Introduction
Cultivated rice (Oryza sativa L.) was domesticated from the Asian wild species O. rufipogon Griff. (Oka, Reference Oka1988). O. rufipogon grows mainly in swamps, ditches, canals and ponds under natural tropical conditions (Fig. 1 and Supplementary Fig. S1). Compared with cultivated rice, wild rice has spikelets with long barbed awns (bristle-like organs at the tips of the lemmas) on panicles and the matured grains with hulls (hereafter seeds) shed by themselves (Fig. 2). Many studies have been carried out to clarify the genetic regulation of seed awning using interspecific populations between wild and cultivated rice species, and three major loci, namely An-1, LABA1 and RAE2, were identified (Luo et al., Reference Luo, Liu, Zhou, Gu, Huang, Shangguan, Zhu, Li, Zhao, Wang, Zhao, Wang, Wang, Sang, Wang and Han2013; Hua et al., Reference Hua, Wang, Tan, Fu, Liu, Xiao, Zhu, Fu, Sun, Gu, Cai, McCouch and Sun2015; Bessho-Uehara et al., Reference Bessho-Uehara, Wang, Furuta, Minami, Nagai, Gamuyao, Asano, Angles-Shim, Shimizu, Ayano, Komeda, Doi, Miura, Toda, Kinoshita, Okuda, Higashiyama, Nomoto, Tada, Shinohara, Matsubayashi, Greenberg, Wu, Yasui, Yoshimura, Mori, McCouch and Ashikari2016). At each locus, the wild functional allele induces awn formation. In addition, wild rice has some other minor genes for seed awning, and an awnless phenotype is not produced by a single mutation in wild rice (Ikemoto et al., Reference Ikemoto, Otsuka, Thanh, Phan, Ishikawa and Ishii2017; Amarasinghe et al., Reference Amarasinghe, Kuwata, Nishimura, Phan, Ishikawa and Ishii2020). This suggests that wild rice has a mechanism to prevent awn length reduction by the multiple genetic controls.

Fig. 1. Wild rice grown in tropical Asia. (A) A large population near a paddy field in Phnom Penh, Cambodia. (B) A small patch beside a ditch in Cantho, Vietnam.

Fig. 2. Awns of wild rice. (A) Maturing seeds on the panicles. Left: O. sativa cv. IR36. Right: O. rufipogon acc. W630. Scale bar = 5 cm. (B) Micro-barbs observed on the surface of a wild awn. Micrometer unit = 1 mm. (C) Upper side of a wild seed. The same magnification as in (B).
In wild plants, long awns assist seed dispersal. Chambers and MacMahon (Reference Chambers and MacMahon1994) defined two phases of seed dispersal: seed movement from the parent plant to a surface (Phase I) and subsequent horizontal or vertical movement (Phase II). In wild wheat, awns propel the seeds on the ground and silica hairs covering the awns push the seeds into the soil during the daily humidity cycle (Elbaum et al., Reference Elbaum, Zaltzman, Burgert and Fratzl2007). Similarly, awns with micro-barbs have been found to promote seed dispersal and burial in wild foxtail grass (Kulic et al., Reference Kulic, Mani, Mohrbach, Thaokar and Mahadevan2009; Wolgemuth, Reference Wolgemuth2009). In wild rice, mature seeds have strong dormancy enabling survival in the tropical dry season, and they germinate the following rainy season (Oka, Reference Oka1988). The efficient dispersal of the seeds directly contributes to the expansion of the distribution area in the next generation. Wild rice has seeds with long barbed awns; however, we do not well-understand their roles on seed dispersal under natural conditions.
In the present study, we prepared seeds with different awn lengths and conducted seed dispersal experiments under simulated natural conditions as observed in the tropical Asian habitat. In Phase I of seed dispersal, seed detachment from the parent plant and seed falling angle in the air were examined. In Phase II, seed slipping ability into small spaces and horizontal movement on the ground and in water were investigated. In each condition, seed dispersal performance was quantified to clarify the roles of awns in wild rice.
Materials and methods
Plant material
A long-awned accession of O. rufipogon W630, provided by the National Institute of Genetics, Japan, was used in this study. This accession was previously examined in domestication and genetic studies (Ishii et al., Reference Ishii, Numaguchi, Miura, Yoshida, Thanh, Htun, Yamasaki, Komeda, Matsumoto, Terauchi, Ishikawa and Ashikari2013; Ikemoto et al., Reference Ikemoto, Otsuka, Thanh, Phan, Ishikawa and Ishii2017).
Seed dispersal experiments
In tropical Asia, O. rufipogon grows mainly in wet areas, and the matured seeds naturally shed on the ground or into shallow water. To evaluate the roles of wild rice awns in seed dispersal in Phases I and II, the following five conditions were set for the flowering spikelets and mature seeds with different awn lengths.
(1) Seed detachment from the parent plant (Phase I). The first seed movement from the parent plant (Phase I) is a detachment of matured seed from the panicle. In wild rice, panicle flowering begins with spikelets in the upper part of the panicle and is complete in about 10 days. And it takes about 2 weeks for each seed to mature. To evaluate the role of awns in seed detachment from the parent plant, 16 plants of O. rufipogon W630 were grown in pots under natural conditions. From August 21–28, two panicles that had started flowering were selected from each plant, and the awns of the spikelets were cut to give four kinds of awn lengths; full, half, quarter and no awns (Fig. 3). In each awn treatment, eight panicles (two panicles each from four plants) were prepared, and the spikelets that flowered in the first 7 days were painted different colours to mark the flowering dates. Then, the days to shedding from flowering were examined for the matured seeds by daily observation. The average value of two panicles was taken as the days to seed shedding for each plant, and the overall averages were compared among the four groups with different awn lengths.
(2) Falling angle of mature seed on muddy ground (Phase I). Wild rice plants grow in swampy areas and panicles emerge vertically at a heading stage (Fig. 1). Their mature seeds sometimes fall on muddy ground (Supplementary Fig. S1A, B). In rice, embryos develop in the glumes on the side opposite the awns. Therefore, the angles of inclination to the ground are important for seeds to self-plant the embryo side down on the muddy surface. To examine whether the awns are associated with the falling angles of mature seeds, long awns (about 10 cm) of mature seeds were cut, and four seed groups with different awn lengths were prepared: full (about 10 cm), half (about 5 cm), quarter (about 2.5 cm) and no (0 cm) awns (Fig. 4). The seeds were dropped in a vertical position from 1 m above the muddy ground (Supplementary Fig. S2A), and the angles of inclination to the ground were scored on a scale of 0–5: 0, 0–15°; 1, 15–30°; 2, 30–45°; 3, 45–60°; 4, 60–75°; 5, 75–90° (Supplementary Fig. S2B, C). The average score of ten seeds was calculated for each awn treatment with ten replications.
(3) Ability to slip into small spaces covered by plants (Phase II). Wild rice is often found in weedy wetland areas. The shed seeds must pass through the surface covered with stems and leaves of other plants (Supplementary Fig. S1C, D). This corresponds to the vertical movement of seed dispersal in Phase II. To inspect the ability of awned seeds to slip into small spaces, an apparatus with four layers (at either 2- or 4-cm intervals) of plastic mesh was prepared (Supplementary Fig. S3). The mesh size was 4 mm, which exceeded the seed width (about 2.5 mm) but was less than the seed length (about 7.5 mm). Seeds with four variations of awns were dropped in a vertical position from 1 m above the apparatus. Slipping ability was scored based on the number of mesh sheets passed through, namely 0 (stopped on the first mesh) to 4 (passed all four mesh sheets). The seed trial (ten seeds with ten replications for each awn group) was the same as in the above falling angle experiment.
(4) Horizontal movement on the ground (Phase II). A fourth experiment was conducted to examine the horizontal movement of awned seeds on the ground. In the dry season, wild rice seeds are often found half-buried in the ground (Supplementary Fig. S1E, F). Wild rice has many small upward silica barbs on the awn surface, and these barbs direct the direction and movement of the seeds (Hua et al., Reference Hua, Wang, Tan, Fu, Liu, Xiao, Zhu, Fu, Sun, Gu, Cai, McCouch and Sun2015). The horizontal movement of awned seeds was examined using a reciprocal shaker with a swing of 5 cm (SR-1; TAITEC Co., Japan). Because awnless seeds move easily by rolling on a flat surface, a V-shaped plastic rod (2 × 2 cm at the edge, 80 cm in length) covered with filter papers (3MM; Whatman, UK) was set on the shaker (Supplementary Fig. S4). First, seed movement was checked at three shaking speeds (150, 175 and 200 rpm) using 100 seeds each for the four awn treatments: full, half, quarter and no awns. Then, at two appropriate speeds (175 and 200 rpm), the average velocity (in 80 cm) of ten moving seeds was calculated for each awn group, with ten replications (Supplementary Movie S1).
(5) Horizontal movement in water (Phase II). The mature seeds of wild rice growing near a small river or ditch usually drop into the water (Supplementary Fig. S1G, H). Seeds are also covered with water in flooded areas. These seeds may spread horizontally with water movement. Therefore, an experiment on seed movement in water was designed using a circulating water apparatus (Nagashi-Somen; Kano Co., Japan) (Supplementary Fig. S5). For two water speeds (5 and 15 cm s−1), the velocity of the seeds with four length variations of awns (full, half, quarter and no awns) was estimated based on the distance moved in 60 s (Supplementary Movie S2). The average velocity of five seeds was first calculated for each awn treatment, and the overall averages were compared with four replications.
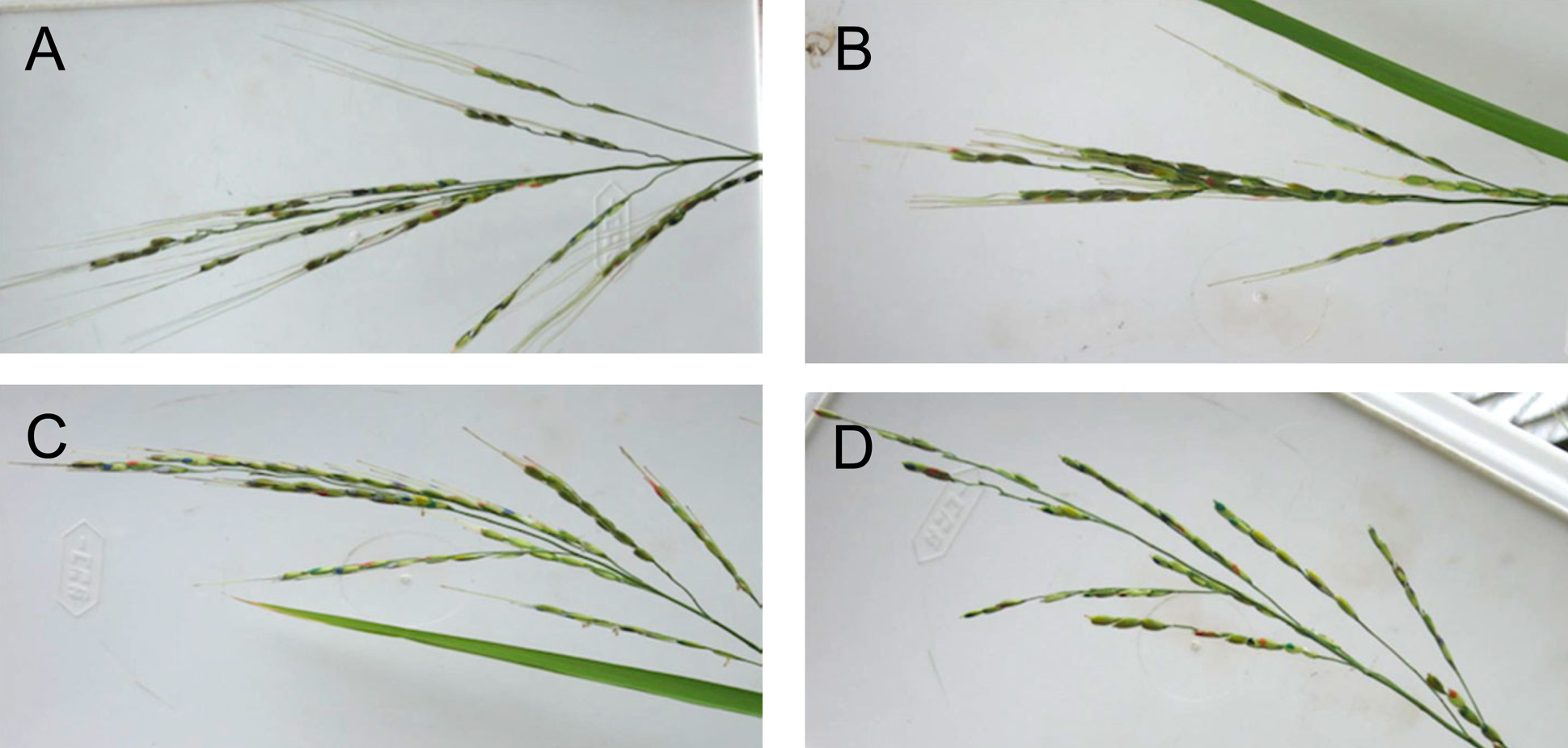
Fig. 3. Panicles observed for seed detachment. Awns of spikelets were cut to give four awn lengths: (A) full, (B) half, (C) quarter and (D) no awns. Spikelets were painted different colors to mark the flowering dates.
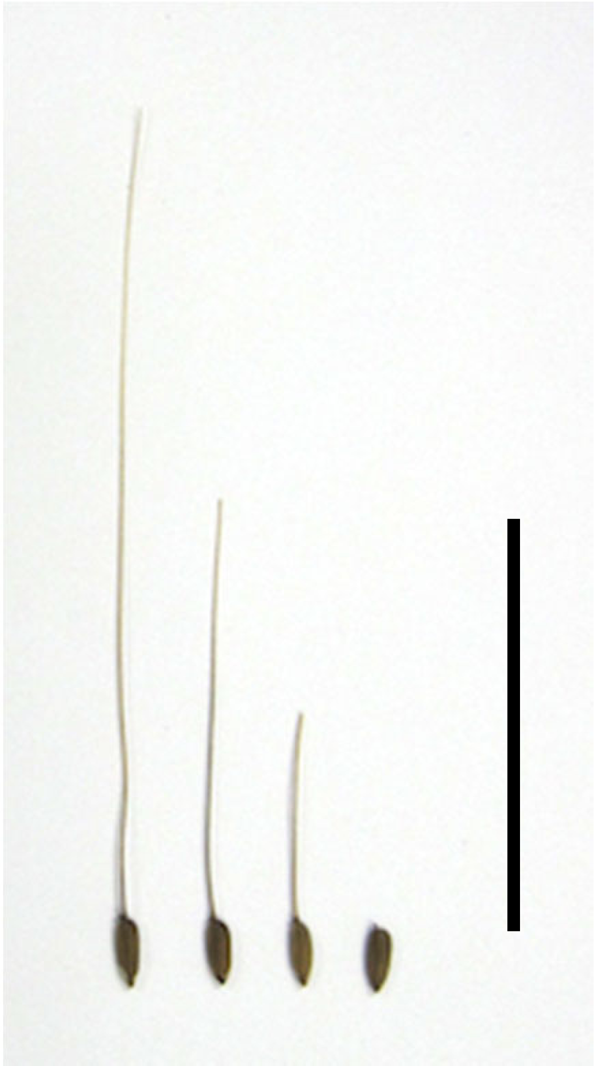
Fig. 4. Seeds treated to give different awn lengths: full, half, quarter and no awns. Scale bar = 5 cm.
Statistical analysis
The significance of the overall averages among the four groups with different awn lengths was tested using the Tukey–Kramer test, with P < 0.05 indicating statistical significance.
Results
Seed dispersal experiments
(1) Seed detachment from the parent plant. A total of 1799 spikelets (or fertile seeds) from 32 panicles were observed (Supplementary Table S1), and the average numbers of spikelets in a panicle for the full, half, quarter and no awn treatments were 55.6, 62.1, 53.8 and 53.4, respectively. The plant group with no awns had the longest average days to seed shedding (15.41 days), followed by that with quarter awns (14.72 days). Similar values were observed for the groups with half (14.46 days) and full (14.49 days) awns. The days to seed shedding were significantly longer (P < 0.05, Tukey–Kramer test) only for the group with no awns (Fig. 5A).
(2) Falling angle of mature seed on muddy ground. Supplementary Table S2 summarizes the scores of the experiment on seed falling angle. The scores for the full, half, quarter and no awn groups were 4.51, 4.08, 2.94 and 0.40, respectively. The falling angle scores of the full and half awn groups were similar, while the quarter and no awn groups, each differed significantly from the others (Fig. 5B).
(3) Ability to slip into small spaces. Supplementary Table S3A shows the average scores of ten seeds for the four awn groups. In both experiments at 2- and 4-cm intervals, the scores of the full and half awn groups exceeded 3.16, showing that many seeds passed all mesh sheets. For the seeds with quarter awns, the overall average scores were 2.73 and 2.09 at 2- and 4-cm intervals, respectively. On the other hand, most awnless seeds could not pass the first mesh sheet at both intervals, the scores for no awns were less than 0.4. At both intervals, the full and half awn groups did not differ significantly, but detected the quarter and no awn groups differed significantly (P < 0.05, Tukey–Kramer test; Fig. 5C, D). The t-tests were additionally carried out between the scores at two intervals in each awn group. The significant result (P < 0.01) was detected only for the seeds with quarter awn length (Supplementary Table S3B).
(4) Horizontal movement on the V-shaped rod. At first, seed movement was compared at three shaking speeds (Supplementary Table S4A). We observed almost half of the awnless seeds did not move at 150 rpm. Therefore, the average velocity (for 80 cm) of the moving seeds at 175 and 200 rpm was further calculated for each awn group (Supplementary Table S4B). Compared to the quarter and awnless seeds, significantly higher seeds (about 14 cm s−1 at 175 rpm and about 20 cm s−1 at 200 rpm; P < 0.05, Tukey–Kramer test) were recorded for the seeds with full and half awns at both shaking speeds (Fig. 6A, B).
(5) Horizontal movement in water. The averages for the four groups with different awn lengths are shown in Supplementary Table S5. At a water speed of 5 cm s−1, only full awn seeds moved smoothly, whereas all seeds moved at a speed of 15 cm s−1. The average seed velocities were significantly faster (P < 0.05, Tukey–Kramer test) in the full awn group at a water speed of 5 cm s−1 and the full and half awn groups at a water speed of 15 cm s−1 (Fig. 5C, D).
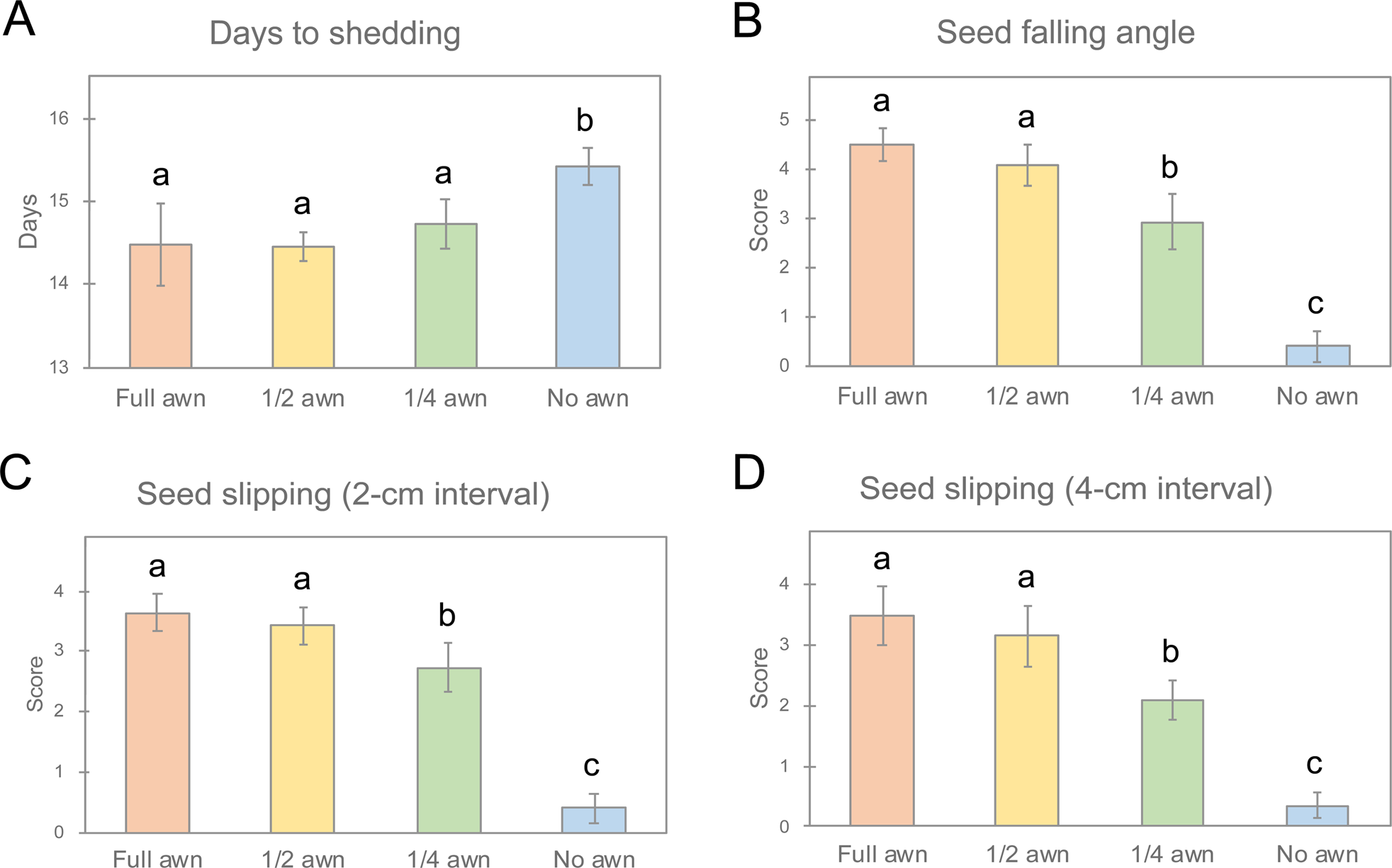
Fig. 5. Comparisons of the means of the groups with full, half, quarter and no awns. (A) Days to shedding. (B) Seed falling angle. (C, D) Seed slipping scores at 2- and 4-cm intervals, respectively. Means labelled with different letters differ significantly (Tukey–Kramer test, P < 0.05).
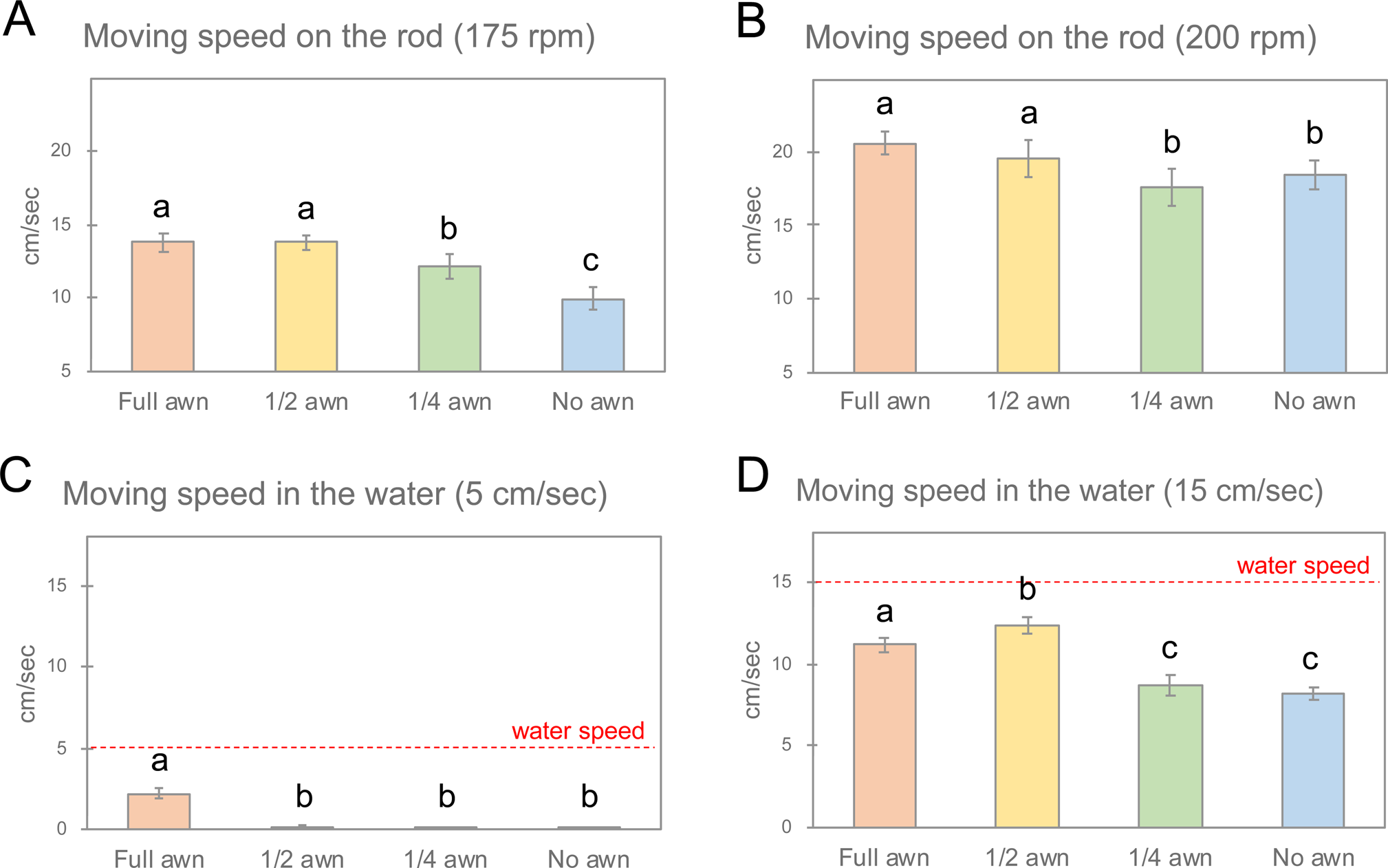
Fig. 6. Comparisons of the means of the groups with full, half, quarter and no awns. (A, B) Seed moving speeds on the rod at shaking speeds of 175 and 200 rpm, respectively. (C, D) Seed speeds in the water under water flow speeds of 5 and 15 cm s−1, respectively. Means labelled with different letters differ significantly (Tukey–Kramer test, P < 0.05).
Discussion
Seed shattering is an important characteristic enabling wild rice to survive under natural conditions. Wild rice starts to form an abscission layer between the grain and pedicel in the early stage of spikelet development (Li et al., Reference Li, Zhou and Sang2006). Once the abscission layer formation is completed, the matured seeds are ready to detach from the panicles. In this study, the average days to seed shedding were 14.49 and 14.46 for full and half awn plants, respectively. These were about 1 day shorter than that for no awn plants (15.41 days). Although the plants with quarter awns had a slightly higher average (14.72 days), the difference in days to shedding differed significantly between the awned and awnless groups (Fig. 5A). These results demonstrate that awns enhance the detachment of matured seeds from the panicles in the initial seed dispersal step of Phase I.
Detached seeds fall through the air, and the falling form is critical for the first contact with the ground. Therefore, we examined the falling angle of the seeds and prepared four seed groups with different awn lengths (Fig. 4). According to the angle scores, the seeds with full and half awns fell almost vertically, those with quarter awns dropped with a slight inclination, and most awnless seeds rotated randomly as they fell (Fig. 5B). This suggests that awns regulate vertical orientation via air resistance. The falling angles of the seeds directly affect self-planting in muddy ground. Namely, awned seeds can push the embryo side down into the mud. The vertical form may enable seeds to squeeze to the surface when the ground is covered with other plants. In the seed slipping experiments, the awned seeds passed many mesh layers, while most awnless seeds were blocked by the first sheet (Fig. 5C, D). Interestingly, for sheet intervals wider than the seed length (for seeds with quarter awns at 4-cm interval), the slipping scores decreased significantly (Supplementary Table S3B). These results indicate that long awns facilitate the first vertical penetration to the ground.
After seed dispersal in Phase I, mature seeds must undergo subsequent horizontal movement. Because wild rice grows in wet areas, seeds fall to the ground or into water. In the dry season, water is drained and the ground surface becomes hard. Therefore, we examined the horizontal movement of seeds on hard ground and in water. In the shaker experiments, awned seeds moved more than awnless seeds on the rod (Supplementary Table S4A). Although small hairs were observed on the seed surface (Fig. 2C), half of the awnless seeds could not move at a shaking speed of 150 rpm. At higher speeds of 175 and 200 rpm, most of the seeds moved, suggesting that the anisotropic friction produced by upward awn barbs and seed hairs allowed the seeds to move. During the forward movement of the shaker, the upward barbs and hairs hooked the surface, and they were released and slipped during the backward movement. The strength of the friction also reflected the seed velocity. Therefore, significantly faster speeds were observed for the seeds with more barbs (i.e. longer awns; Fig. 6A, B). In the water flow experiments, the seeds with long awns also tended to move faster (Fig. 6C, D). Interestingly, the seeds moved awn-forward, as though the seeds were pulled by the long awns (Supplementary Movie S2). Awns probably have a lower specific gravity than the seed bodies in the water. In both experiments, the awned seeds showed advantages in horizontal movements on the ground and in the water, implying that the awns are also necessary for seed dispersal in Phase II.
This study quantified the roles of awns experimentally in Phases I and II of seed dispersal (Chambers and MacMahon, Reference Chambers and MacMahon1994). In most of the experiments under simulated natural conditions, seeds with full awns showed the best performance for seed dispersal, suggesting that wild rice keeps long awns to survive under natural conditions. Similarly, seeds with half awns showed better contribution for seed dispersal. In wild rice, seed awning is dominantly controlled by wild functional alleles at several loci, and the plants with the drastic reduction of awn length are hardly produced (Luo et al., Reference Luo, Liu, Zhou, Gu, Huang, Shangguan, Zhu, Li, Zhao, Wang, Zhao, Wang, Wang, Sang, Wang and Han2013; Hua et al., Reference Hua, Wang, Tan, Fu, Liu, Xiao, Zhu, Fu, Sun, Gu, Cai, McCouch and Sun2015; Bessho-Uehara et al., Reference Bessho-Uehara, Wang, Furuta, Minami, Nagai, Gamuyao, Asano, Angles-Shim, Shimizu, Ayano, Komeda, Doi, Miura, Toda, Kinoshita, Okuda, Higashiyama, Nomoto, Tada, Shinohara, Matsubayashi, Greenberg, Wu, Yasui, Yoshimura, Mori, McCouch and Ashikari2016; Ikemoto et al., Reference Ikemoto, Otsuka, Thanh, Phan, Ishikawa and Ishii2017; Amarasinghe et al., Reference Amarasinghe, Kuwata, Nishimura, Phan, Ishikawa and Ishii2020). In other wild grass species, long awns were also reported to assist seed dispersal and burial (Garnier and Dajoz, Reference Garnier and Dajoz2001; Li et al., Reference Li, Yin, Yang, Yang, Qian, Zhou, Zhang, Du and Yang2015; Ntakirutimana et al., Reference Ntakirutimana, Xiao, Xie, Zhang, Zhang, Wang and Yan2019). Since these seed movements are essential for the successful seed propagation, their awn lengths may be maintained under similar genetic controls in wild rice.
This study demonstrates how barbed awns contribute to successful seed propagation in wild rice. Through the experiments under simulated natural conditions as observed in the tropical Asian habitat, long awns of wild rice were well-studied to have a positive effect on seed dispersal in many phases. The present results may provide fundamental information for the ecological studies on wild propagation strategies and domestication studies on awnlessness in rice.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S096025852000046X.
Acknowledgements
We thank the National Institute of Genetics (National Bioresource Project), Japan, for providing the seeds of wild rice. This work was supported in part by a Grant-in-Aid from the Japanese Society for Promotion of Science (No. 18H02178).
Conflicts of interest
None declared.








