Introduction
The seed germination process is a critical period because seedling survival probability and growth depend on temperature and water availability after germination (Bradford, Reference Bradford2002). Temperature strongly regulates germination, since it affects root protrusion capacity and speed when water is available (Probert, Reference Probert and Fenner2000; Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013).
Germination can be quantified by mathematical models based on linearity principles of the inverse relationship between time and germination of a given fraction of a seed population, in response to temperature (Dahalal and Bradford, Reference Dahal and Bradford1994; Bradford, Reference Bradford2002; Soltani et al., Reference Soltani, Galeshi, Kamkar and Akramghaderi2008). The models are based on cardinal temperatures and thermal time or heat units. Cardinal temperatures are called minimum or base (T b) and maximum or ceiling (T max), where below T b or above T max, germination processes do not occur, while the optimum temperature (T o) is the reciprocal temperature, where the germination rate (R (g)) is at maximum (Alvarado and Bradford, Reference Alvarado and Bradford2002).
Models based on cardinal temperatures are fundamental and widely used to make germination accurate predictions for species survival. Models have been used to study weeds (Soltani et al., Reference Soltani, Baskin, Baskin, Soltani, Galeshi, Ghaderi-Far and Zeinali2016), cultivated species (Kamkar et al., Reference Kamkar, Al-Alahmadi, Mahdavi-Damghani and Villalobos2012; Parmoon et al., Reference Parmoon, Moosavi, Akbari and Ebadi2015; Derakhshan et al., Reference Derakhshan, Bakhshandeh, Siadat, Moradi-Telavat and Andarzian2018) and native species (Cochrane et al., Reference Cochrane, Hoyle, Yates, Wood and Nicotra2014; Mattana et al., Reference Mattana, Sacande, Bradamante, Gomez-Barreiro, Sanogo and Ulian2018). In studies of Brazilian tree species of different biomes, such as Cerrado, Drymaria cordata (Cardoso and Pereira, Reference Cardoso and Pereira2009) and Buchenavia tomentosa (Correa et al., Reference Correa, Silva, Silva, Camili, Silva and Coelho2020); for Amazonian species (Daibes and Cardoso, Reference Daibes and Cardoso2018; Daibes et al., Reference Daibes, Amoêdo, Moraes, Fenelon, Silva, Vargas and Frigeri2019) and for Caatinga species, such as Cenostigma microphyllum (Gomes et al., Reference Gomes, Oliveira, Araujo, Seal and Dantas2019) and Myracroduon urundeuva (Oliveira et al., Reference Oliveira, Silva, Araujo, Costa, Gomes, Matias and Dantas2019a), thermal models proved to be useful to elucidate ecological aspects of species survival and local adaptation.
The Brazilian Cerrado is the richest tropical savannah in terms of plant diversity and one of the most important ecosystems of vascular plant flora in the world (Colli et al., Reference Colli, Vieira and Dianese2020); at the same time, it is also one of the most threatened ecosystems (Strassburg et al., Reference Strassburg, Brooks, Feltran-Barbieri, Iribarrem, Crouzeilles, Loyola and Balmford2017). The Cerrado is located in the Brazilian central region and is characterized by well-divided annual dry and wet seasons (Reys et al., Reference Reys, Camargo, Grombone-Guaratini, Teixeira, Assis and Morellato2013), with highest temperatures during the rainy season, when seeds can germinate.
Regarding the future projections of global temperature impact in the ecosystems, temperature increase of up to 4.8°C is expected (IPCC, 2014). In the Cerrado, especially, the rains may be reduced by 20% over the years in central areas, and expectations show increased drought periods until 2100 (Marengo et al., Reference Marengo, Jones, Alves and Valverde2009). As a consequence of temperature increase, patterns of distribution of some species to less arid regions are expected, where there is inevitable risk of species extinction (Oliveira et al., Reference Oliveira, Staggemeier, Faria, de Oliveira and Diniz-Filho2019b).
Thermal amplitude for seed germination is related to ecosystem climatic characteristics (Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015), in that the environment in which seeds germinate determines the survival of seedlings and the natural selection of species in a habitat (Donohue et al., Reference Donohue, Casas, Burghardt, Kovach and Willis2010; Marques et al., Reference Marques, Atman, Silveira and Lemos-Filho2014). Therefore, climatic changes can impact species survival with restricted distribution in seasonal environments, especially those that need water to germinate after dispersal, making them more susceptible to population reduction and extinction in case of future temperature increase and rainfall reduction.
Information about seed germination is not often precisely described in models, especially those that simulate the potential impacts of climate change (Dürr et al., Reference Dürr, Dickie, Yang and Pritchard2015). However, germination response thermal modelling is essential for understanding plant and ecosystem resilience more accurately, especially to evaluate the proportion of species that will be affected under climate change scenarios of increased temperatures. In this context, the objectives of this study were (1) to quantify seed germination of 14 species occurring in the Cerrado, based on thermal time; (2) to verify the effectiveness of non-linear models and dent-like function to predict thermal time and (3) to relate climatic characteristics with the vulnerability of species in the face of global climate change and within the context of seed ecology.
Material and methods
Seed collection and regional climate characterization
Mature seeds of 14 species (Table 1) that frequently occur in warmer areas of the Brazilian Cerrado were collected after natural dispersal and tested in 2018 and 2019; the seeds were tested again in 2020 (supplementary Fig. S3). Collection areas included the Federal University of Mato Grosso (Universidade Federal de Mato Grosso – UFMT) campus in Cuiabá, urban areas and preserved dry micro-forests in the Cerrado areas of the state of Mato Grosso in central-western Brazil. Most of the selected species belong to the Fabaceae and are trees; all species, both trees and shrubs display natural seed dispersal in the dry season, from 1 to 3 months before the beginning of the rainy season.
Table 1. Ecological aspects, type and treatment for breaking seed dormancy, mass of a thousand seeds and seed water content of 14 species collected in the Brazilian Cerrado and found in other biomes

Numbers in parentheses represent standard deviation. Species occurrence in biomes is described in Flora do Brasil (2020).
A, Amazonia; Ca, Caatinga; C, Cerrado; MA, Mata Atlântica; Pa, Pampa; Pan, Pantanal; AP, preservation areas; PY/Esc, mechanic scarification with a pyrograph; ND, non-dormant; MTS, mass of a thousand seeds; SWC, initial seed water content.
Fruits and seeds were collected immediately after natural dispersal and taken to the laboratory. Seeds were removed from fruits and categorized as viable, predated or visually unviable. After separation, seeds were stored in a laboratory environment (25 ± 3°C temperature and 50 ± 5% relative humidity) for up to 2 weeks before analysis.
The mass of a thousand seeds for each species was determined by weighing eight repetitions of 100 seeds and by using estimates based on the frequency and standard sample error (Brasil, Reference Brasil2009). Three replicates of 0.5 and 1.0 g (for species with small and large seeds, respectively) were used to determine initial water content by drying the seeds in an oven for 24 h at 105 ± 3°C (Brasil, Reference Brasil2009). The Cerrado's climate is classified as Aw (Köppen classification), with a rainy season from October to April (late spring to early autumn) and drought from May to September (late autumn to early spring) (Reys et al., Reference Reys, Camargo, Grombone-Guaratini, Teixeira, Assis and Morellato2013). Maximum and mean temperatures do not fluctuate widely throughout the year in the region, making rainfall the main variable (Fig. 1). In the studied area, at the start of the rainy season (October to December), when seeds can germinate, minimum, mean and maximum daily temperatures are 22.5, 27.2 and 35°C, respectively, over the last 20 years (2000–2020) (Fig. 1). Minimum temperatures in winter are 16.5°C. Already, extreme air temperatures of 37.4–41.6°C have been recorded during the last 20 years in the rainy season (October to April), which occurs generally in consecutive days after the rains have begun, but atypical years can occur.
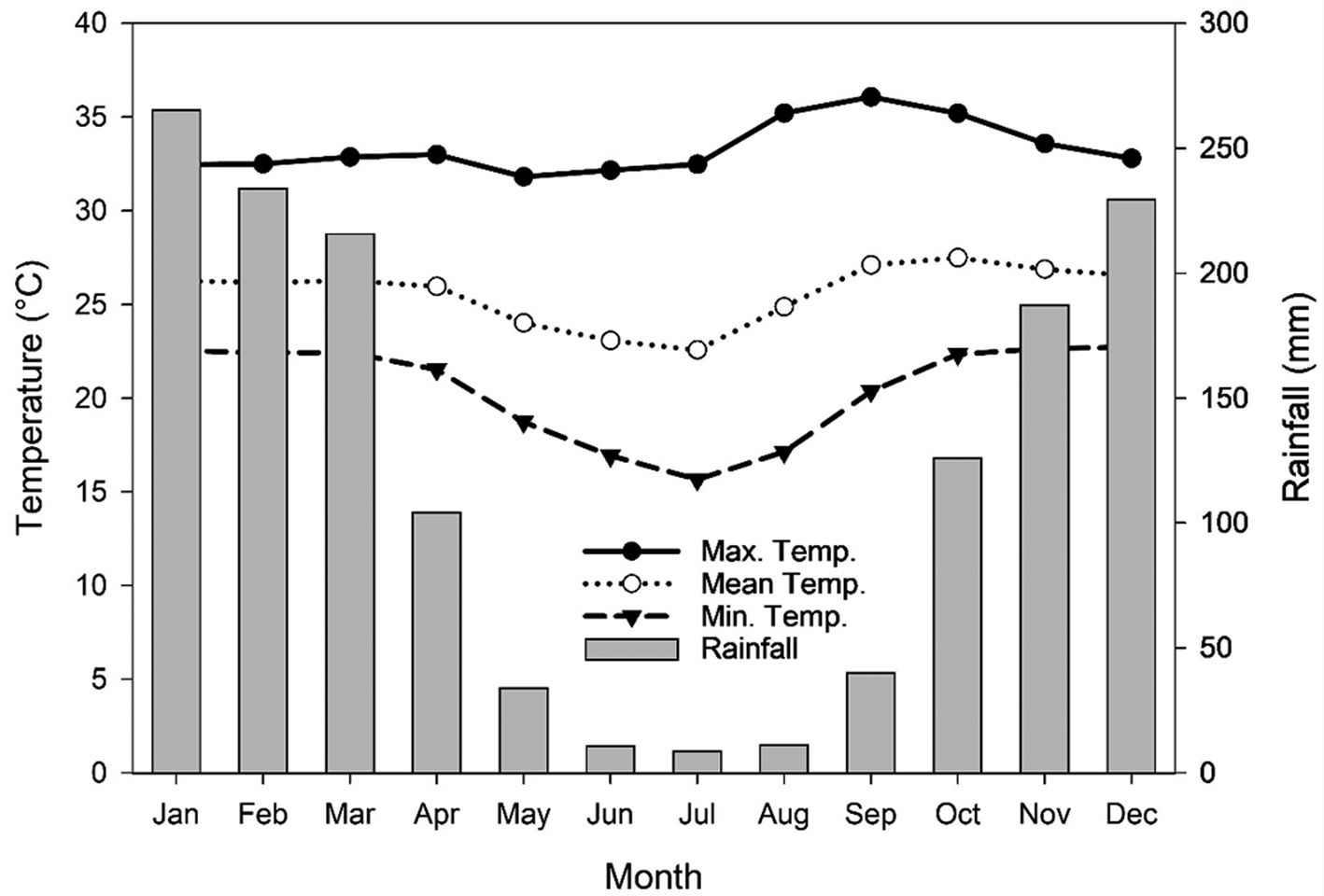
Fig. 1. Maximum, mean and minimum air temperatures (Max. Temp., Mean Temp. and Min. Temp., respectively) and rainfall of the Cerrado region in Mato Grosso from 2000 to 2019. Data were obtained from the INMET (Instituto Nacional de Meteorologia, Brazil).
Effect of temperature on seed germination
Four replicates of 25 seeds were placed to germinate at constant temperatures of 10, 15, 20, 25, 30, 35, 40, 45 and 50°C in BOD (biological oxygen demand) incubators with a 12-h photoperiod (led light intensity of 60 μmol m−1 s−1). Seeds of species already reported in the literature or evaluated in preliminary tests that presented physical dormancy (PY) (Table 1) were subjected to mechanical scarification with a pyrograph. In the case of Tachigali vulgaris seeds, it was necessary to remove the seed dispersal structure, due to the high fungal attack and mortality in the germination test.
Seeds were sown between filter papers and kept in rolls in an incubator, while seeds of Senna multijuga, Guazuma ulmifolia and Physocalymma scaberrimum were sown in acrylic boxes on germination paper, because the seeds are very small. Papers in both boxes and rolls were moistened with distilled water until saturation and kept moist until the end of the germination test. Boxes were sealed with plastic film and the rolls placed in plastic bags to avoid the loss of moisture, during the evaluation period.
For fast germinating species, germinated seeds were counted every hour and for slow germinating seeds daily. Radicle protrusion ≥2 mm was the germination criterion, and germination was counted until constant. Soaked seeds that did not germinate were transferred to an incubator at 25°C, and germinated seeds counted after 2 weeks. Then, non-germinated seeds were crushed to verify viability, and seeds with a soft structure in the crushing test were classified as dead.
Statistical analysis
Cardinal temperatures and thermal time models were obtained from estimates of time to seed germination of 50% of the seed population (T 50) at each temperature. Accumulated germination progress curves were plotted as a function of time, and T 50 was determined using non-linear equations for each temperature repetition and each species.
Germination rate (R (g)) was calculated based on the germination percentage of incubated seeds in each tested repetition, defined as the inverse of T 50:
where R 50 is the germination rate and T 50 is the time to seed germination of 50% of the population. For determining cardinal temperatures, the following model was considered:
where f(T) is the correction factor and R max is the maximum germination rate, which are obtained in the optimal temperature range for each species. 1/R max, therefore, indicates the minimum time required for seed germination in the optimal range. Dent-like function was used to adjust models and to obtain cardinal temperatures, which are expressed as follows:
where T is the incubation temperature (°C); T b is the base temperature; T 01 is the lowest optimum temperature; T 02 is the highest optimum temperature and T max is the maximum germination limit temperature. The equation parameters were obtained by non-linear regressions representative of the R 50 as a function of temperatures using SigmaPlot v. 14.0 (Systat Software Inc., San Jose, CA, USA).
Assuming that cardinal temperatures did not vary between seed population fractions for all species, thermal time models were adjusted to the sub- (equation 4) and supra-optimal (equation 5) temperatures, expressed as follows:
where θ sub(g) is the thermal time to seed germination at sub-optimal temperatures; t g is the time to seed germination of g fraction of the population; θ supra(g) is the thermal time to seed germination at supra-optimal temperatures and T max is the maximum temperature for germination. Germination curve in the optimal region (between T 01 and T 02) was obtained based on t g and thermal time, which is expressed as follows:
Thermal time models at sub- and supra-optimal temperatures were adjusted using the Weibull model, with four parameters (equation 7):
where G is the germination percentage, and a, b, c and x 0 are constants in the model. Models were run using SigmaPlot v. 14.0.
Future projections for seed germination under possible climate changes
Based on the IPCC (International Panel on Climatic Change) global predictions (IPCC, 2014), four RCP (Representative Concentration Pathway) scenarios were considered: RCP 2.6, RCP 4.5, RCP 6.0 and RCP 8.5, which represent estimated greenhouse gas emissions and increased future global temperatures since 2050 until 2100. For each RCP, optimistic and pessimistic scenarios were considered.
To estimate the effects of increased global temperature on the seed germination of 14 Cerrado species, the temperature data recorded by meteorological stations located in the warmer areas of the Cerrado in the state of Mato Grosso were analysed. The number of days with temperatures equal to or greater than 40, 42 and 45°C was obtained from data in INMET (2000–2020), and based on all RCP scenarios, the possible future increase in the number of days could also be estimated at the specified temperatures.
Results
Estimates of cardinal temperatures were T b between 3.51 and 16.51°C and T max between 34.99 and 50.15°C (Table 2) in both considered years. In general, the optimal temperature range for seed germination of most studied species was between 30 and 35°C. Enterolobium contortisiliquum and Samanea tubulosa showed germination in a greater thermal range (~4 to 50°C) and for a higher germination rate between 37 and 44°C. S. multijuga, G. ulmifolia and Jacaranda mimosifolia showed the lowest thermal requirement range to reach T 50 (~15 to 40°C).
Table 2. Dent-like function and thermal time model parameters for seed germination of 14 species occurring in the Brazilian Cerrado

Values in parentheses represent standard deviations.
a Thermal time in °C h.
b Seeds with PY were scarified with a pyrography before immediately starting the test.
c Thermal time for seeds without dispersion structure. T b, base temperature; T 01, lowest optimal temperature; T 02, highest optimal temperature; T max, maximum temperature limit for seed germination; R max, maximum germination rate. Thus, 1/R max represents the minimum time required for seeds to reach T 50 at optimal temperatures; G, final germination percentages at sub- or supra-optimal temperatures; R 2, coefficient of determination of the regression; θ 50, thermal time for seed germination of 50% of the seed population.
Dent-like function was adjusted well to the germination rate of most species as reflected by the high coefficients of determination (R 2 from 0.93 to 0.99) in observed and predicted values (Fig. 2). Likewise, the 2020 data were well fitted with previous rate-based dent-like models, showing that the germination rate models were useful to estimate germination thermal parameters in the different years of collection. Thus, reliable thermal estimates for sub-optimal models and for most of the supra-optimal models were obtained, as well as good estimates of germination curves (supplementary Fig. S4).

Fig. 2. Predicted (lines) and observed values (symbols) of the seed germination rate of species occurring in the Cerrado at constant temperatures for a fraction of 50% of the seed population. Lines represent the dent-like function adjusted to the data and error bars represent standard deviations.
The T 50 at optimal temperatures ranged from hours to days and for most species, between 1 and 5 d. Senegalia polyphylla and S. tubulosa had the lowest T 50, at 21.41 and 27.77 h, respectively, and for Amphilophium elongatum, the highest T 50 (20.7 d). All species showed a high germination percentage (> 90%) at sub-optimal temperatures and sizeable germination (> 50%) at supra-optimal temperatures.
Thermal time varied widely between species, both at sub- and supra-optimal temperatures, and there was no pattern or relationship between species. S. polyphylla had the shortest thermal time (484.37 and 108.12°C h, at sub- and supra-optimal temperatures, respectively), while A. elongatum had the highest (399.92 and 126.17°C d). For both species, seeds placed to germinate at temperatures above T max reached 100% of mortality until 10 d, and few species showed dormancy below T b.
Based on IPCC analyses up until 2100 (IPCC, 2014), minimum temperatures during the rainy season in the Cerrado are expected to increase between 22.8 and 27.3°C; mean temperatures would be between 27.3 and 31.8°C, and maximum temperatures between 35.3 and 39.8°C. The maximum number of days with temperatures above 40°C during the rainy season would be 13 ± 8.04 in the most optimistic scenario and 60 ± 4.43 in the most pessimistic RCP 8.5 scenario (Table 3).
Table 3. Predicted mean temperature and number of days with extreme temperatures during the rainy season, and minimum temperature during winter in the central region of the Cerrado, based on global temperature increase in optimistic and pessimistic RCP scenarios (IPCC, 2014)

Mean and minimum temperature expectations based on the last 20 years (2000–2020) and days with extreme temperatures on the last 5 years (2016–2020) in the central regions of the Cerrado.
Op, optimistic scenario; Pe, pessimistic scenario; ND 40, 42 and 45, number of days with temperatures greater than 40, 42 and 45°C, respectively.
Discussion
Species showed a wide difference in thermal requirement and thermal range for germination, where E. contortisiliquum, S. tubulosa (from <10°C to close to 50°C), S. polyphylla, Peltogyne confertiflora and T. vulgaris (from <10°C to close to 45°C) were the least sensitive to extreme temperatures and at the same time, the most dispersed in diverse ecosystems of Brazil (Table 1). The other species are less distributed and frequent in the Cerrado and showed germination generally up to 40°C.
For the most studied species, the maximum rate and high germination percentage between 40 and 45°C show that high temperatures are not a limiting factor for seed germination in the present time, especially the more widely distributed species. Therefore, water availability in the soil with the first rains is the main factor affecting seed germination in the Cerrado. For seeds dispersed during the dry season in the Cerrado, competition would occur at the beginning of the wet season, with the first rains, since the seeds would be able to germinate only in that period, regardless of germination phenology (Marques et al., Reference Marques, Atman, Silveira and Lemos-Filho2014). Therefore, differences in thermal requirement for germination between studied species prevent interspecific competition and can determine the co-existence of species in the environment in the present time. This is due to evolutionary divergence in germination characteristics, which reduces the probability of competition and facilitates species co-existence in populations (Grubb, Reference Grubb1977).
Bauhinia campestris (endemic to the Cerrado), J. mimosifolia and P. scaberrimum, widely distributed in the central of Brazil, showed germination thermal range consistent with Cerrado temperatures, which restricts the occurrence in ecosystems with daily temperatures below 15 or 20°C at the time of germination, possibly making germination null or very slow. Therefore, temperature possibly limits the distribution of these species to specific habitats with a thermal range similar to the Cerrado.
Thermal characteristics are imposed by the climate in each environment. It is possible that response to environmental factors, particularly germination temperature, can explain the geographic and ecological area of species distribution (Donohue et al., Reference Donohue, Casas, Burghardt, Kovach and Willis2010; Marques et al., Reference Marques, Atman, Silveira and Lemos-Filho2014). This relationship was also found in tropical bromeliad seeds, where the temperature range and light requirement for germination were associated with the habitat characteristics of adult plant occurrence (Marques et al., Reference Marques, Atman, Silveira and Lemos-Filho2014). For Amazonian species, cardinal temperatures and ecological distribution do not appear to have a strong relationship (Daibes et al., Reference Daibes, Amoêdo, Moraes, Fenelon, Silva, Vargas and Frigeri2019). Although climate has been recognized as the main factor in diversity patterns between biomes (Hawkins et al., Reference Hawkins, Field, Cornell, Currie, Guégan, Kaufman and Porter2003; Field et al., Reference Field, O'brien and Whittaker2005), other factors, such as soil properties, also dictate the diversity of communities and size of populations (Arruda et al., Reference Arruda, Fernandes-Filho, Solar and Schaefer2017).
A. elongatum seeds also germinated in a lower temperature range and showed high mortality above 35°C. This is due to the higher thermal time required (399.22°C d), which makes the seeds remaining in the soil for longer times and becomes susceptible to pathogens, which was confirmed in the germination tests at 35°C. For A. elongatum, in particular, dispersal in the Cerrado and in climatic conditions with high daily temperatures (≥35°C) makes its distribution more restricted.
Future projections for seed germination under climate changes
Despite the greater diversity of the Cerrado being in the herbaceous layer (Furley, Reference Furley1999), few studies have used thermal modelling to describe the germination of woody species, especially in climate change scenarios. IPCC global forecasts predict temperature increases of up to 4.8°C (IPCC, 2014); consequently, the increase in days with extreme temperatures (Table 3) will certainly affect the Cerrado. At the same time, regional models indicate rising temperatures in central Brazil throughout the year, especially in autumn and spring; in winter, minimum temperatures will be between 21 and 23°C, until 2100 (Marengo et al., Reference Marengo, Jones, Alves and Valverde2009). Rainfall in the central region of Brazil may also be reduced by at least 20%, leading to more intense dry seasons in the future (Marengo et al., Reference Marengo, Jones, Alves and Valverde2009). Therefore, seeds less tolerant to high temperatures may be affected, making them susceptible to extinction in the future of Cerrado.
For Libidibia ferrea, E. contortisiliquum, S. tubulosa, Platymenia foliolosa, B. campestris, G. ulmifolia and Cochlospermum regium, which possess PY, and for S. polyphylla, P. confertiflora and T. vulgaris, which are non-dormant, but which are widely distributed, even in the most pessimistic scenarios of increased mean air temperature, no damage to germination is expected, but the seeds will germinate faster (supplementary Table S4). Possibly, seeds on the soil surface may be more susceptible than buried seeds, because the soil surface will reach higher temperatures.
S. multijuga, J. mimosifolia and P. scaberrimum also will germinate faster in the optimistic scenario of RCP (supplementary Table S4); however in the more pessimistic scenario, the seeds will need more days to germinate and will face temperatures above the optimum range and days with extreme temperatures that will increase the rate of seed degradation. For A. elongatum, especially, even in optimistic scenarios, there are risks of seed mortality in its habitat, because it showed optimal germination limit (T 02 of 29.04°C) below the medium temperature increase estimated in most scenarios (Table 3). Average daily temperature increase can be more harmful to A. elongatum, especially in RCP 8.5, which can increase the time of seeds in the soil by more than 10 d (supplementary Table S4).
J. mimosifolia, P. scaberrimum and A. elongatum are non-dormant seeds dispersed at the beginning of the rains, which showed high thermal requirement for germination. Time dispersal is the main mechanism for controlling the germination of non-dormant seeds in the Cerrado (Salazar et al., Reference Salazar, Goldstein, Franco and Miralles-Wilhelm2011; Escobar et al., Reference Escobar, Silveira and Morellato2018). Seeds in tropical savannas are not long-lived and lose viability in storage at warm temperatures (Salazar et al., Reference Salazar, Goldstein, Franco and Miralles-Wilhelm2011; Escobar and Cardoso, Reference Escobar and Cardoso2015). At the same time, woody non-dormant seeds did not form persistent soil seed banks in the savannas, which suggest that the seeds must germinate in the dispersal season (Salazar et al., Reference Salazar, Goldstein, Franco and Miralles-Wilhelm2011). Thus, species that do not present dormancy after dispersal and need a higher thermal requirement for germination may have an increased risk of mortality in the future.
Consequences of temperature increase and reduction of the rainfall volume in the most pessimistic scenarios will cause species to change their distribution to transitional regions of biomes with a favourable climate, mainly for non-dormant seeds with a degree of vulnerability. Thus, a greater displacement of the most sensitive species to more humid and milder temperatures in the Cerrado can be expected, as is predicted for certain species (Oliveira et al., Reference Oliveira, Staggemeier, Faria, de Oliveira and Diniz-Filho2019b; Velazco et al., Reference Velazco, Villalobos, Galvão and de Marco-Júnior2019). In species of Eugenia, a decrease of richness in Cerrado areas has been verified from the past to the present and tends to continue, in the future, where the species will move towards the south of the Cerrado and other biomes (Oliveira et al., Reference Oliveira, Staggemeier, Faria, de Oliveira and Diniz-Filho2019b). However, changes can be so severe and rapid in populations that there may be a decrease in species richness and extinction at local and regional levels, with greater risk for endemic species (Strassburg et al., Reference Strassburg, Brooks, Feltran-Barbieri, Iribarrem, Crouzeilles, Loyola and Balmford2017).
The PY cycle may also be changed in the Cerrado. Minimum air temperatures in winter below 20°C possibly control seed sensitivity in seeds with PY in tropical savannas, as occurs in S. multijuga (Rodrigues-Junior et al., Reference Rodrigues-Junior, Baskin, Baskin and Garcia2018). In addition, high temperatures increase PY break (Probert, Reference Probert and Fenner2000; Baskin and Baskin, Reference Baskin and Baskin2014; Dayrell et al., Reference Dayrell, Gonçalves-Alvim, Negreiros, Fernandes and Silveira2015). Therefore, changes in winter temperatures may result in a greater number of insensitive seeds with PY in the soil, impairing the recruitment of seedlings in the germination season in the future, if minimum air temperatures rise to 21.3°C (Table 3). At the same time, temperature increase may cause an intense break of PY in sensitive seeds, which will be susceptible in the soil if there is reduced or delayed rainfall.
In conclusion, the seeds displayed great variation in thermal time requirements, showing a higher thermal range for species widely distributed, in both years. Non-dormant seeds with higher thermal time to germination have a higher risk of extinction if pessimistic scenarios of temperature increase occur. Temperature rise decreases relative air humidity of the Cerrado areas and will increase the frequency of fires before the rains, increasing the risk of seed mortality in the soil seed bank before they have a chance to germinate. At the same time, the lack of basic biological knowledge prevents adequate assessment of the degree of threat to most species in the biome (Colli et al., Reference Colli, Vieira and Dianese2020). Thus, this study contributes to understand the seed ecology of the Cerrado trees and shrubs in the current and future climate and calls for the adoption of reforestation practices in degraded areas as a way to maintain species diversity in the Cerrado. Studies on the reduction of rainfall frequency (which are relevant in assessing the tolerance of seeds to water stress) are necessary as well, in order to help predict species survival, mainly because climatic change may occur faster than species will be able to adapt in the Cerrado.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S0960258521000131.
Acknowledgements
The authors thank the undergraduate students from the Laboratório de Sementes at the Universidade Federal de Mato Grosso supported by PIBIC/CNPq scholarships for their assistance in field collection and laboratory analysis. Thanks are also extended to Rosiane A. P. Guimarães for field collection and technical support.
Financial support
This work was funded by a Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) scholarship to A. Ribeiro Correa.
Conflicts of interest
The authors declare no conflict of interests.








