Introduction
Physical dormancy in seeds is caused by a water-impermeable seed or fruit coat (Baskin and Baskin, Reference Baskin and Baskin1998), and this class of dormancy is known to occur in seeds of all or most species of Fabaceae of the temperate and arctic zones, and in many of those in the tropical/subtropical zone (Baskin and Baskin, Reference Baskin and Baskin1998, Reference Baskin and Baskin2005). For these seeds to germinate, the water-impermeable layer(s) must become permeable. Depending on the species, physical dormancy can be broken in different ways (Baskin and Baskin, Reference Baskin and Baskin1998). However, the mechanism underlying the breaking of physical dormancy in seeds of legumes is still controversial, especially with regard to initial site of water entry into the seed. In many species, the lens is believed to be the primary site of water entry (Serrato Valenti et al., Reference Serrato Valenti, Vries and Cornara1995; Baskin et al., Reference Baskin, Baskin and Li2000; Baskin, Reference Baskin2003). Seeds of Albizia lophantha (Dell, Reference Dell1980), Acacia kempeana (Hanna, Reference Hanna1984) and Sesbania punicea (Manning and Van Staden, Reference Manning and Van Staden1987) imbibed water only via the lens after pretreatment with boiling water, and a concentrated sulphuric acid treatment made seeds of Astragalus cicer permeable only at the lens (Miklas et al., Reference Miklas, Townsend and Ladd1987). However, Egley (Reference Egley1979) reported that covering the lens of hot-water pretreated seeds of the legume Crotalaria spectabilis with petroleum jelly did not prevent germination. Burns (Reference Burns1959) reported that palisade cells of the hilum in Lupinus angustifolia seeds were destroyed by acid scarification, and that water entered through the hilum, but not through the lens. Morrison et al. (Reference Morrison, Auld, Rish, Porter and McClay1992, Reference Morrison, McClay, Porter and Rish1998) showed that dry-heating caused a disruption of the seed coat at the lens of some legumes, while in other species some region other than the lens was the site of water entry.
More recently, Taylor (Reference Taylor2004, Reference Taylor2005) proposed four recognizable stages in the seed-softening process: impermeable, preconditioned, slowly permeable at some point on the testa, and rapidly permeable at the lens. In this model, a minute opening, possibly at some place in the testa other than the lens, allows moisture to enter seeds slowly before the lens is ruptured, resulting in rapid imbibition. Hu et al. (Reference Hu, Wang, Wu and Baskin2008) also reported that seeds of Sophora alopecuroides first became slowly permeable in the hilum or extrahilar region and not in the lens, but longer scarification treatments also cracked the lens, causing seeds to enter the fast imbibition stage. These results imply that very slow imbibition could easily be overlooked in many studies due to relatively short periods of time allowed for imbibition to occur. Thus, results and interpretations from previous research may vary, depending on whether or not pretreated seeds entered the fast imbibition stage and on the various periods of time (from 12 h to 1 month) used to determine whether seeds would imbibe (Hanna, Reference Hanna1984; Serrato Valenti et al., Reference Serrato Valenti, Cornara, Ghisellini and Ferrando1994, Reference Serrato Valenti, Vries and Cornara1995; Morrison et al., Reference Morrison, McClay, Porter and Rish1998; Ma et al., Reference Ma, Cholewa, Mohamed, Peterson and Gijzen2004; Das and Saha, Reference Das and Saha2006). To determine the underlying mechanism of water entry into seeds after various treatments, frequent observations need to be made during the dormancy-breaking process, and various periods of imbibition need to be used.
Sesbania sesban and Vigna oblongifolia (Fabaceae, Papilionoideae) were selected for study. S. sesban is an important legume fodder tree, widely distributed throughout tropical Africa and Asia, and its seeds have physical dormancy that can be broken by hot-water treatment (Kusekwa et al., Reference Kusekwa, Msafiri, Mwilawa, Ngowi, Kyamanywa, Ulime, Kategile and Adoutan1993; Wang and Hanson, Reference Wang and Hanson2008). V. oblongifolia is another important tropical and subtropical agricultural species, used as a pulse, forage, vegetable, green manure, or erosion-control plant, and it has a high percentage of physically dormant seeds that are made water permeable by hot-water or sulphuric acid pretreatment (Wang et al., Reference Wang, Hanson and Mariam2007).
The specific objectives of our study were to determine: (1) if there are differences in the responses of physically dormant seeds of S. sesban and V. oblongifolia to dormancy-breaking treatments; (2) the initial site of water entry into seeds during imbibition following various dormancy-breaking treatments; (3) the morphological changes (testa cracks) that occur in physically dormant seeds following various dormancy-breaking treatments; and (4) how imbibition time affects identification of the primary site of water entry when seeds are in the slow or rapid imbibition stages following various dormancy-breaking treatments.
Materials and methods
Seed source
Seeds of S. sesban and V. oblongifolia were obtained from a seed gene bank at the International Livestock Research Institute in Ethiopia in August 2007. After seeds were received at Lanzhou University, they were stored in paper envelopes at 4°C until used.
Treatments
Seeds of S. sesban and V. oblongifolia were subjected to the following treatments: (1) four replicates of 50 seeds each were scarified with concentrated H2SO4 for 1, 3 and 15 min and then washed with tap water for 10 min; and (2) four replicates of 50 seeds each were soaked in water at 80°C for 3 min and then washed with tap water.
Point of water entry during imbibition
To determine the point of water entry, 500 seeds of S. sesban and of V. oblongifolia were treated with concentrated sulphuric acid for 1, 3 and 15 min. After each acid scarification treatment for each species, four replicates of 25 seeds each were subjected to the following treatments: (1) no blockage applied (control); (2) blockage material (Vaseline™) applied to hilum area; (3) Vaseline™ applied to lens area; (4) Vaseline™ applied to lens + hilum area; and (5) Vaseline™ applied to extrahilar region, which is defined as the whole seed coat except the hilum and lens area. In addition, whole seeds of S. sesban and V. oblongifolia, pretreated with sulphuric acid for 30 min, were completely covered with Vaseline™, and no seeds imbibed after 14 d, indicating that Vaseline™ could completely inhibit seed imbibition. The same blocking experiment was conducted on seeds of S. sesban and V. oblongifolia that had been soaked in water at 80°C for 3 min.
After Vaseline™ had been applied to treated seeds of the two species, seeds were placed on two sheets of filter paper (Shuangquan-11, China) in closed 12-cm-diameter Petri dishes and incubated in darkness at 25°C for 14 d. For each dish, 10 ml of distilled water were used. The number of imbibed and non-imbibed seeds in each dish was monitored daily. Each seed was weighed at 24-h intervals, and a seed was recorded as imbibed when the amount of water taken up exceeded 50% of its initial mass; in most cases the amount of water greatly exceeded 50% when a seed was recorded as imbibed. At a 50% increase in mass, there were visible changes in seed colour and in size/volume, and clearly, seeds were imbibing rapidly.
Seed-surface features
After receiving dormancy-breaking treatments, seeds of S. sesban and V. oblongifolia were dried at room temperature for 24 h. For both species, surface features of seeds from each dormancy-breaking treatment and control were examined using scanning electron microscopy (SEM); 10 seeds were used for each treatment and control. All samples were coated with gold and examined with a JSM-6380LV (JEOL, Japan) scanning electron microscope at 20 kV.
Statistical analysis
Data were analysed with SPSS 13.0 for Windows statistical software system (SPSS Inc., Chicago, Illinois, USA). All percentage data were arcsine transformed to equalize variances. To test the effects of blockage on seed imbibition following the dormancy-breaking treatments, analysis of variance (ANOVA) and Duncan's multiple range tests were used to compare means. All data are presented as means of replicates (n = 4).
Results
Point of water entry during imbibition of treated seeds
Sesbania sesban
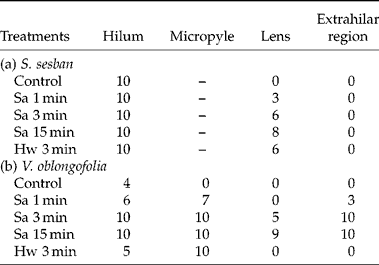
Hilum blockage (compared to no blockage) on seeds receiving 1, 3 or 15 min of acid scarification significantly reduced the percentage of seeds imbibed after 1 d, but not after 14 d (Table 1). For seeds soaked at 80°C for 3 min, hilum blockage (compared to no blockage) had no effect on the percentage of imbibed seeds after both 1 and 14 d. Blockage of the lens (or blockage of hilum + lens) for seeds receiving all dormancy-breaking treatments completely inhibited seed imbibition after 1 and 14 d, except for 2% imbibition of seeds receiving 15 min acid scarification. Blockage of the extrahilar region (compared to no blockage) significantly reduced the percentage of seeds imbibed after 1 d, but there were no differences after 14 d (Table 1).
Table 1 Percentage of imbibed seeds of S. sesban and V. oblongifolia after 1 and 14 d incubation in darkness at 25°C. Different areas of the seed coat (hilum, lens and extrahilar region) were blocked with Vaseline™ following acid scarification or a hot-water dormancy-breaking treatment. Values sharing a common small letter in each row for a given day (1 or 14) are not significantly different at P≤0.05, using Duncan's multiple range tests. Sa, Sulphuric acid; Hw, 80°C hot water

Vigna oblongifolia
Hilum blockage (compared to no blockage) of V. oblongifolia seeds receiving different dormancy-breaking treatments significantly reduced the percentage of imbibed seeds after both 1 and 14 d, except for the 15 min sulphuric acid scarification treatment, which showed no differences (Table 1). Lens blockage (compared to no blockage) of seeds receiving 1 and 3 min (but not 15 min) of acid scarification significantly reduced the percentage of seeds imbibed after 1 d, but not after 14 d. Effects of lens blockage (compared to no blockage after 14 d) on imbibition of seeds treated with 80°C water declined between 1 and 14 d, i.e. it reduced the percentage of seed imbibed by 91% after 1 d, but only by 15% after 14 d. Extrahilar region blockage had no effect on seed imbibition after 14 d except for the 80°C water treatment, in which there was a 20% reduction in percentage of imbibed seeds. Except for 3 min acid scarification, there was no difference in percentage of imbibition for seeds with hilum blocked or with hilum plus lens blocked (Table 1).
Seed surface features
For all seeds examined, three regions of the seed coat could be distinctly identified: (1) a hilum with a groove in the middle (the micropyle lies on one side of the hilum and is covered by the hilum in seeds of S. sesban); (2) the lens, which is on the opposite end of the hilum from the micropyle; and (3) the extrahilar region, which is defined as the area not occupied by the lens and hilum (Fig. 1a, Fig. 2a).

Figure 1 Scanning electron micrographs of the S. sesban seed coat following different treatments. (a–c) Seeds without treatment: (a) entire seed showing the position of the hilum (H) and lens (L); (b) hilum with cracks (HC) and opening hilum groove (HG); (c) an intact lens. (d–f) Seed treated for 15 min with concentrated sulphuric acid: (d) hilum with HC and HG; (e) lens with cracks (LC); (f) extrahilar region (ER, see text for explanation). (g–i) Seeds treated with 80°C water for 3 min: (g) hilum with HC and HG; (h) lens with LC; (i) extrahilar region.

Figure 2 Scanning electron micrographs of the V. oblongifolia seed coat following different treatments. (a–c) Seeds without treatment: (a) showing the relative position of the micropyle (M), hilum (H) and lens (L); (b) micropyle with closed funicular lid (FL); (c) lens. (d–g) Seed treated for 1 min with concentrated sulphuric acid: (d) hilum with hilum cracks (HC) and opening hilum groove (HG); (e) micropyle without the FL; (f) lens; (g) extrahilar region with cracks (EC). (h–k) Seed treated for 3 min with concentrated sulphuric acid: (h) hilum with HC and HG; (i) cracked micropyle (MC); (j) lens with cracks (LC); (k) extrahilar region with cracks (EC). (l–o) Seed treated for 15 min with concentrated sulphuric acid: (l) hilum with HC and HG; (m) cracked micropyle; (n) lens with cracks; (o) extrahilar region with cracks. (p–r) seeds treated wiht 80°C water for 3 min: (p) hilum with HC and HG; (q) micropyle without FL; (r) lens remains intact.
Sesbania sesban
Although all examined seeds of S. sesban had cracks in the hilum, acid scarification and hot-water treatment seemed not to produce more or wider cracks than those in the control seeds (Fig. 1b, d, g). The lens and extrahilar regions remained intact in control seeds (Fig. 1a, c). When seeds received acid scarification for 1 min, about 30% of them cracked in the lens. As acid scarification was increased to 3 and 15 min (Fig. 1e), the percentage of seeds with a cracked lens increased to 60% and 80%, respectively (Table 2). However, no changes were observed in extrahilar regions in any of the seeds examined in the various dormancy-breaking treatments (Fig. 1f, i, Table 2). Hot-water treatment caused only the lens to crack, and no changes were observed in other regions (Fig. 1h, i).
Table 2 Effect of sulphuric acid (Sa) and hot water (Hw) on number of seeds with cracks in the hilum, micropyle, lens and extrahilar regions. Seeds were examined by scanning electron microscopy; 10 seeds were examined for each treatment. –, no data
Vigna oblongifolia
Unlike seeds of S. sesban, the micropyle is distinct from the hilum in seeds of V. oblongifolia, and in non-treated seeds the micropyle with a funicular lid is tightly closed (Fig. 2a, b). After acid scarification for 1 min, seeds cracked in the hilum (Fig. 2d), and the funicular lid disappeared, with a minute opening in the micropyle area (Fig. 2e). Also, small eroded areas appeared in the extrahilar region in some seeds (30%) (Fig. 2g), but no changes were observed in the lens (Fig. 2f versus c, Table 2). When the treatment time was increased to 3 min, the hilum cracked further (Fig. 2h), and the micropyle was destroyed (Fig. 2i). About 50% of the seeds cracked in the lens, and more small eroded areas could be seen in the extrahilar region in all seeds (Fig. 2j, k, Table 2). When treatment time increased to 15 min, most seeds cracked in the hilum, micropyle and lens, and eroded areas in the extrahilar regions increased in size and depth (Fig. 2l–o, Table 2). After the hot-water treatment, seeds cracked in the hilum (Fig. 2p), and the funicular lid of the micropyle disappeared (Fig. 2q). However, no effects were observed on other areas of the seed coat (Fig. 2r).
Discussion
Consistent with most of the previous research in legume species (Baskin et al., Reference Baskin, Baskin and Li2000), lens blockage completely inhibited imbibition of S. sesban seeds on a wet substrate for 14 d, while blockage of the hilum or extrahilar region had no effect on imbibition, regardless of the dormancy-breaking treatment (Table 1). Thus, the lens was the site of water entry into S. sesban seeds, and scarification treatments broke physical dormancy by acting on the lens area. This conclusion is supported by SEM results, which indicate that seeds cracked only in the lens in response to the dormancy-breaking treatments (Fig. 1, Table 2). Although all S. sesban seeds, including those in the control, had cracks in the hilum, they may not be deep enough to allow water entry, because no seeds with a covered lens imbibed (Table 1). These results are consistent with those for seeds of S. punicea (Graaff and Van Staden, Reference Graaff and Van Staden1983; Manning and Van Staden, Reference Manning and Van Staden1987), which imbibed only through the lens after hot-water pretreatment. Although Das and Saha (Reference Das and Saha2006) reported that the structure of hilum and micropyle, observed under SEM, was different between dormant and non-dormant seeds of S. cannabina, they found no direct correlation between seed structure and imbibition.
In contrast to seeds of S. sesban, the hilum region rather than the lens is the initial site of water entry into seeds of V. oblongifolia pretreated with sulphuric acid for 1 min. In these seeds, blocking the hilum area almost completely inhibited imbibition (Table 1), and SEM images revealed that most of them had cracks in the hilum and adjacent micropyle (Table 2, Fig. 2a, d, h, l, p). However, in seeds of V. oblongifolia acid scarified for 3 min, water may enter seeds through the lens or extrahilar region because hilum blockage was only partly effective in inhibiting water uptake (Table 1). SEM results showed that many seeds of V. oblongifolia were cracked in the lens and extrahilar area (Table 2, Fig. 2f, g, k, n, o). When seeds of V. oblongifolia were acid scarified for 15 min, they had cracks over their entire surface, and water entered via the hilum, lens and extrahilar region. Thus, the hilum area is the first site acted on by short acid scarification treatment and allows water entry into the seed, while longer treatments may further act on other sites and allow water entry into seeds through different areas. These results for seeds of V. oblongifolia are consistent with those on seeds of Sophora alopecuroides (Hu et al., Reference Hu, Wang, Wu and Baskin2008), which suggested that the hilum is the initial site of water entry into seeds after acid scarification for 1 min. However, when seeds of S. alopecuroides were acid scarified for 15 min, the lens cracked, thus allowing seeds to enter the fast imbibition stage.
After hot-water treatment of V. oblongifolia seeds, both the hilum and extrahilar regions were sites for initial uptake of water because blockage of the hilum and of the extrahilar area significantly reduced the percentage of imbibed seeds. SEM images showed that most seeds cracked in the hilum area after treatment; however, there were no visible cracks in the extrahilar region in any of the seeds examined (Fig. 2). Although Ma et al. (Reference Ma, Cholewa, Mohamed, Peterson and Gijzen2004) reported that fluorescent dyes penetrated seeds of soybean through minute cracks in the cuticle of the seed coat, in the present study cracks were not observed even under 5000 × magnification (image not shown). In hot-water treated seeds of V. oblongifolia, we found a minute opening in the micropyle region that could be involved in seed imbibition; however, due to its close proximity to the hilum, we were not able to distinguish between the role of micropyle and hilum during water uptake in the blocking experiment.
It has been suggested that cracks in the seed coat produced by heat treatment result from seed coat expansion and contraction due to the increase from room temperature to 80°C (hot water) (Hanna, Reference Hanna1984; Zeng et al., Reference Zeng, Cocks, Kailis and Kuo2005). Cracks in acid-scarified seeds may be attributed to both chemical erosion and temperature changes. We found that cracks resulting from sulphuric acid scarification and hot-water treatment were distinctly different, implying that various treatments make seeds permeable in different ways (Fig. 2k, m, p). The cracks occurring in the lens of S. sesban or in the hilum region of V. oblongifolia suggest that these sites are the physically weakest part of the testa, and thus more easily broken by treatments (Serrato Valenti et al., Reference Serrato Valenti, Vries and Cornara1995; Morrison et al., Reference Morrison, McClay, Porter and Rish1998; Baskin et al., Reference Baskin, Baskin and Li2000).
If only the imbibition data of S. sesban seeds for the first day were considered, other points on the seed (hilum, extrahilar regions) in addition to the lens could also be assumed to be points of some water entry. That is, blocking these points significantly reduced the percentage of imbibed seed, partly because the blockage material is water repellant and thus slowed down the rate of imbibition.
Also, if imbibition data of V. oblongifolia for only the first day were considered, the conclusion would be entirely different from that of 14-d imbibition data. For seeds of V. oblongifolia, imbibiton data for the first day indicate that each point of the seed acid scarified for 1 min is responsible for seed imbibition, because blockage on hilum, lens and extrahilar region significantly reduced the percentage of seed imbibition (Table 1). These data indicate that imbibition via the hilum is a much slower process than that via the lens. After 14 d, imbibition was reduced more by blockage of the hilum than by blockage of the lens. Thus, the short period of imbibition (1 d) would not have detected such imbibition only via the hilum, and it would have underestimated the number of seeds that became permeable.
Taylor (Reference Taylor2005) suggested that the length of time utilized for imbibition tests depends on the species. For some slowly imbibing seeds, a 14-d test period may fail to identify all the seeds that potentially can imbibe. In our study, determination of the entry point of water into the seed, as well as the final percentage of imbibed seeds, was affected by the duration of the test. A 2-week period was optimal for the two species used in our study because no increase in percentage of imbibed seeds was observed when the imbibition period was increased to 1 month (data not shown).
Taylor (Reference Taylor2004, Reference Taylor2005) suggested that minute openings in the hilum or extrahilar region (not lens) are the initial site of water entry into seeds after physical dormancy is broken, thus allowing seeds to enter the slow imbibition stage. Results from our studies on seeds of V. oblongifolia and from those on seeds of S. alopecuroides (Hu et al., Reference Hu, Wang, Wu and Baskin2008) support Taylor's hypothesis. However, results from studies on S. sesban seeds do not support his hypothesis because in this species the lens, rather than the hilum or extrahilar sites, is the first site to become permeable after scarification treatment. Thus, the conclusion of Morrison et al. (Reference Morrison, McClay, Porter and Rish1998) that not all legume seeds have the same response to dormancy-breaking treatments is supported by our research.
Although our results provide new insight into how common laboratory treatments break physical dormancy, they do not necessarily tell us how seeds respond to natural dormancy-breaking factors in the field, which would require further research.
Acknowledgements
We thank Mr Zhang Baolin for his help in the seed collection and field work and Professor Jerry Baskin for his help with the manuscript. The study was funded by the National Key Basic Research Special Foundation Project of China (2007CB108904) and Natural Science Foundation of China (30771532).






