Introduction
The development of seeds involves several phases: morphogenesis, growth and reserve deposition, maturation and desiccation, prior to dispersal from the mother plant. Development is regulated by a complex network of endogenous signalling pathways which respond to environmental cues (Kermode, Reference Kermode2005; Gutiérrez et al., Reference Gutiérrez, Van Wuytswinkel, Castelain and Bellini2007; Matilla, Reference Matilla2007; Bentsink and Soppe, Reference Bentsink and Soppe2008; Sorefan et al., Reference Sorefan, Girin, Liljegren, Ljung, Robles, Galván-Ampudia, Offrinaga, Friml, Yanofsky and Østergard2009; De Smet et al., Reference De Smet, Lau, Mayer and Jürgens2010). The result is a viable and autonomous organism which will germinate when internal and environmental conditions are suitable (Black et al., Reference Black, Bewley and Halmer2006; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008a; Preston et al., Reference Preston, Tatematsu, Kanno, Hobo, Kimura, Jikumaru, Yano, Kamiya and Nambara2009; Nonogaki et al., Reference Nonogaki, Bassel and Bewley2010).
The transition from dormancy to germination is a critical control point leading to the initiation of vegetative growth. Dormancy is an important trait that was altered during domestication of wild species (Vaughan et al., Reference Vaughan, Balázs and Heslop-Harrison2007) and its physiology appears to be similar among different species (Bentsink et al., Reference Bentsink, Hanson, Hanhart, Blankestijn-de Vries, Coltrane, Keizer, El-Lithy, Alonso-Blanco, de Andrés, Reymond, van Eeuwijk, Smeekens and Koornneef2010; Linkies et al., Reference Linkies, Graeber, Knight and Leubner-Metzger2010). It has been proposed that natural variation in seed dormancy in Arabidopsis is controlled by different genetic and molecular pathways rather than by epistatic interactions (Bentsink et al., Reference Bentsink, Hanson, Hanhart, Blankestijn-de Vries, Coltrane, Keizer, El-Lithy, Alonso-Blanco, de Andrés, Reymond, van Eeuwijk, Smeekens and Koornneef2010). Although the adaptive significance of dormancy is evident in wild plants, this state poses a practical problem in seeds of commercial crops (e.g. cereals; reviewed by Matilla, Reference Matilla2007; Fang and Chu, Reference Fang and Chu2008). Dormant seeds can remain alive in a state of very low hydration for extended periods without losing vigour, until environmental cues such as cold stratification (a pre-chilling treatment of fully imbibed seeds) or afterripening (AR) (a period of dry storage also called post-harvest storage) trigger the release from dormancy (Bentsink and Koornneef, Reference Bentsink and Koornneef2008).
Although seed dormancy has been studied extensively during the past century, there is still limited information available on the physiological and molecular mechanisms that break it. Understanding dormancy or its loss in model plants is fundamental to developing new strategies to control the trait in commercial crops. This review focuses on novel knowledge acquired during the past decade on the molecular and physiological mechanisms involved in the breaking of seed dormancy by dry AR.
Dry AR, a means to dormancy loss under low moisture conditions
AR is the loss of the dormancy achieved by exposure of a seed to a specific set of environmental conditions after maturation and separation from the mother plant (Foley, Reference Foley2001; Black et al., Reference Black, Bewley and Halmer2006). The conditions that facilitate AR vary with the species. For example, some species respond to warm, dry conditions (i.e. dry AR), others to cool, moist conditions (i.e. stratification or chilling) and some respond to both sets of conditions (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Finkelstein et al., Reference Finkelstein, Reevers, Ariizumi and Steber2008). The time required to complete AR is also variable between species. Dry AR does not bring about an abrupt change from a dormant to a fully germinable state; rather, seeds in a population become more responsive to a set of conditions within which they are able to germinate (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Carrera et al., Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008a, Reference Holdsworth, Finch-Savage, Grappin and Jobb).
Dry AR occurs in non-imbibed viable seeds under very low water potentials (i.e. less than 0.1 g H2O (g dry weight)− 1; Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007). In tobacco and pine seeds there appear to be cells with different degrees of hydration in the dry, mature state (Manz et al., Reference Manz, Müller, Kucera, Volke and Leubner-Metzger2005; Terskikh et al., Reference Terskikh, Feurtado, Ren, Abrams and Kermode2005). Consequently, the moister cells could be involved in the alterations promoted by dry AR. Oil content of a dry seed is a parameter that seems to influence dry AR because it affects water potential (Manz et al., Reference Manz, Müller, Kucera, Volke and Leubner-Metzger2005). Dry AR does not occur in very dry seeds because it requires seed moisture above a threshold value. This threshold moisture content is species-specific and is lower in oil-storing seeds than in starchy seeds because the former contain less bound water at any particular relative humidity. The conditions that generate optimal low-hydration values for dry AR of several species have been determined (Leubner-Metzger, Reference Leubner-Metzger2005).
Modelling of germination
Seeds may persist in the soil for months or even years until the environmental conditions, interacting with the seeds' physiological sensory mechanisms, trigger germination. Some mathematical models have been developed to describe the germination and dormancy behaviour of seeds on a population basis (Bradford, Reference Bradford2002). Their application has provided a comprehensive physiological explanation for the responses of seed germination to temperature and other physiological parameters. Hydrotime (Ht) and hydrothermal-time (HTt) models can be used to study the dry AR status (Bradford, Reference Bradford2002; Bradford et al., Reference Bradford, Benech-Arnold, Côme and Corbineau2008). Both provide information about how physiological and environmental factors interact to regulate dormancy release as well as speed of germination (Alvarado and Bradford, Reference Alvarado and Bradford2002; Allen, Reference Allen2003; Bradford, Reference Bradford2005; Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007). Although the HTt model does not identify the biochemical mechanism(s) by which water potential (Ψ) of a seed is regulated, it clearly points to necessary biochemical and molecular investigations. That is, the parameters of the HTt model can be used to quantitatively characterize and compare the physiological status of seed populations under different environmental conditions or from different genetic backgrounds (Chantre et al., Reference Chantre, Batlla, Sabbatini and Orioli2009, Reference Chantre, Sabbatini and Orioli2010). Ht and HTt have revealed that, at a given temperature, the timing and percentage of germination in a seed population are controlled by the difference between a physiologically determined Ψ threshold and the seed Ψ. Seed dormancy is a reflection of high values of the Ψ threshold, and conditions that break dormancy (e.g. dry AR) shift the Ψ distribution to lower values (Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007). Progressive loss of dormancy in a seed population may be related to a progressive decrease in the seed Ψ(50) (Bradford, Reference Bradford, Kigel and Galili1995). Dormancy release due to AR in seeds of the winter annual weeds Bromus tectorum and Elymus elymoides (Meyer et al., Reference Meyer, Debaene-Gil and Allen2000; Bair et al., Reference Bair, Meyer and Allen2006) has been described through a reduction in Ψ(50) values. Hydrothermal AR time models for Bromus tectorum (Bair et al., Reference Bair, Meyer and Allen2006) and Lolium rigidum (Steadman et al., Reference Steadman, Bignell and Ellery2003a, Reference Steadman, Crawford and Gallagherb) indicate that AR occurs at a moisture-content range of 5–20% (fresh weight basis) (Fig. 1). The AR conditions for Arabidopsis thaliana Cabo Verde Island (Cvi) accession seeds are 20°C and 33% relative humidity, with a measured moisture content of 7% (Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007). The discovery that dry AR rate is a linear function of storage temperature above a specific base temperature led to the use of thermal-time (Tt) to describe rates of dormancy loss. Using a Tt approach, Steadman et al. (Reference Steadman, Bignell and Ellery2003a, Reference Steadman, Crawford and Gallagherb) found that the rate of dormancy loss in L. rigidum seeds is a positive linear function of dry AR temperature. Dry AR was characterized by a progressive increase in the mean maximum germination temperature and a reduction in the Tt requirements for germination at suboptimal temperature (Chantre et al., Reference Chantre, Batlla, Sabbatini and Orioli2009). Recently, it was demonstrated that dry AR can be adequately described as a Tt response, and that this AR–Tt developed model can be used for predicting both the extent and timing of Lithospermum arvense germination under field conditions (Chantre et al., Reference Chantre, Sabbatini and Orioli2010).

Figure 1 Conceptual diagram proposing how storage water potential influences dry afterripening (AR) in Bromus tectorum seeds as indicated by changes in Ψb(50) per unit thermal time. HTAR, hydrothermal AR time; TAR, thermal AR time. (Adapted from Bair et al., Reference Bair, Meyer and Allen2006).
AR alters gene expression in dry viable seeds
At the end of maturation, the seed has residual low moisture content (i.e. ~5–15%, depending on the species). Whether or not there is transcriptional activity in these seeds is debatable. Reorientation of the genetic programme during dry AR occurs in dry seeds, but its significance or importance in dormancy relief by dry AR, is unknown (Chibani et al., Reference Chibani, Ali-Rachedi, Job, Job, Jullien and Grappin2006; Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007; Carrera et al., Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008; Holdsworth et al., Reference Holdsworth, Finch-Savage, Grappin and Job2008b; Holman et al., Reference Holman, Jones, Russell, Holman, Jones, Russell, Medhurst, Úbeda-Tomás, Talloji, Márquez, Schmuths, Tung, Taylor, Footitt, Bachmair, Theodoulou and Holdsworth2009). Using cDNA amplified fragment length polymorphism (AFLP) to identify transcripts differentially expressed in the dry seeds before and after dry AR, it appears that both gene transcription and translation are possible in Nicotiana plumbaginifolia and Hordeum vulgare (Bove et al., Reference Bove, Lucas, Godin, Oge, Jullien and Grappin2005; Leymarie et al., Reference Leymarie, Bruneaux, Gibot-Leclerc and Corbineau2007). Because the AtDOG1 gene decreases in abundance during dry AR and this gene expression is abscisic acid (ABA)-dependent, it was hypothesized that AtDOG1 is associated with the induction of primary dormancy during seed development (Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007). AtDOG transcripts are present in dormant and AR dry seeds and decline upon imbibition of the latter (Bentsink et al., Reference Bentsink, Jowett, Hanhart and Koornneef2006, Reference Bentsink, Hanson, Hanhart, Blankestijn-de Vries, Coltrane, Keizer, El-Lithy, Alonso-Blanco, de Andrés, Reymond, van Eeuwijk, Smeekens and Koornneef2010; Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007; Graeber et al., Reference Graeber, Linkies, Müller, Wunchova, Rott and Leubner-Metzger2010). In dry seeds of Sisymbrium officinale, which are highly susceptible to AR, dry AR increased the expression of SoGA3ox2 transcripts, and greatly reduced those of SoACS7 (Iglesias-Fernández and Matilla, Reference Iglesias-Fernández and Matilla2009). This indicates that the preparation for radicle protrusion during AR requires strong stimulation of gibberellic acid (GA) synthesis and has a lesser requirement for the stimulation of ethylene (ET) synthesis. Cell wall modifying enzymes like endo-β-mannanases (MAN) are also involved in germination of seeds such as Datura ferox (Nonogaki et al., Reference Nonogaki, Bassel and Bewley2010) and A. thaliana, in which transcripts of three (AtMAN5, 6 and 7) of the eight members of the AtMAN family are localized in the micropylar endosperm during germination of these seeds. Moreover, AR seeds of knock-out lines of these genes showed a lower germination rate than the wild type (Wt) (Iglesias-Fernández et al., Reference Iglesias-Fernández, Rodríguez-Gacio, Barrero-Sicilia, Carbonero and Matilla2010). Leubner-Metzger (Reference Leubner-Metzger2005) demonstrated that there is a transient low level of transcription and translation of the β-1,3-glucanase gene during tobacco seed AR, leading to the release of dormancy. Local hydrated pockets within cells or tissues of dormant seeds might allow changes in gene expression in the dry state during AR (Leubner-Metzger, Reference Leubner-Metzger2005). However, it remains to be confirmed that gene activity exists in these particular localized areas.
Signalling of ROS is related to dry AR
Expression analyses have shown that the transition from late embryogenesis to germination occurs gradually and therefore characteristics of both co-exist during the transition (Nakabayashi et al., Reference Nakabayashi, Okamoto, Koshiba, Kamiya and Nambara2005). Seed development, germination and post-germination seedling growth are well-regulated processes that involve high metabolic activity and generation of reactive oxygen species (ROS) (Bailly, Reference Bailly2004). During desiccation seeds must face severe oxidative stress and show particularly high levels of expression of genes involved in ROS detoxification, such as catalases and superoxide dismutases (SOD) (Bailly, Reference Bailly2004; Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008). It has been suggested that during storage seeds accumulate free radicals, which must be detoxified during dormancy, dry AR and germination to prevent damage to proteins, membranes and DNA (Kibinza et al., Reference Kibinza, Vinel, Côme, Bailly and Corbineau2006; Garnczarska et al., Reference Garnczarska, Bednarski and Jancelewicz2009). ROS production during seed storage in the dry state was documented by Pukacka and Ratajczak (Reference Pukacka and Ratajczak2005). ROS accumulation during seed maturation has been associated with seed desiccation and during germination with metabolic activity, gene expression and with programmed cell death (PCD) in the aleurone layer of wheat seeds (Pulido et al., Reference Pulido, Dominguez and Cejudo2009). ROS could be one of the signals responsible for induction of antioxidant gene expression, causing a substantial increase of the respective enzymatic activities of SODs in developing and germinated maize seeds (Foyer and Noctor, Reference Foyer and Noctor2005; Mylona et al., Reference Mylona, Polidoros and Scandalios2007). Thus ROS production is likely continuous and in mature seeds is initiated immediately after harvest. Interestingly, the amount of CATA1 transcript, one of the four genes encoding catalase in sunflower, declines during dry AR, raising the possibility that the H2O2-scavenging capacity of non-dormant seeds is low during early imbibition. Non-dormant imbibed seeds of sunflower contain much higher H2O2 amounts than the dormant ones (El-Maarouf-Bouteau et al., Reference El-Maarouf-Bouteau, Job, Job, Corbineau and Bailly2007). In agreement with this, Oracz et al. (Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007) have shown that dry AR in sunflower is associated with accumulation of ROS in the embryonic axes; they measured the content of H2O2 and ![]() during dormancy release, recording increases of 100% and 50%, respectively. They suggested that AR is associated with lipid peroxidation. In Bidens pilosa, ROS appear to be implicated in the alleviation of seed dormancy, and reagents that generate hydroxyl radicals and superoxide partially replaced the requirement for AR (Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010).
during dormancy release, recording increases of 100% and 50%, respectively. They suggested that AR is associated with lipid peroxidation. In Bidens pilosa, ROS appear to be implicated in the alleviation of seed dormancy, and reagents that generate hydroxyl radicals and superoxide partially replaced the requirement for AR (Whitaker et al., Reference Whitaker, Beckett, Minibayeva and Kranner2010).
In addition to gene alterations promoted in the dry seed by AR, there may be protein modifications, as well. ROS can alter the function of seed proteins through modification of their redox state, or, for example, carbonylation (El-Maarouf-Bouteau et al., Reference El-Maarouf-Bouteau, Job, Job, Corbineau and Bailly2007; Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007; El-Maarouf-Bouteau and Bailly, Reference El-Maarouf-Bouteau and Bailly2008). Carbonylation is the most commonly occurring oxidative protein modification (for a review, see Møller et al., Reference Møller, Jensen and Hansson2007) and inhibits or alters protein activities and intensifies their susceptibility to proteolytic attack. There are no indications that carbonylation is reversible. The task of identifying the causal factors behind age-dependent increased carbonylation has proved difficult. In sunflower seeds, ROS accumulation clearly leads to carbonylation of specific embryo proteins, not only during dry AR (Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007), but also during artificial breaking of dormancy by cyanide application (HCN; a compound that triggers ROS accumulation because it is an inhibitor of SOD, catalase and carbonylation) or methylviologen (a ROS-generating compound) (Bethke et al., Reference Bethke, Libourel, Reinöhl and Jones2006; Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007, Reference Oracz, El-Maarouf-Bouteau, Bogatek, Corbineau and Bailly2008; El-Maarouf-Bouteau and Bailly, Reference El-Maarouf-Bouteau and Bailly2008). The hypothesis that HCN interacts with ROS-producing pathways has been supported by data on intracellular ROS production in response to HCN treatment (Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Kranner, Bogatek, Corbineau and Bailly2009). In dry, mature Arabidopsis seeds, most of the carbonylation occurred in the storage proteins, and carbonylation of a number of other proteins increased strongly during germination although the germinated seeds still gave rise to vigorous plantlets (Job et al., Reference Job, Rajjou, Lovigny, Belghazi and Job2005; Møller et al., Reference Møller, Jensen and Hansson2007). Interestingly, the carbonylation level of some proteins decreased during AR, e.g. a 20S proteasome α-subunit (Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007). This observation is consistent with previous data showing a requirement for proteasome activity in sunflower embryos for both the breaking of dormancy by ethylene (ET) and the progression of germination. In summary, Oracz et al. (Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007) proposed a novel mechanism for seed dormancy release involving a change in proteome oxidation, resulting from an accumulation of ROS during dry AR.
NADPH oxidases (NOX) catalyse the production of ROS (Swanson and Gilroy, Reference Swanson and Gilroy2009) and in plants the NOX homologues have been named ‘respiratory burst oxidases’ (Rboh) (Sagi and Fluhr, Reference Sagi and Fluhr2006). However, it is not known what roles Rboh, a major producer of superoxide in germinating Arabidopsis seeds, play in seeds. It has been demonstrated, however, that the A. thaliana AtRbohB gene, which is expressed in the embryo and not in the endosperm, plays a role in AR and seed germination, AR being prevented in the AtrbohB mutant which also shows reduced protein carbonylation (Müller et al., Reference Müller, Carstens, Linkies, Torres and Leubner-Metzger2009). The AtrbohB pre-mRNA is alternatively spliced in fresh and AR seeds and this is ABA-dependent. It was hypothesized that the alternative splicing is used by seeds to react quickly to changes in their environment, especially to stress, in which ABA and ROS signalling play a major role (Müller et al., Reference Müller, Carstens, Linkies, Torres and Leubner-Metzger2009).
In addition to its role as a cofactor, nicotinamide adenine dinucleotide (NAD+) is involved in signalling and gene-regulation pathways in eukaryots (Hunt et al., Reference Hunt, Lerner and Zeigler2004). Nicotinamide (NIC) is transformed to nicotinic acid by nicotinamidase (NICase; EC 3.5.1.19) and participates in the Arabidopsis salvage pathway of NAD biosynthesis (Wang and Pichersky, Reference Wang and Pichersky2007). NICase activity has also been detected in embryos of germinating mungbean; both exogenous NIC and NAD+ inhibit seed germination, and a link between depth of seed dormancy and NAD+ content has been demonstrated, using a null mutant for NICase (Zheng et al., Reference Zheng, Hayashibe and Ashihara2005; Hunt et al., Reference Hunt, Holdsworth and Gray2007). Arabidopsis NIC2 is expressed at relatively high levels during seed maturation but is low at 24 h after imbibition (HAI) (Nakabayashi et al., Reference Nakabayashi, Okamoto, Koshiba, Kamiya and Nambara2005). In addition, the NAD+ concentration in fresh seeds of different Arabidopsis ecotypes with low, intermediate and high levels of dormancy, respectively (Cvi, Col-0 and Ws), was proportional to the degree of seed dormancy, whereas that of NADP+ was inversely proportional (Alonso-Blanco et al., Reference Alonso-Blanco, Bentsink, Hanhart, Blankestijn-de Vries and Koornneef2003; Hunt and Gray, Reference Hunt and Gray2009). While the amount of NADP+ does not change between fresh and AR seeds; that of NAD+ declines significantly during AR in the Cvi accession. Thus in Arabidopsis it appears that the depth of seed dormancy is also related to NAD+/NADP+ or NAD+/NADH+ ratios (Hunt et al., Reference Hunt, Holdsworth and Gray2007; Hunt and Gray, Reference Hunt and Gray2009). Analysis of the Arabidopsis NICase mutant (nic2-1), whose seeds contain a higher amount of NADP+, showed reduced NICase activity and ABA hypersensitivity as compared to Wt seeds. This suggests a role for NICase activity in regulating seed germination potential. Seeds of the Cvi accession, which have deep seed dormancy, had a relatively high NICase activity, which appeared to be reduced by AR (Hunt et al., Reference Hunt, Holdsworth and Gray2007; Hunt and Gray, Reference Hunt and Gray2009). This suggests that NICase activity is not directly correlated with depth of seed dormancy.
The relationship between dry AR, temperature and nitrate signalling
Higher plants, and some organs and cell components, exhibit a range of responses to the temperature of their environment (Penfield, Reference Penfield2008; Penfield and Hall, Reference Penfield and Hall2009), although the temperature sensors are unknown. It is widely accepted that temperature is the major environmental factor governing changes in the depth of dormancy in seeds in temperate environments (Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000; Probert, Reference Probert and Fenner2000; Baskin and Baskin, Reference Baskin and Baskin2004; Black et al., Reference Black, Bewley and Halmer2006). Dry AR is highly temperature-dependent (Donohue, Reference Donohue2002; Steadman et al., Reference Steadman, Bignell and Ellery2003a; Bair et al., Reference Bair, Meyer and Allen2006). In species that produce seeds in the spring/summer and that germinate in autumn, a period of prolonged desiccation often leads to a loss of primary dormancy that is present when the mature seed is shed. Although soil temperature is generally considered to be the primary environmental factor regulating dormancy, the effect of temperature on dormancy release and induction may also be modulated by soil moisture (Bair et al., Reference Bair, Meyer and Allen2006; Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2006). Bromus tectorum, a winter annual, requires dry AR for dormancy release, but very low soil moisture contents slow the rate at which dormancy is lost (Bair et al., Reference Bair, Meyer and Allen2006). Ecologically, a dry AR requirement prevents seeds from germinating during the hottest periods of the summer when there is a risk of seedling death due to inadequate rainfall. In some species, dry AR must precede stratification in order to break dormancy for at least a proportion of the seed population (Garvin and Meyer, Reference Garvin and Meyer2003). From this perspective, dry AR broadens the optimal temperature range for germination, accelerating radicle protrusion as compared with non-ripened seeds (Foley, Reference Foley2001; Corbineau and Côme, Reference Corbineau, Côme, Nicolás, Bradford and Pritchard2003; Bair et al., Reference Bair, Meyer and Allen2006; Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007; Iglesias-Fernández and Matilla, Reference Iglesias-Fernández and Matilla2009). Thus, temperature, together with seed moisture status, affects dry AR (Foley, Reference Foley2008).
Nitrate (![]() ) is an important nitrogen source for plants, so much so that some (i.e. nitrophilous plants) live in soils enriched in this anion.
) is an important nitrogen source for plants, so much so that some (i.e. nitrophilous plants) live in soils enriched in this anion. ![]() is also involved in plant signalling and controlling various aspects of development (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005; Krouk et al., Reference Krouk, Crawford, Coruzzi and Tsay2010), but its mechanism of action is still largely unclear.
is also involved in plant signalling and controlling various aspects of development (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005; Krouk et al., Reference Krouk, Crawford, Coruzzi and Tsay2010), but its mechanism of action is still largely unclear. ![]() releases dormancy of some seeds (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005; Bethke et al., Reference Bethke, Libourel, Reinöhl and Jones2006), negatively affecting early ABA synthesis (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004; Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009) or light requirement (Batak et al., Reference Batak, Devi, Giba, Grubisi, Poff and Konjevic2002). When applied to dormant seeds of Arabidopsis Cvi accession it results in a decrease in ABA content and prevents its de novo synthesis, which normally occurs in the absence of
releases dormancy of some seeds (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005; Bethke et al., Reference Bethke, Libourel, Reinöhl and Jones2006), negatively affecting early ABA synthesis (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004; Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009) or light requirement (Batak et al., Reference Batak, Devi, Giba, Grubisi, Poff and Konjevic2002). When applied to dormant seeds of Arabidopsis Cvi accession it results in a decrease in ABA content and prevents its de novo synthesis, which normally occurs in the absence of ![]() and in the presence of light (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004). Cross-talk between light and
and in the presence of light (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004). Cross-talk between light and ![]() signalling has also been related to the breaking of seed dormancy (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005).
signalling has also been related to the breaking of seed dormancy (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005). ![]() nutrition of the mother plant affects dormancy of seeds, and their depth of dormancy is inversely correlated to endogenous maternal
nutrition of the mother plant affects dormancy of seeds, and their depth of dormancy is inversely correlated to endogenous maternal ![]() content (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005). In Arabidopsis seeds, the gene AtNRT2.7, which encodes a tonoplast nitrate transporter, is involved in
content (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005). In Arabidopsis seeds, the gene AtNRT2.7, which encodes a tonoplast nitrate transporter, is involved in ![]() accumulation (Chopin et al., Reference Chopin, Orsel, Dorbe, Chardon, Truong, Miller, Krapp and Daniel-Vedele2007). This gene is expressed in developing seeds, particularly at the end of seed maturation, its transcripts peaking in dry seeds. Physiological analyses of Arabidopsis plants defective in the dual-affinity nitrate transporter NRT1.1 (i.e. nrt1.1) and NRT2.7 (i.e. nrt2.7), have revealed a possible involvement of these transporters in
accumulation (Chopin et al., Reference Chopin, Orsel, Dorbe, Chardon, Truong, Miller, Krapp and Daniel-Vedele2007). This gene is expressed in developing seeds, particularly at the end of seed maturation, its transcripts peaking in dry seeds. Physiological analyses of Arabidopsis plants defective in the dual-affinity nitrate transporter NRT1.1 (i.e. nrt1.1) and NRT2.7 (i.e. nrt2.7), have revealed a possible involvement of these transporters in ![]() accumulation and in the intensity of seed dormancy and signalling (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005; Chopin et al., Reference Chopin, Orsel, Dorbe, Chardon, Truong, Miller, Krapp and Daniel-Vedele2007). Recently, it was demonstrated that an intact two-component complex of AtNRT2.1 and AtNAR2.1 (AtNRT3.1), localized in the plasma membrane, is the form that is active in the inducible high-affinity
accumulation and in the intensity of seed dormancy and signalling (Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2005; Chopin et al., Reference Chopin, Orsel, Dorbe, Chardon, Truong, Miller, Krapp and Daniel-Vedele2007). Recently, it was demonstrated that an intact two-component complex of AtNRT2.1 and AtNAR2.1 (AtNRT3.1), localized in the plasma membrane, is the form that is active in the inducible high-affinity ![]() transport system (Yong et al., Reference Yong, Kotur and Glass2010). During stratification, Arabidopsis Cvi accession seeds first become sensitive to
transport system (Yong et al., Reference Yong, Kotur and Glass2010). During stratification, Arabidopsis Cvi accession seeds first become sensitive to ![]() , then to cold, and finally to light (Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007). Results by Iglesias-Fernández and Matilla (Reference Iglesias-Fernández and Matilla2009) on the germination rate in seeds of the nitrophilous plant Sisymbrium officinale have shown that
, then to cold, and finally to light (Finch-Savage et al., Reference Finch-Savage, Cadman, Toorop, Lynn and Hilhorst2007). Results by Iglesias-Fernández and Matilla (Reference Iglesias-Fernández and Matilla2009) on the germination rate in seeds of the nitrophilous plant Sisymbrium officinale have shown that ![]() requirements diminished with AR; this suggests cross-talk between dry AR and
requirements diminished with AR; this suggests cross-talk between dry AR and ![]() signalling. Exogenous
signalling. Exogenous ![]() increased the induction of AtCYP707A transcript and accelerated the ABA decline at 6 HAI, conferring to the CYP707A2 gene a central role in controlling seed dormancy in response to
increased the induction of AtCYP707A transcript and accelerated the ABA decline at 6 HAI, conferring to the CYP707A2 gene a central role in controlling seed dormancy in response to ![]() (Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009). This work highlights the importance of the regulation of ABA degradation rather than ABA synthesis in controlling the Arabidopsis seed dormancy response to
(Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009). This work highlights the importance of the regulation of ABA degradation rather than ABA synthesis in controlling the Arabidopsis seed dormancy response to ![]() . Likewise, transcript profiling of imbibed seeds with or without
. Likewise, transcript profiling of imbibed seeds with or without ![]() showed that exogenous
showed that exogenous ![]() led to a higher expression of
led to a higher expression of ![]() -responsive genes, whereas endogenous
-responsive genes, whereas endogenous ![]() led to a profile similar to that of stratified or AR seeds (Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009).
led to a profile similar to that of stratified or AR seeds (Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009).
AR acquisition triggers alterations in ABA metabolism
Once a viable seed is afterripened, it is ready to germinate. This is considered to be determined by the balance of negative and positive effects of the hormones ABA and GA, respectively (Yamaguchi et al., Reference Yamaguchi, Kamiya, Nambara, Bradford and Nonogaki2007; Bentsink and Koornneef, Reference Bentsink and Koornneef2008; Yamaguchi, Reference Yamaguchi2008; Rodríguez-Gacio et al., Reference Rodríguez-Gacio, Matilla-Vázquez and Matilla2009; Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009; Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). It has been suggested that dry AR and loss of dormancy are two distinct processes, because ABA-deficient, non-dormant Arabidopsis seeds still displayed transcriptome changes characteristic of dry AR after several months of storage at room temperature. Thus, dry AR and dormancy were considered genetically separate pathways and ABA contributed only to the induction and maintenance of dormancy of imbibed seeds, and was not required for AR (Carrera et al., Reference Carrera, Holman, Medhurst, Dietrich, Footitt, Theodoulou and Holdsworth2008; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008a).
The NCED6 and NCED9 genes, which are involved in ABA synthesis, play a major role in the control of seed development, including dormancy, while the CYP707A gene family is involved in ABA inactivation, playing a critical role in dormancy release in several species (Gubler et al., Reference Gubler, Millar and Jacobsen2005; Rodríguez-Gacio et al., Reference Rodríguez-Gacio, Matilla-Vázquez and Matilla2009; Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009; Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). Reverse-genetic analyses in Arabidopsis seeds (Kushiro et al., Reference Kushiro, Okamoto, Nakabayashi, Yamagishi, Kitamura, Asami, Hirai, Koshiba, Kamiya and Nambara2004; Okamoto et al., Reference Okamoto, Kuwahara, Seo, Kushiro, Asami, Hirai, Kamiya, Koshiba and Nambara2006) indicate that (1) AtCYP707A2 expression in the dry seed, controls the rapid decline in ABA during imbibition (between 6 and 12 HAI); (2) AtCYP707A1 and AtCYP707A3 are involved in germination; (3) the Atcyp707a2 mutant overaccumulates ABA in dry seeds and the high ABA content is maintained after seed imbibition; and (4) freshly harvested Atcyp707a2 seeds exhibit hyperdormancy.
The ABA content of dry seeds of the Arabidopsis line Col (non-dormant) is much lower than that of Cvi (highly dormant), which in turn is only slightly higher than in those of dry afterripened Cvi seeds (non-dormant) (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004). The ABA content of dormant tobacco seeds was strongly reduced during AR (Grappin et al., Reference Grappin, Bouinot, Sotta, Miginiac and Jullien2000). Dry AR itself had little effect on the ABA content of embryos of dry barley seeds. However, a decrease in ABA content and a rise in phaseic acid occurred during imbibition of AR seeds as compared to those that were not AR (Jacobsen et al., Reference Jacobsen, Pearce, Poole, Pharis and Mander2002). Phaseic and dihydrophaseic acids also accumulated in dry AR Arabidopsis (Col) seeds at 24 HAI (Kushiro et al., Reference Kushiro, Okamoto, Nakabayashi, Yamagishi, Kitamura, Asami, Hirai, Koshiba, Kamiya and Nambara2004). In barley, HvNCED1 (an ABA biosynthesis gene) was not affected by dry AR but is probably involved in preventing germination in non-optimal conditions of light and temperature (Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008). Millar et al. (Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006) showed in barley and in the Arabidopsis ecotype C24 that (1) AtCYP707A2 and ABA 8′-hydroxylase (HvABA8′OH-1) genes were induced in dry AR seeds as compared to dormant seeds; (2) HvABA8′OH-1-mRNA was preferentially localized in the coleorhiza; it occurred in barley AR seeds but not in 12-h-imbibed dormant ones. Therefore, ABA signalling appears to take place in the coleorhiza and is affected by dry AR (Barrero et al., Reference Barrero, Talbot, White, Jacobsen and Gubler2009). These authors point to a pivotal role for the HvABA8′OH-1 gene in controlling dormancy and that the action of its enzyme product may be confined to the coleorhiza. Thus this region of the embryo may play a major role in maintaining dormancy by acting as a barrier to root emergence, and AR potentiates molecular changes related to ABA metabolism and sensitivity that ultimately lead to degradation of the coleorhiza, and completion of germination as radicle emergence. On the other hand, ABA metabolism occurred also in the endosperm of Arabidopsis (Okamoto et al., Reference Okamoto, Kuwahara, Seo, Kushiro, Asami, Hirai, Kamiya, Koshiba and Nambara2006) and constitutive overexpression of a CYP707A gene in transgenic Arabidopsis resulted in a decreased ABA content in mature dry seeds and a much shorter AR period to overcome dormancy. Conversely, mutating the CYP707A2 gene resulted in seeds that required longer AR. On the other hand, greater induction of the AtCYP707A gene in Col than in Cvi may be related to the greater reduction of ABA content in the former (Preston et al., Reference Preston, Tatematsu, Kanno, Hobo, Kimura, Jikumaru, Yano, Kamiya and Nambara2009). Finally, by using two Arabidopsis ABA metabolism mutants (i.e. ABA-deficient mutant aba2 and ABA overaccumulating cyp707a1a2a3 triple mutant) Okamoto et al. (Reference Okamoto, Tatematsu, Matsiu, Morosawa, Ishida, Tanaka, Endo, Mochizuki, Toyoda, Kamiya, Shinozaki, Nambara and Seki2010) demonstrated that a cluster of ABA-upregulated genes partially resembled those of dormant genes, whereas ABA-downregulated genes partially overlapped with dry AR-regulated genes. This elegant work shows the complexity of cross-talk between ABA and AR in seeds. Gerjets et al. (Reference Gerjets, Scholefield, Foulkes, Lenton and Holdsworth2010), analysing six wheat varieties, also indicated no obvious relationship between ABA function and dry AR in wheat.
An ABA-insensitive seed-germination mutant chotto1 (cho1) has been identified and characterized (Nambara et al., Reference Nambara, Suzuki, Abrams, McCarty, Kamiya and McCourt2002). It carries a mutation in a gene encoding a double APETALA2 repeat transcription factor. CHO1 is expressed in imbibed seeds and requires ABI4 (Nambara and Marion-Poll, Reference Nambara and Marion-Poll2005). Imbibed dry AR seeds were affected in their content of ABA, of GA4, jasmonate, salicylate and N6-(D2-isopentenyl) adenine (iP). Dry AR also affected the expression of genes involved in ABA and GA metabolism beyond seed dormancy release, and the pattern of phytohormone and transcript contents indicated that briefly stored cho1 mutant seeds mimicked fully dry AR Wt-seeds (Yano et al., Reference Yano, Kanno, Jikumaru, Nakabayashi, Kamiya and Nambara2009). These results indicate that CHO1 acts downstream of ABA to repress GA biosynthesis during seed germination.
AR also affects ethylene responses
ET also positively influences germination potential (Matilla and Matilla-Vázquez, Reference Matilla and Matilla-Vázquez2008). However, the role of this hormone is less evident than that of ABA and GA (Vandenbussche and Van der Straeten, Reference Vandenbussche and Van Der Straeten2007; Stepanova and Alonso, Reference Stepanova and Alonso2009). ET is not the hormone that triggers the decisive steps during the appearance and elimination of dormancy, but rather is part of a complex network of interacting signals involved in dormancy, the details of which are currently being investigated. ET seems to act antagonistically with ABA during dormancy termination, but acts in concert with GA to promote germination (Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005; Bentsink and Soppe, Reference Bentsink and Soppe2008; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008a; Matilla and Matilla-Vázquez, Reference Matilla and Matilla-Vázquez2008). Recently, it has been suggested that in afterripened S. officinale seeds GA is more important than ET during early imbibition (up to 6 HAI), while ET is more important later during germination (Iglesias-Fernández and Matilla, Reference Iglesias-Fernández and Matilla2010).
Concluding remarks
Seed dormancy can be broken by dry storage. This process, called dry AR, is genetically determined and enables the seed to germinate upon subsequent imbibition. Some mechanisms that regulate dry AR have been investigated in depth, but many gaps still remain. The alterations triggered by dry AR itself already occur in the dry seed, but its effects also continue during and after imbibition. Dry AR increases germination potential by enhancing the sensitivity of seeds to factors that promote germination, such as light and GA, and reduce sensitivity to those that inhibit germination, such as ABA. The lowering of ABA content in imbibed seeds is strongly influenced by dry AR and the length of its application, thus promoting germination. In barley and A. thaliana, AR treatments led to an up-regulation of CYP707A expression, resulting in a decline of ABA in the imbibed seed. In addition, transcriptome analysis of A. thaliana Cvi-0 seeds has revealed that the breaking of seed dormancy by dry AR is associated with changes in the expression of ABA- and GA-metabolism genes, such as the CYP707A2 and the GA3ox1 genes (Fig. 2). However, transcriptome analysis of non-dormant aba-deficient1 or aba-insensitive1 mutants has demonstrated that dry AR functions independently of the ABA signalling pathway. In Arabidopsis (Col) dry AR affects the transcript levels of genes involved in ABA and GA metabolism beyond seed dormancy release, i.e. dry AR affects the expression of a large number of genes even in the non-dormant mutants and continues to reduce ABA responsiveness even though the seed is completely released from primary dormancy.
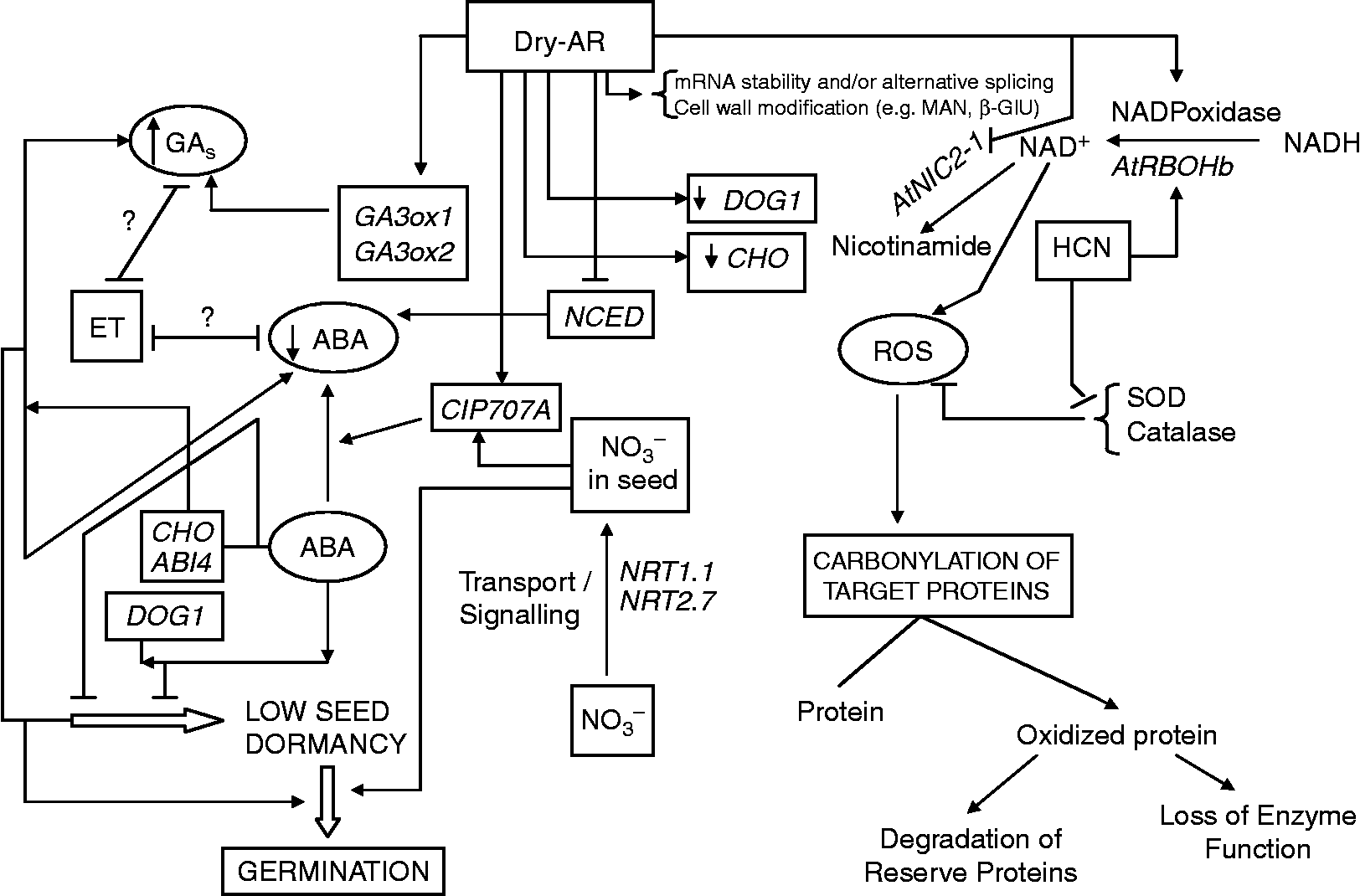
Figure 2 Known implications of dry afterripening on the breaking of seed dormancy, and germination.
Quantitative trait locus (QTL) analysis of Arabidopsis has identified the DOG1 gene, a major player in the control of seed dormancy that requires ABA for its expression (Fig. 2). DOG1 transcripts, that decrease during dry AR more strongly than in non-AR germinating seeds, are localized in the radicle and micropylar endosperm of Lepidium sativum and, as in Arabidopsis, the transcripts are rapidly degraded upon imbibition. This strongly suggests a novel role(s) for DOG1 in germination timing (e.g. negatively regulating AR).
In addition, ROS (i.e. ![]() and H2O2) have been proposed to produce important effects on seed germination and dormancy. Some of these involve the modification of proteins (e.g. carbonylation), and their relationship with dry AR is at present under study. A large number of hormonal (i.e. ET) and non-hormonal (i.e. HCN, NAD+) signals are involved in seed ROS production. NAD+ content appears to be a good indicator of the depth of seed dormancy and nic2-1 mutant seeds contain high NAD+ amounts, diminished NICase activity, ABA hypersensitivity and delayed dry AR. Synthesis of ROS during dry AR causes a notable degradation of key proteins by carbonylation. Seeds of the Arabidopsis NADPH-oxidase mutant (AtrbohB) germinate more slowly when subjected to dry AR, and the ROS-production genes (i.e. NADPHox and NOX) are differentially affected during dry AR.
and H2O2) have been proposed to produce important effects on seed germination and dormancy. Some of these involve the modification of proteins (e.g. carbonylation), and their relationship with dry AR is at present under study. A large number of hormonal (i.e. ET) and non-hormonal (i.e. HCN, NAD+) signals are involved in seed ROS production. NAD+ content appears to be a good indicator of the depth of seed dormancy and nic2-1 mutant seeds contain high NAD+ amounts, diminished NICase activity, ABA hypersensitivity and delayed dry AR. Synthesis of ROS during dry AR causes a notable degradation of key proteins by carbonylation. Seeds of the Arabidopsis NADPH-oxidase mutant (AtrbohB) germinate more slowly when subjected to dry AR, and the ROS-production genes (i.e. NADPHox and NOX) are differentially affected during dry AR.
Cho1 mutant seeds are more sensitive to dry AR than Wt seeds. The cyp707a2-1 cho1-3 double mutant (as well as 35S::CHO1 lines) indicate that the primary action of CHO1 is to regulate ABA responsiveness. Likewise, in both Wt and cho1 mutants, dry AR affects the transcript levels of ABA metabolism (ZEP, NCED9, CYP707A2, CYP707A3) and GA metabolism (GA3ox1, GA3ox2) genes after imbibition, even after seed dormancy is completely broken by dry AR. This indicates that, in dry-stored seeds, dry AR progresses beyond seed-dormancy release. Dry AR affects gene expression in non-dormant aba1 mutants. Moreover, CHO1 participates in the regulation of endogenous ABA and GA balance by affecting transcription of these hormone-metabolism genes in imbibed seeds (Fig. 2).
Acknowledgements
We thank Professor P. Carbonero (Centro de Biotecnología y Genómica de Plantas, Madrid, Spain) and Professor J. Derek Bewley for their critical reading of this manuscript and suggestions. The authors wish to apologize to all those seed scientists whose manuscripts have not been directly mentioned due to space constraints. We thank our colleagues for sending their most representative publications, their hypotheses on the mechanism of AR and valuable comments that led to the improvement and updating of this review. This work was financially supported by a grant from Ministerio de Ciencia e Innovación (MICINN, Spain) (CGL2009-11425). R.I.-F. is supported by a postdoctoral contract from Consolider CSD2007-00057 in the CBGP (Centro de Biotecnología y Genómica de Plantas, Madrid, Spain).


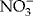 ) or nicotinamide adenine dinucleotide (NAD
) or nicotinamide adenine dinucleotide (NAD

