Introduction
Plant species exhibit different sensitivities to light environments for seed germination. Seeds of many species are indifferent to light conditions, whereas others can germinate only in light, only in the darkness or to higher percentages in light than in darkness and vice versa (Baskin and Baskin, Reference Baskin and Baskin2014). Light conditions provide important information about the optimal time and place for seedling establishment. The chances of successful establishment may be determined by whether the germinating seed is buried in the soil or is on the soil surface. If seeds are buried, the precise depth is crucial for seedling emergence (Bond et al., Reference Bond, Honig and Maze1999). Seed mass may represent a constraint for seedling emergence of small-seeded species. Small seeds are therefore more likely to require light for germination, which ensures that germination does not occur too deep in the soil for seedling emergence (Pons, Reference Pons and Fenner2000). Thus light response and seed mass could have co-evolved (Milberg et al., Reference Milberg, Andersson and Thompson2000). However, solar irradiance penetrates to only 4–5 mm into the soil in physiologically significant quantities (Tester and Morris, Reference Tester and Morris1987).
Photoinhibition of seed germination (PISG) refers to the suppression or retardation of germination under white (day) light conditions, with a high photon flux density (PFD) and it is considered to be a high irradiance response (HIR) sensu Górski and Górska (Reference Górski and Górska1979). The inhibitory effect of a high PFD on seed germination has been demonstrated in a number of species, even those that are otherwise positively photoblastic (Pons, Reference Pons and Fenner2000). Indeed, the inhibitory effect on seed germination in light-requiring species may be particularly effective under extremely high levels of natural lighting (Corbineau and Côme, Reference Corbineau and Côme1982). In this context, PISG has been traditionally interpreted as a physiological adaptation to avoid germination at or near the soil surface, which protects seedlings from dehydration and exposure to extremely high temperatures (Koller et al., Reference Koller, Sachs and Negbi1964; Thanos et al., Reference Thanos, Georghiou, Douma and Marangaki1991). An interesting implication of this adaptation is the formation of a third seed bank type apart from the well-established canopy and soil seed banks: the soil-surface seed bank (Thanos et al., Reference Thanos, Fournaraki and Makri2005). As a result, non-dormant seeds of photoinhibited species may persist at the soil surface and eventually germinate only when incorporated in the soil (Fenner and Thompson, Reference Fenner and Thompson2005). Not only light quantity but also light quality may influence the seeds' responses to light. Irradiance is attenuated by the plant canopy and/or by the leaf litter, leading to light enriched in far red (FR; Vazquez-Yanes et al., Reference Vazquez-Yanes, Orozco-Segovia, Rincon, Sanchez-Coronado, Huante, Toledo and Barradas1990). Hence, from an ecological point of view PISG under white light or natural daylight conditions occurs only in seeds exposed to direct, rather than transmitted, daylight (Pons, Reference Pons and Fenner2000).
PISG has been known for over a century (Heinricher, Reference Heinricher1903; Remer, Reference Remer1904). Kinzel (Reference Kinzel1913–1926) found that out of the 964 species he studied, light favoured seed germination in 672 species and inhibited germination in 258. However, despite the importance from evolutionary, ecological and agricultural perspectives, inhibition of seed germination under artificial white light or natural, non-shaded daylight has received little attention. This shortage of studies is in sharp contrast to the extensive literature concerning the other two major light germination responses, namely promotion by light in the laboratory or in the field (e.g. on the soil surface; Milberg et al., Reference Milberg, Andersson and Thompson2000) and inhibition by plant canopy-filtered light (Pons, Reference Pons and Fenner2000 and literature cited therein). Therefore, in this review the occurrence of PISG at seed, species and environmental levels has been explored and analysed from a physiological, ecological and evolutionary perspective.
Materials and methods
Data source
A dataset was compiled based on information in the Baskin and Baskin (Reference Baskin and Baskin2014) book and on information from a literature search that was electronically conducted within the ISI Web of Science and Google Scholar databases, using the following keywords: ‘dark germination’, ‘seed germination photoinhibition’, ‘seed germination light inhibition’, ‘soil emergence’. Studies were included in the analysis only when it was clear that a source of (white) light was used and if the germination percentage or rate in darkness was statistically significantly higher than that in light. Only data for optimal or near-optimal conditions for germination were used. A 30% final germination limit under dark conditions was applied to retain only records with a reasonably good germination response and are listed in Table 1. The discarded records are listed in supplementary Table S1.
Table 1. Photoinhibited taxa, seed properties, plant traits and PISG index (Pi).
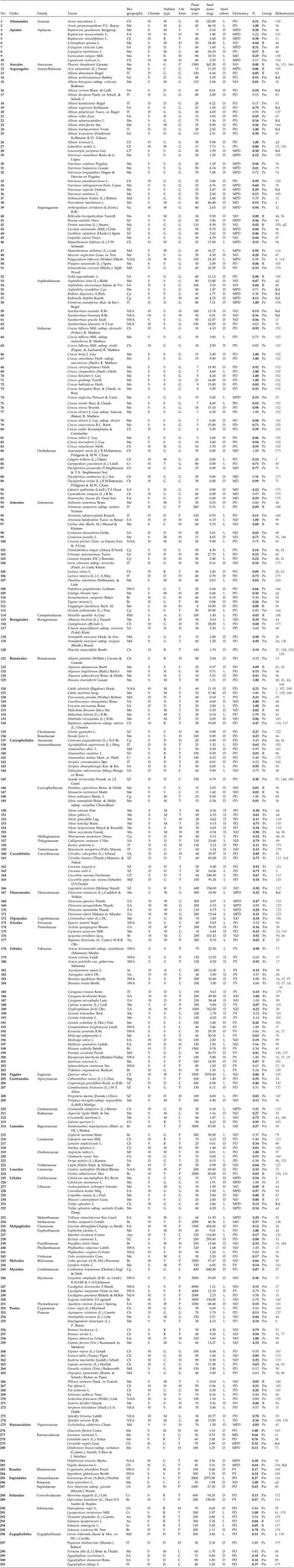
Taxonomic information was standardized against The Plant List Database (http://www.theplantlist.org/). Biogeographical range follows the Floristic Regions of Takhtajan (Reference Takhtajan1986): Am, Amazonian; An, Andean; Br, Brazilian; Cb, Circumboreal; Cp, Cape; CP, Chile-Patagonian; Cr, Caribbean; EA, Eastern Asiatic; In, Indian; IT, Irano-Turanian; Md, Madrean; Me, Mediterranean; Mg, Madagascan; Ml, Malesian; NAA, North American Atlantic; NEA, Northeast Australian; Nz, Neozeylandic; RM, Rocky Mountain; SA, Saharo-Arabian; SWA, Southwest Australia; SZ, Sudano-Zambezian. Climate: D, dry; H, humid; S, seasonal. Life forms: P, Phanerophytes; C, Chamaephytes; H, Hemicryprophytes; G, Geophytes (Cryptophytes); T, Therophytes. Habitats: A, freshwater; M, coastal; W, woodlands; T, tropical forests; G, grasslands; H, high-mountain vegetation; S, low-sized shrublands; C, cliffs and walls; D, deserts; R, agricultural and ruderal habitats. Seed colour: L, light; D, dark. Seed dormancy classes: MD, Morphological; MPD, Morphophysiological; PY, Physical; PD, Physiological; ND, Nondormancy. P i: numbers in bold = final dark germination exceeds 70%. Group: Ps, strongly photoinhibited taxa (Pi exceeds 0.5); Pw, weakly photoinhibited taxa (Pi exceeds 0.1 but not 0.5); R: light reduces only the rate of germination. Reference (s): numbers in alphabetical order within Table 1 are represented in brackets at the end of each citation in References; Kd, Filip Vandelook, Rosemary Newton, Angelino Carta unpublished data; u1, Angelino Carta unpublished data; u2, Costas Thanos, unpublished data; u3, Filip Vandelook unpublished data.
For each taxon, final germination percentage and, when available, rate in light and in darkness were recorded, as well as optimal temperature for germination, dormancy type, seed mass, life form, phylogeny, plant height, habitat type, climate and biogeographic range, all of which are known to more or less influence responses of seeds to light. Moreover, as the photoreceptors (phytochromes) are located in the embryo, the optical properties of the seed-covering structures were taken into account. Thus, in this review, seed colour was also recorded and included in the analysis. Most of this information was gathered from the original publications, but when not available it was retrieved from the literature (e.g. Tutin et al., Reference Tutin, Heywood, Burges, Valentine, Walters and Webb1964–1980; Western Australian Herbarium, 1998; Flora of China Editorial Committee, 1994+; Flora of North America Editorial Committee, 1993+).
Taxonomic information was standardized against The Plant List (2013), and phylogenetic classification followed Angiosperm Phylogeny Group (APG) IV (Stevens, Reference Stevens2001; APG, 2016). Biogeographical range followed the Floristic Regions of Takhtajan (Reference Takhtajan1986), and climate was defined according to the terrestrial ecoregions of the world (Olson et al., Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D'Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001). Plant species richness per ecoregion was derived from the estimates made by Kier et al. (Reference Kier, Mutke, Dinerstein, Ricketts, Küper, Kreft and Barthlott2005). The Seed Information Database (Royal Botanic Gardens Kew, 2016) was queried for information on a specific seed trait, when this was not available in the original publication, and for the seed mass for the world flora.
Data analysis
The PISG index (Pi) was defined as:
where GD is final germination percentage in darkness and GL is final germination percentage in light. Thus, Pi can have values between 0 and 1, with 0 corresponding to equal germination percentages in light and darkness, and 1 to germination occurring only in the dark.
For analytical purposes, we classified the species into three levels of habitat moisture (dry = 1, moist = 2, wet = 3) and light (shaded = 1, semi-shaded = 2, open = 3) conditions. Then, we fitted generalized linear models (GLMs, logit link function, binomial distribution) to analyse the effect of seed germination conditions (mean temperature and temperature regimes), environmental traits (habitat moisture and light) and plant traits (height and seed mass) on Pi. The analyses were also run for the subgroup of taxa with final germination ≥ 70%, but since the results were similar only those from the entire dataset (final germination ≥ 30%) are presented here.
The frequency distribution of seed mass in our dataset was compared with that of the world flora by means of a Kolmogorov–Smirnov two sample test. Whether both samples follow a normal distribution was assessed using a Kolmogorov–Smirnov single sample test. For graphical and analytical purposes, we estimated the probability density function of both samples applying the Kernel density estimation. This function does not assume any underlying distribution for the variable (non-parametric technique) but is extremely helpful in evaluating the underlying distribution of a continuous variable as its definite integral over its support set (the area under the density estimate) must equal 1. Hence, the area between two given values under a Kernel density estimate curve returns an estimated probability of the variable.
To assess whether observed frequencies of categorical variables (taxonomic ranks, biogeography, climate, habitat type, life form, seed colour and seed dormancy) differed significantly from theoretical expectations, we used simple χ2 tests. A χ2 test of independence was applied to determine whether there was a significant association between two categorical variables. All analyses were performed with the software R (R Development Core Team, 2015).
Results and Discussion
Occurrence
Records were examined for a total of 413 taxa. The requirements for PISG were not met for 112 taxa (supplementary Table S1). Thus, the final dataset contains 301 photoinhibited taxa, belonging to 59 families and 27 orders. The clear majority of these plants germinated to >70% in darkness (232 taxa; their Pi values shown in bold in Table 1). In 46 taxa, final dark germination ranges from 50 to 70%, and in only 23 taxa final dark germination ranges between 30 and 50%. Of the 301 taxa, 141 were strongly photoinhibited, with a Pi >50% (Ps; Table 1) while for 31 taxa, light significantly reduced germination rate without affecting final germination (R; Table 1).
Although PISG is not confined to specific major clades or biogeographical regions and habitats, some patterns have been identified. In particular, most of the photoinhibited seeds are dark coloured and relatively larger than those of the world flora (Fig. 1). They belong to non-woody plants (74%) and occur in open and dry habitats (73%; Fig. 2) at mid-latitudes (non-tropical) under a seasonal or arid climate (Fig. 4). Overall, the data suggest that PISG occurs in most biogeographical realms (excluding the Oceanic and the Antarctic, Fig. 4). Furthermore, PISG is not a limited, monophyletic phenomenon but occurs in 27 angiosperm orders (40% of all APG orders, Fig. 3).

Figure 1. Kernel density estimates of seed mass for the world flora (blue line; Royal Botanic Gardens Kew, 2016) and for the photoinhibited flora (red line; present study). Both distributions are significantly different (P < 0.001 based on Kolmogorov–Smirnov two sample test). The vertical dashed lines correspond to seed mass thresholds separating seeds whose germination is light stimulated (<0.96 mg), photoinhibited (>0.96 and <26.76 mg) and indifferent to light (>26.76 mg). The red peak, coinciding approximately with 0.002 mg, corresponds to Orchidaceae and Orobanchaceae taxa. Red areas correspond to seed mass ranges where there is a higher representation of photoinhibited taxa than of the world flora, and blue areas to those where photoinhibited taxa are less represented.

Figure 2. Proportion of photoinhibited taxa per seed dormancy class (a), life form (b) and habitat type (c). For explanation of the abbreviations see the footnote of Table 1.

Figure 3. Photoinhibition of seed germination as percentage per order of families (left) and genera (right) with documented PISG. Phylogeny follows APG IV (Stevens, Reference Stevens2001; APG et al., 2016).

Figure 4. Percentage of photoinhibited taxa in each climatic region within each biogeographical realm (terrestrial ecoregions of the world; see Olson et al., Reference Olson, Dinerstein, Wikramanayake, Burgess, Powell, Underwood, D'Amico, Itoua, Strand, Morrison, Loucks, Allnutt, Ricketts, Kura, Lamoreux, Wettengel, Hedao and Kassem2001) calculated based on plant richness estimates from Kier et al. (Reference Kier, Mutke, Dinerstein, Ricketts, Küper, Kreft and Barthlott2005). Realms: Australasia, Antarctic, Afrotropics, IndoMalay, Nearctic, Neotropics, Oceania, Palearctic. Climatic regions: tropical humid, tropical dry, temperate humid, temperate montane, cold, tropical semi-arid, temperate semi-arid, montane, polar, mediterranean, arid.
While compiling the database and assembling the available literature, a reasonable question arose. How many species in the world flora can be expected to exhibit PISG? To answer this inquiry, we need to know the approximate number of taxa with known germination behaviour. A reliable proxy for this is the 15,311 taxa in SID (Royal Botanic Gardens Kew, 2016). On the basis of the total number of taxa in Table 1, we estimate a frequency of 2% for PISG, a moderate estimate compared with the one (26.8%) reported by Kinzel (Reference Kinzel1913–1926). Furthermore, by simple extrapolation, we can expect a total number of ca 6000 taxa [i.e. 0.02 × ca 300,000 angiosperms according to Christenhusz and Byng (Reference Christenhusz and Byng2016)] with PISG in the world flora.
Seed mass
Seed mass distributions for PISG (301 taxa) and the world flora (34,395 taxa; Royal Botanic Gardens Kew, 2016) are normal (Kolmogorov–Smirnov single sample test, P < 0.001), but their probability density estimates mostly do not overlap (Fig. 1). Indeed, the photoinhibited group has significantly (Kolmogorov–Smirnov two sample test, P < 0.001) larger seeds (mean 3.09 mg) than that of the world flora (mean 1.34 mg). Furthermore, seed mass has a standard deviation of 2.22 compared with 3.38 for the world flora and the probability for the photoinhibited seed mass curve in the range 0.96–26.76 mg (c in Fig. 1), is significantly higher (65.3%) than that of the world flora. Thus, while almost two-thirds of PISG taxa fall within this range, the corresponding value for the world flora is 45.9%. A secondary, minor peak of the PISG curve lies in the range 0.0003–0.0078 mg (a in Fig. 1). This ‘hump’ represents ca 3.4% of the PISG taxa and corresponds to members of Orchidaceae and Orobanchaceae listed in the dataset. Furthermore, within the ranges 0.0078–1 mg and >26.76 mg (b and d in Fig. 1, respectively), the PISG curve is lower than that of the world flora distribution. Thus, PISG is certainly under-represented in these particular ranges, arguably as the result of the well-known, relative predominance of light-requiring (e.g. Grime et al., Reference Grime, Mason, Curtis, Rodman, Band, Mowforth, Neal and Shaw1981; Pons, Reference Pons and Fenner2000) and light-indifferent (e.g. Milberg et al., Reference Milberg, Andersson and Thompson2000; Pearson et al., Reference Pearson, Burslem, Mullins and Dalling2002) seeds, respectively.
Indeed, the lower value of the 0.96–26.76 mg range is consistent with the threshold of 1 mg, below which seeds are more likely to have a light-stimulated germination (Pons, Reference Pons and Fenner2000), and with the approximate cut-off (1.5 mg) between species that require light for germination and those that do not, which was identified by Jankowska-Blaszczuk and Daws (Reference Jankowska-Blaszczuk and Daws2007) while studying the impact of red:far red ratio on temperate forest herbs. The higher value of the 0.96–26.76 mg range suggests that seeds larger than ca 27 mg should be indifferent to light/dark conditions, since their seed mass may not represent a constraint for the emergence of germinated seeds. While studying the germination ecology of neotropical pioneers, Pearson et al. (Reference Pearson, Burslem, Mullins and Dalling2002) found that the maximum mass of species likely to show a significant positive germination response to irradiance was 0.7, whereas the mean seed mass of genera containing light indifferent species was 22.7 mg, confirming both thresholds identified in the present study.
For species with relatively medium-sized seeds, in which seed mass is well above the assumed thresholds for light requirement, PISG is a rather more advantageous mechanism compared with a light requirement (Skourti and Thanos, Reference Skourti and Thanos2015). This is because seedling emergence from burial depths below the upper 4–5 mm of the soil [where light is present in physiologically significant quantities (Tester and Morris, Reference Tester and Morris1987)] can take place before the seed reserves are depleted. This might also explain why large seeds are correlated with dry and seasonal habitats, i.e. they can be buried as deeply as their particular seed reserves allow seedling emergence (Leishman et al., Reference Leishman, Wright, Moles, Westoby and Fenner2000; Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008; Vandelook et al., Reference Vandelook, Verdú and Honnay2012). On the other hand, large seed size is also an adaptation to aseasonal and moist habitats (Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003), reducing the likelihood of desiccation-induced mortality in desiccation-sensitive or recalcitrant seeds (Daws et al., Reference Daws, Gamene, Glidewel and Pritchard2004). Large seed mass for recalcitrant seeds, which does not represent a constraint for emergence when buried and indifference to light for seeds larger than ca 27 mg or PISG for seeds with masses of between ca 1 and 27 mg, may therefore be adaptations to promote germination of buried seeds. It can thus be hypothesized that the ecological conditions that favour recalcitrance also select for large seed size.
Seeds of terrestrial orchids usually germinate better in darkness than in light (e.g. Waes and Debergh, Reference Waes and Debergh1986; Zettler and McInnis, Reference Zettler and McInnis1994; Wang et al., Reference Wang, Baskin, Cui and Du2009), although PISG in terrestrial orchids is by no means universal (e.g. Oliva and Arditti, Reference Oliva and Arditti1984; Dutra et al., Reference Dutra, Johnson, Kauth, Stewart, Kane and Richardson2008). Orchids, and maybe other dust-seeded taxa as well, are clearly an exception to the 1 mg lower threshold of PISG. This is probably due to their specific germination ecology in association with fungi. Not only are seedlings from these dust-seeds less likely to run out of reserves before reaching the soil surface, but burial in the soil can also bring seeds into fungus-rich substrates (Zettler and McInnis, Reference Zettler and McInnis1994). Light responses have been much less studied in epiphytic orchids, but at least one species, Cyrtopodium punctatum, is photoinhibited (Dutra et al., Reference Dutra, Kane and Richardson2009). Much work is still needed to find general patterns in PISG in orchids. Also, most orchid seed germination experiments have been performed in asymbiotic and sterile laboratory conditions. However, burial experiments with seeds of Dactylorhiza maculata, Epipactis helleberoni (van der Kinderen, Reference van der Kinderen1995) and Cephalanthera damasonium (Roy et al., Reference Roy, Gonneau, Rocheteau, Berveiller, Thomas, Damesin and Selosse2013) have shown that seeds of these orchids can germinate, in the absence of light, up to 7–10 cm deep in the soil. Both D. maculate and E. helleborine have been shown to germinate better in the dark than in light in laboratory conditions (Waes and Debergh, Reference Waes and Debergh1986), but a control experiment testing seed germination in exposed conditions in nature is missing.
Seed colour
Seed coats can be intensely pigmented, which reduces the PFD and alters the spectral composition of the light inside the seeds (Widell and Vogelmann, Reference Widell and Vogelman1988). Most of the photoinhibited seeds were dark coloured (65%), confirming previous findings that PISG was related to black or dark-coloured seeds (Thanos, Reference Thanos, Hendry and Grime1993; Thanos et al., Reference Thanos, Fournaraki and Makri2005; Fournaraki, Reference Fournaraki2010). It has been reported that this optical property of the dark seed coat reduces light transmission to phytochrome in the embryo of seeds (Widell and Vogelmann, Reference Widell and Vogelman1988). However, in our dataset, dark seeds were significantly over-represented in seasonal climates (χ2 test of independence, P < 0.001) and it is possible that the ‘abundance’ of dark seeds could be a consequence of habitat selection (P < 0.01) or phylogenetic inheritance at the order, family and genus levels (P < 0.001, see also ‘Phylogeny’ section below), rather than a convergent evolution of seed coat colour and PISG. For example, the most important synapomorphy for Asparagales, first used by Huber (Reference Huber1969) as a unifying character in the order, is the characteristic black colour of the seeds caused by phytomelanin incrustation of the seed coat. This black substance is also common among seeds of Asteraceae (Stevens, Reference Stevens2001).
In Brassicaceae, seed colour seems to be related to geographical distribution through an association between high temperatures and light-coloured seed coats, with a few exceptions such as the brownish seeds of Mediterranean species (Van Deynze et al., 1992). If this is the case for other species, the high percentage of dark seeds may be due to the geographical distribution of photoinhibited seeds and not to the seed coat properties themselves. The above hypothesis is corroborated by results in Arabidopsis (Debeaujon et al., Reference Debeaujon, Leon-Kloosterziel and Koornneef2000), in which seed pigmentation mutants (less pigmented seeds) exhibit a higher capacity to germinate in darkness.
Seed germination
Plants in frost and drought conditions are more likely to have dormancy than species in milder and wetter environments (Jurado and Flores, Reference Jurado and Flores2005). In tropical rainforests, nondormancy is more frequent than in any other vegetation zone, and when temperate broad-leaved evergreen forests, deciduous forests, steppes, matorral and cold deserts are compared, non-dormancy decreases with a decrease of precipitation and temperature (Baskin and Baskin, Reference Baskin and Baskin2014). The great majority of photoinhibited species included in the present study have been reported to be dormant, with only ca 20% of them being non-dormant (Fig. 2a). In particular, 52% of the photoinhibited species possess some degree of physiological dormancy (PD; Fig. 2a). These data are not surprising, since PD is the most common class of dormancy among seed plant species (Baskin and Baskin, Reference Baskin and Baskin2014).
The percentage of photoinhibited species with morphophysiological dormancy (MPD) is relatively high (15%; Fig. 2a), and this is similar to the expected percentage of temperate, herbaceous species with MPD (Baskin and Baskin, Reference Baskin and Baskin2014). A possible reason for this is that MPD is common among monocots and geophytes, which was the main photoinhibited life form in our dataset (Fig. 2b). Perhaps unexpectedly, some physically dormant (PY) species also have photoinhibited (9%) seeds. While there is emerging evidence that species with PY from fire-prone habitats show this kind of response to light (e.g. Turner et al., Reference Turner, Merritt, Baskin, Dixon and Baskin2005), seed germination of species with PY is generally considered as neither suppressed nor promoted by the presence of light (Baskin and Baskin, Reference Baskin and Baskin2014).
Some authors reported that PISG is stronger at higher germination temperatures (Thanos et al., Reference Thanos, Georghiou and Skarou1989; Bell et al., Reference Bell, Rokich, McChesney and Plummer1995). However, the present review has not considered the possible dependence of photoinhibition on incubation temperature, i.e. promotion by light at certain temperatures and inhibition by others, in the same species and under similar light conditions (Fournaraki, Reference Fournaraki2010).
Life form and plant height
Non-woody species were the most common among the photoinhibited taxa, with most of them being geophytes (37%) followed by therophytes, i.e. annuals (19%) (Fig. 2b). These two life forms are significantly associated with arid and seasonal climates (χ2 test of independence, P < 0.05). Geophytes are by far over-represented in the present study, considering that on a global scale they represent only 4% of the flora (Cain, Reference Cain1950). Even in the Cape Floristic Region, the most geophyte-rich area on earth, they reach only about 23%. Also, it has been recently suggested that PISG is a common germination characteristic among geophytes from relatively dry habitats (Skourti and Thanos, Reference Skourti and Thanos2015 and literature cited therein).
Phanerophytes (woody plants), which typically prevail in tropical semi-arid forests, are also represented in the dataset (14%), and this life form is most common in tropical semi-arid forests. A significant association between life forms and habitat types was also found (χ2 test of independence, P < 0.001): with shrubs prevailing in scrubland (31%) and deserts (28%); geophytes in scrubland (40%) and grassland (25%); and annuals in ruderal (32%), maritime (22%), desert (20%) and grassland habitats (17%).
Logistic regression predicted a higher occurrence of PISG in relatively small plants (Table 2). However, Grime et al. (Reference Grime, Mason, Curtis, Rodman, Band, Mowforth, Neal and Shaw1981) detected no consistent relationship between germination in the dark and average height of seed release (i.e. plant height). Considering that plant height is also related to life form, and in relevance to the leaf-height-seed (LHS) strategy scheme proposed by Westoby (Reference Westoby1998), further analyses are needed to confirm whether seedling establishment of smaller plants benefits from photoinhibition of seeds. Although an elaborate analysis of the relationship of plant height, leaf area and seed mass, under the light of photoinhibition may be promising, it is beyond the scope of this review.
Table 2. Simple generalized linear models (GLMs, logit link function and binomial distribution) results for the effect of alternating temperature and mean temperature used in the germination experiments, plant height, seed mass, habitat light and habitat moisture on the Pi

For analytical purposes, we classified the species into three categories according to habitat moisture (dry = 1; moist = 2; wet = 3) and habitat light (shaded = 1; semi-shaded = 2; open = 3).
Phylogeny
Gymnosperms have not been unequivocally proven to include photoinhibited taxa, despite a limited number of reports (on Abies amabilis, A. lasiocarpa and Pinus monticola, Li et al., Reference Li, Burton and Leadem1994; Tsuga mertensiana, Edwards and El-Kassaby, Reference Edwards and El-Kassaby1996; Podocarpus latifolius, Bussmann and Lange, Reference Bussmann and Lange2000). They are not included in the dataset because the PISG or the methodology were either not clearly explained or were dubious. Therefore, we can postulate that PISG seems to be apomorphic to flowering plants. Moreover, there are no published photoinhibition reports for the ANA grade and only few records in the Magnoliids (specifically in the Laurales), while PISG is quite widespread in eudicots and especially in monocots.
The percentages of families and genera with photoinhibited taxa, superimposed onto the phylogenetic framework, showed that the distribution of PISG across the seed plants occurs more frequently in certain lineages than others (Table 1, Fig. 3). The single most represented order is Asparagales (27%), followed by Fabales (9%), Asterales (7%), Caryophyllales (7%) and Poales (7%). The most represented families are Amaryllidaceae (9%), Fabaceae (8%), Asteraceae (7%), Iridaceae (7%), Poaceae (6%) and Asparagaceae (5%). Four of these families are monocots and three belong to Asparagales. The total number of monocot taxa is 122, or 40.5% among plants with PISG, a considerable divergence from the estimated 26.1% of monocots in the world flora (of eudicots plus monocots; Christenhusz and Byng, Reference Christenhusz and Byng2016).
Overall, PISG is clearly not monophyletic and shows a large degree of homoplasy across seed plants. Thus, this trait is shared by taxa belonging to distinct clades, due to convergence, parallelism or reversal. Consequently, while seed germination behaviour may often be inferred from embryo morphology (Baskin and Baskin, Reference Baskin and Baskin2004) and other conservative seed morphological traits (Corner, Reference Corner1976), a summarization based on shared morphological and functional seed characteristics by taxa belonging to the same lineage is not possible for PISG.
At present, the ancestral PISG state is not known. In particular, it is unclear whether PISG is ancient or a recent acquisition. Our understanding of the molecular mechanisms is incomplete, but there is evidence that, as for seed dormancy (Willis et al., Reference Willis, Baskin, Baskin, Auld, Venable, Cavender-Bares, Donohue and Rubio de Casas2014), most of the molecular pathways controlling PISG are common among seed plants. Thus, the information gained to date, suggests that PISG is likely to have evolved independently in different lineages (homoplasy).
Habitat and climate
Logistic regression revealed that Pi is strongly associated with open habitats (Table 2). Scrubland is the most represented habitat type overall (27%; Fig. 2c), mainly in Southwest Australian (75% of taxa) and Mediterranean regions (45%). However, scrublands consist primarily of a mosaic of habitats with different degree of vegetation cover. Thus, many (herbaceous) species reported as growing in scrublands could perhaps generally be defined as species growing in open habitats. In fact, grassland is the third most represented habitat category (14%) after deserts (16%). These results are not unexpected, since light-inhibited germination traditionally has been interpreted as an adaptive mechanism for plants inhabiting sandy, coastal habitats (Thanos et al., Reference Thanos, Georghiou and Skarou1989, Reference Thanos, Georghiou, Douma and Marangaki1991; Bell et al., Reference Bell, Plummer and Taylor1993; Delipetrou, Reference Delipetrou1996), deserts (Koller, Reference Koller1956; Barbour, Reference Barbour1968; Gutterman, Reference Gutterman, Black, Bewley and Halmer2006) and semi-arid and open disturbed habitats (Thanos et al., Reference Thanos, Fournaraki and Makri2005). In scrublands, grasslands and deserts (61% of taxa in our dataset), and in open and dry habitats in general (73% of taxa), water availability is limited, even temporarily, thus making germination strategies that avoid seedling desiccation, crucial for successful germination and subsequent seedling establishment in these environments. Additionally, Carta et al. (Reference Carta, Probert, Moretti, Peruzzi and Bedini2014) argued that PISG coupled with epicotyl dormancy protects seedlings of Crocus neglectus growing in Mediterranean montane grasslands from frost damage in early winter.
Interestingly, a significant association between habitat types and plant orders has been found: deserts (Asparagales, Asterales, Caryophyllales and Cucurbitales), grasslands (Asparagales, Asterales, Boraginales, Liliales and Ranunculales), coastal dunes (Apiales, Asparagales, Asterales, Brassicales, Caryophyllales, Gentianales and Poales), ruderals (Solanales), scrubland (Asparagales, Caryophyllales, Fabales, Liliales and Myrtales), tropical woodlands (Solanales and Malpighiales) and woodlands (Asparagales and Dioscoreales).
From an ecogeographical point of view, there is a clear pattern that can be mainly attributed to climatic conditions. It is noteworthy that PISG is absent from both humid tropical and cold areas, whereas regions with seasonal and especially arid climates host the majority of photoinhibited taxa (Fig. 4). However, a bias in the distribution may be due to the low number of studies conducted outside Eurasia. We speculate that PISG became more frequent mainly in mid-latitude seasonal climates and in coincidence of palaeoclimatic events related to the Neogenic orogenesis, leading to the expansion of open habitats and the establishment of modern deserts (Patterson and Givnish, Reference Patterson and Givnish2002). However, ancestral state reconstructions based on a worldwide phylogenetic comparative study should be encouraged to elucidate the patterns behind PISG evolution.
Presumed mechanisms
A rigorous discussion of the molecular and photomorphogenetic mechanisms that modulate PISG is beyond the scope of this review. Nevertheless, it must be noted that phytochrome has been routinely implicated in PISG although the involvement (coaction) of another photoreceptor cannot be excluded entirely (Casal and Sánchez, Reference Casal and Sánchez1998). PISG can be described graphically by photoinhibition curves (final germination vs log fluence rate), which are usually linear (Thanos, Reference Thanos, Hendry and Grime1993). Furthermore, PISG certainly belongs to the HIR class of responses as it requires long durations of irradiation (white, red, far red or blue) and depends on both fluence rate and wavelength. On the other hand, it does not show a red/far red reversibility, nor does it obey the reciprocity law. It should be stressed that, in striking contrast to other HIR, PISG inhibits – rather than promotes – a photomorphogenetic response. A possible mechanism proposed by Thanos et al. (Reference Thanos, Georghiou, Douma and Marangaki1991) attributes this response to phytochrome intermediates that are trapped during seed desiccation in a form that upon seed imbibition can slowly revert to Pfr (active phytochrome) and thus eventually promote dark germination. Furthermore, a sufficiently intense, long irradiation is required to inhibit seed germination by continuously recycling phytochrome between its active and inactive forms and thus obstructing it from acting. A modern approach would probably implicate phytochrome A (light-labile, type I phytochrome) or even phytochrome C, but no relevant experimental investigation has been recently attempted.
Outlook
Although currently the survival value of PISG cannot be measured, the present study offers useful hints to understand its ecological significance. That is, PISG is a physiological adaptation to avoid germination on the soil surface, where conditions may not be suitable for seedling establishment, especially in habitats susceptible to drought (Koller, Reference Koller1956; Thanos et al., Reference Thanos, Georghiou, Douma and Marangaki1991; Bell et al., Reference Bell, Plummer and Taylor1993; Thanos et al., Reference Thanos, Fournaraki and Makri2005). Nevertheless, these conclusions should be treated with caution, since we have considered only the effects of light; other factors, such as temperature, may modify the responses to light considerably and should be investigated in future studies. In contrast to the strong association of PISG to aridity, certain species growing in wet habitats and temperate climates also show PISG, apparently without a clear ecological benefit. A possible explanation is that although phylogeny itself cannot be the single predictor of PISG, this dark germination might have been conserved among related species, despite the fact that it confers no obvious advantage in such habitats (phylogenetic inertia). It is suggested that evolutionary patterns, like PISG, should be further investigated, especially among monocots.
Overall, PISG is probably much more widespread in seed plants than previously thought and special attention should be paid in designing germination experiments under both dark and light conditions and, if possible, using sunlight-type, prolonged illuminations with different levels of irradiance.
Acknowledgements
We are grateful to Jerry Baskin and Hugh W. Pritchard for their valuable comments.
Financial support
The European Native Seed Conservation Network (ENSCONET) Consortium is acknowledged for financial support.
Conflicts of interest
None.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258517000137









