm falling between August and November. It snows from early November to mid-February with a total accumulation of about 60 mm, which is 32% of total annual precipitation (http://qxg.com.cn/n/?cid = 45&nid = 19668&fc = nd). After about mid-March, snow and ice begin to melt and moisture level in the sandy soil gradually increases.
Seed collection and germination tests
Mature seeds of I. tataricum, T. iliensis and S. pusilla were collected from one natural population per species on 24 May 2006 and on 29 May 2008. In 2006, seeds were used after 2 months of dry storage at 4°C. Initial seed water content based on gravitational measurement, according to ISTA (1999), was < 9%. For each species, 1000-seed weights were determined. In 2008, some freshly harvested seeds were tested immediately after collection, and the other seeds were placed in dry storage at 4°C. For each species, freshly matured and dry-stored seeds were surface sterilized with sodium hypochlorite (0.5%, w/v) for 10 min. Four replicates of 50 seeds each were placed on moist (distilled water) filter paper in 9-cm-diameter Petri dishes in incubators equipped with a warm fluorescent light with a red:far-red (R:FR) ratio of 14.5 (HPG-280B Illuminating Incubator; Haerbin Electronic Apparatus Manufactory, Haerbin, China). Photosynthetic photon flux density (PPFD) was approximately 40 μmol m− 2 s− 1 under the 14-h daily photoperiod. Germination was recorded daily, and seeds were considered germinated when the radicle emerged from the seed coat. For the three species studied, the time from the beginning to the end of germination varied from 4 to 5 weeks (unless otherwise specified), depending on germination conditions. Seeds were tested for germination at constant temperatures of 4, 5, 10, 15, 20, 25, 30, 35°C, or 14-h fluctuating cycles of 10/4°C or/and 15/4°C (day/night). To determine the effects of light on germination, seeds were given red and far-red light treatments and also placed in darkness. For incubation in darkness, Petri dishes containing 2-month-old dry-stored (afterripened) seeds were wrapped with four layers of black cloth. For red and far-red light treatment, Petri dishes with afterripened seeds were put in an incubator equipped with red lamp-houses or far-red lamp-houses (R and FR, 65 μmol m− 2 s− 1) (LED-R, Tokyo Rikakikai Co., Ltd, Japan). For white light treatments, the germination test was performed in the incubator described above. A green safe light (Walck et al., Reference Walck, Baskin and Baskin2000) was used to examine the dark-incubated seeds.
Effects of water availability on germination
To determine the effects of water potentials on germination, solutions of polyethylene glycol (PEG) 8000 were prepared to produce water potentials (0, − 0.49, − 1.0, − 1.54 and − 3.17 MPa); these were determined using an isopiestic psychrometer at 5°C (Boyer and Knipling, Reference Boyer and Knipling1965). Then, 10 ml of the PEG-8000 solution was placed into each Petri dish containing two pieces of Whatman No.1 filter paper and 20 seeds. Each Petri dish was wrapped with transparent plastic film to maintain a constant humidity. The water potential inside Petri dishes with distilled water was assumed to be 0 MPa. Germination tests were carried out in light at 5°C For all tests, afterripened (2006) seeds were used.
To determine the effects of soil moisture content on germination, sandy top soil (e.g. 0–10 cm) was collected from the habitat of the three species. The soil was dried at 103°C for 24 h and then triturated with a glass pestle. Later, a portion of the pretreated soil was weighed and placed into 9-cm-diameter glass Petri dishes. Distilled water was added to the soil to obtain a specific moisture content of 3.1%, 5.3%, 12.7%, 15.6%, 17.1% or 21.4%, and then seeds were sown on the soil. Four replicates of 20 fresh (2008) seeds and of 20 dry stored (2008) seeds were used. All Petri dishes were wrapped with a transparent plastic bag to reduce evaporation. Petri dishes were placed in an incubator and maintained at 12-h 10°C (light)/12-h 4°C (dark), 70–85% relative humidity (RH), and PPFD of 40 μmol m− 2 s− 1 at the soil level from a fluorescent lamp during the photoperiod. Germinated seeds were counted every 3 d for 30 d.
Statistical analyses
Prior to analysis, percentages were arc–sin square-root transformed. However, values in Figs 1 and 3 are of untransformed data. Means were compared by both the least significant difference (LSD) intervals method at P < 0.05, and one-way ANOVA and Duncan multiple comparison tests, carried out using SPSS 12.0 software package (SPSS Inc., Chicago, Illinois, USA).
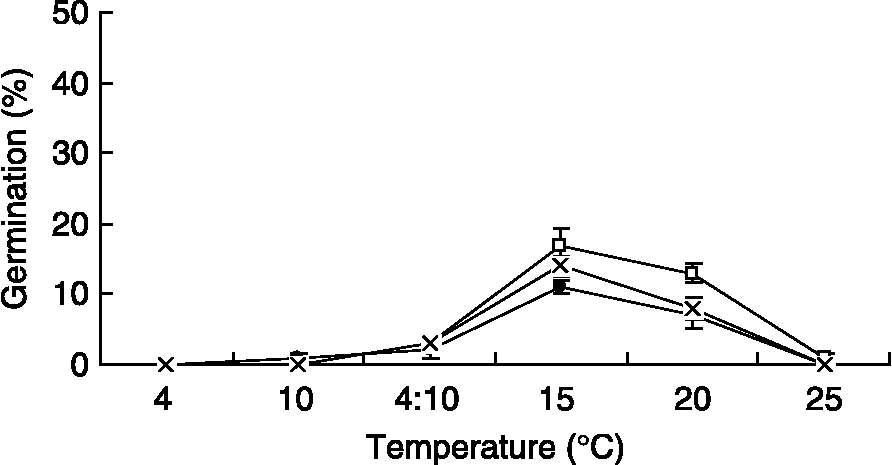
Figure 1 Final germination percentages of freshly matured seeds of Ixiolirion tataricum (●), Tulipa iliensis ( × ) and Scorzonera pusilla (□) at various temperatures in a 14-h photoperiod. Values are means ± SD of four replicates. Bars indicate ± SD.
Results
Seed dormancy and effects of temperature on germination
Fresh seeds (1000-seed weights were 3.4 ± 0.1 g, 2.2 ± 0.3 g and 5.7 ± 0.3 g for S. pusilla, T. iliensis and I. tataricum, respectively), which were harvested in 2008, germinated to ≤ 17% at all temperatures tested (Fig. 1). However, 2-month dry-stored seeds of the three species in 2006 germinated almost completely at 4°C (Fig. 2). In detail, I. tataricum germinated to 2, 67, 97 and 100% after 9, 25, 30 and 32 d, respectively. T. iliensis germinated to 1, 57, 79 and 96% after 7, 25, 30 and 39 d, respectively. Also, S. pusilla germinated to 1, 58, 71 and 93% after 7, 25, 30 and 35 d, respectively.
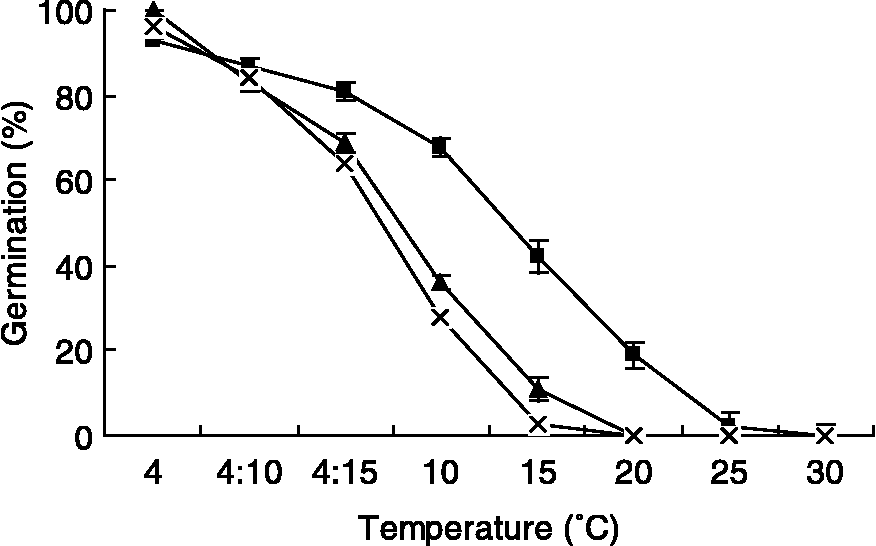
Figure 2 Effect of temperature on final germination percentages of seeds of Ixiolirion tataricum (▲), Tulipa iliensis ( × ) and Scorzonera pusilla (■) stored dry for 2 months and then incubated over a range of temperatures for 60 d in light/dark. Values are means ± SD of four replicates. Bars indicate ± SD.
Temperature had significant effects on the final germination percentages of the three species (F = 9.582, P < 0.0001). For each species, the germination percentage was much higher ( ≥ 93%) at 4°C than that at other temperatures. Inhibitory effects of temperature increased with a temperature increase from 15 to 30°C in S. pusilla and from 4 to 10°C in the other two species (Fig. 2).
Effects of light on germination
There was no significant difference in final germination percentages among the three light-quality treatments (F = 0.126, P>0.05). Germination percentage was 81%, 84% and 78% in white light, red light and darkness, respectively, for S. pusilla; 96%, 96% and 94%, respectively, for I. tataricum; and 92%, 94% and 91%, respectively, for T. iliensis. In addition, light did not significantly affect the germination speed of any of the three species (data not shown).
Effects of water availability on germination
Water potential had significant effects on final germination percentages and germination rates of each of the three species tested at 5°C in light (F = 206.4, P < 0.0001). When water potentials were reduced, final germination percentage decreased dramatically, and no seeds germinated at − 3.17 MPa. Germination percentages were the highest in controls, followed by − 0.49 and − 1.0 MPa (Fig. 3). Moreover, the germination rates of the three species at 0 MPa and − 0.49 MPa were significantly different in time of the onset and end of germination (Fig. 4). Also, soil moisture content strongly impacted final germination percentages of all the three species, and there were significant differences in final germination percentages among different soil moisture contents (Fig. 5). When the soil moisture content increased from 3.1% to 17.1%, the final germination percentages of seeds of these three species gradually increased, but it decreased abruptly between 17.1% and 21.4%.

Figure 3 Germination percentages of seeds of the three species after 60 d at various water potentials. For each species, significant differences (P < 0.01) between treatments according to Duncan's multiple comparisons test are indicated by different lower-case letters. Values are means ± SD. Bars indicate ± SD.

Figure 4 (A) T min (d) and (B) T max (d) for seed germination of Scorzonera pusilla, Ixiolirion tataricum and Tulipa iliensis at two water potentials (0 and − 0.49 MPa). Values with the same superscript letters are not significantly different among treatments for a species at P < 0.05. Values are means ± SD. Bars indicate ± SD.
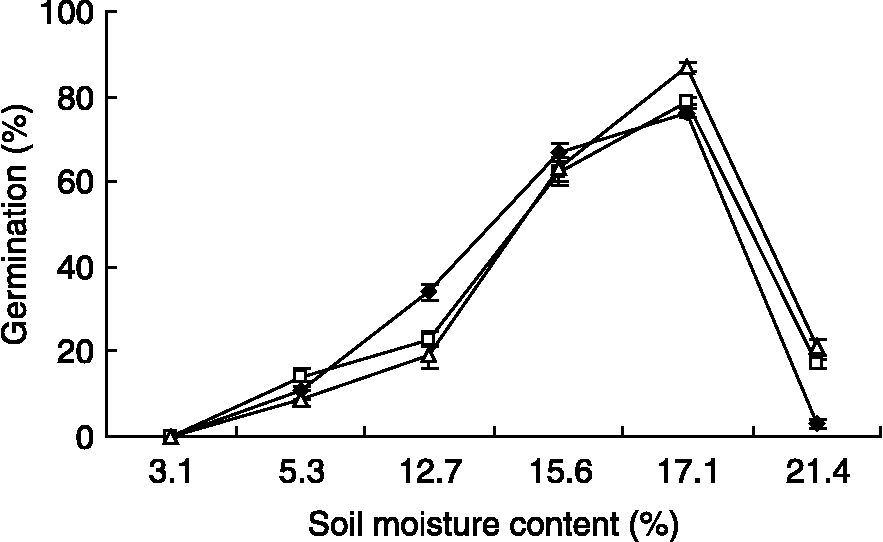
Figure 5 The final germination percentages of Scorzonera pusilla (♦), Ixiolirion tataricum (△) and Tulipa iliensis (□) at various water contents. Values are means ± SD. Bars indicate ± SD.
Discussion
Freshly matured seeds of the three ephemerals were dormant (Fig. 1), but seeds of the three species stored dry for 2 months at 4°C and subsequently tested for germination at 4°C germinated to 93–100%. This sequence of temperatures somewhat simulated the conditions occurring in the field from late autumn to spring. That is, the soil in the field is cold and dry in late autumn, and then, after it snows, presumably seeds are imbibed at temperatures near 0°C, which are effective for cold stratification. In our study, some dormancy breaking may have taken place via afterripening while seeds were dry at 4°C. Also, with the exception of I. tataricum, considerable dormancy break may have occurred during the germination test at 4°C (Fig. 4). Germination of seeds of the three species at low temperatures is consistent with observations made by Pan and Huang (Reference Pan and Huang1995) that seeds of many ephemerals germinated following a very cold winter. Low germination for seeds given 2 months of dry storage at 4°C and then moved to 15°C is probably due to the fact that 15°C is usually too high to be effective for cold stratification (Baskin and Baskin, Reference Baskin and Baskin1998).
Based on the family to which the three species belong, it is likely that seeds of I. tataricum and T. iliensis (Amaryllidaceae and Liliaceae, respectively) have morphophysiological dormancy, and those of S. pusilla (Asteraceae) physiological dormancy (Baskin and Baskin, Reference Baskin and Baskin1998). Sun and Fang (Reference Sun and Fang2000) showed that seeds (with underdeveloped embryos) of 13 species of Liliaceae have morphophysiological dormancy which was broken by cold stratification. Gao et al. (Reference Gao, Li and Xiao1997) showed that dormancy in seeds of Fritillaria thunbergii (Liliaceae) was broken by a 50-day cold stratification period. Also, Zong et al. (Reference Zong, Liu, Bu, Xu and Wu2006) found that 51 species of Asteraceae occurring in an alpine meadow produce physiologically dormant seeds. However, no studies on dormancy class in seeds of T. iliensis, I. tataricum and S. pusilla have been performed.
The light–dark condition had little effect on the germination of I. tataricum, T. iliensis and S. pusilla, i.e. seeds of these species germinated equally well in darkness and when given far-red/red light treatments. Thus, it is expected that non-dormant seeds of the three species could germinate under soil in the field, but this has not been tested. In contrast, Pan and Huang (Reference Pan and Huang1995) reported that seeds of four ephemeral species which grow in the Junggar Basin did not germinate in continuous darkness. Tang et al. (Reference Tang, Tian and Long2009) also pointed out that in Olimarabidopsis pumila (or Arabidopsis pumila), a spring-type ephemeral species in the Gurbantunggut Desert, seed germination was sensitive to light.
In desert environments, where rainfall is rare and uncertain, glycophytes and halophytes respond to water stress in a similar way during the germination stage, i.e. the germination process is delayed by water stress (Khan and Ungar, Reference Khan and Ungar1997). Species with seeds that germinate at low water potentials have the advantage of becoming established in areas where species with drought-sensitive seeds cannot. Wesche et al. (Reference Wesche, Pietsch, Ronnenberg, Undrakh and Hensen2006) reported that seed germination of 26 species from Central Asian steppes depends on rains that are restricted to the summer months between June and August. In the present study, rainfall is infrequent, in particular, from July to the next early March. It is expected that germination of all three species is almost completely suppressed by very low temperature ( < 0°C) in winter (and presence of seed dormancy) when it mostly does not rain but snows. As expected, germination of seeds of the three species was reduced/inhibited by decreased water potentials (Figs 3 and 4) and at low soil moisture contents (Fig. 5). In the field, although there is a very small quantity of rainfall in autumn, wind makes the top soil layer dry very quickly so that water availability is greatly limited for germination. In fact, in the study area soil water content of the top layer (0–5 cm) was < 9% in both October and November (Tang, Reference Tang2008). Therefore, water stress becomes a limiting factor for germination. In view of the rigorous climatic conditions in the field, the probable period for seed germination of the three species is between late March and mid-April, when sufficient water coincides with favourable temperatures and seed dormancy has been broken by cold stratification.
Acknowledgements
We thank Professor Yan Ping, Mr Zhai Wei and Ma Zhen (Shihezi University) for their help in the field. We received necessary assistance from He Huiying and Lan Qinying (Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences) for different aspects of this study. We are very grateful to Carol and Jerry Baskin (University of Kentucky, Lexington, Kentucky, USA) for their critical comments and editing the English. This study was supported financially by the Ministry of Education of China (B08044 & MUC 985-3-3), and the Ministry of Science and Technology of China (2005DKA21006).







