Introduction
Seeds within a population germinate over a period of time even under optimal germination conditions without dormancy. Such characteristics have adaptive value (Donohue, Reference Donohue2002; Mandak et al., Reference Mandak, Bimova and Plackova2006) because germination over time also determines the ability of plant populations to colonize and persist in changing environments (Donohue et al., Reference Donohue, Dorn, Griffith, Kim, Aguilera, Polisetty and Schmitt2005a, Reference Donohue, Dorn, Griffith, Kim, Aguilera, Polisetty and Schmittb). Consequently, assessment of environmental and genetic influences on seed germination should allow for a better understanding of fundamental processes of population establishment, range expansion and geographic differentiation (Donohue, Reference Donohue2005).
There is considerable evidence that germination timing is under some degree of heritable genetic control (Squire et al., Reference Squire, Marshall, Dunlop and Wright1997; Cabin et al., Reference Cabin, Mitchell and Marshall1998; Marshall et al., Reference Marshall, Dunlop, Ramsay and Squire2000). For example, Squire et al. (Reference Squire, Marshall, Dunlop and Wright1997) showed that subpopulations with early and late germination of an oilseed rape cultivar produced progeny with distinct germination characteristics, indicating genetic control of germination rate. Donohue et al. (Reference Donohue, Dorn, Griffith, Kim, Aguilera, Polisetty and Schmitt2005c) detected a significant genotype-by-environment interaction influencing germination timing in recombinant inbred lineages of Arabidopsis thaliana. Some studies in natural populations showed that different germination environments favoured particular genotypes and significant genetic differentiation existed between early and late-germinating seeds (Cabin et al., Reference Cabin, Evans and Mitchell1997; Mandak et al., Reference Mandak, Bimova and Plackova2006). However, the extent to which germination timing is genetically or environmentally controlled remains poorly understood (Donohue, Reference Donohue2005).
Plains rough fescue [Festuca hallii (Vasey) Piper] is the diagnostic, long-lived perennial species in Fescue Prairie, and a North American endemic tetraploid (2n = 4x = 28), presumably outcrossing (Aiken et al., Reference Aiken, Dallwitz, McJannet and Consaul1996 onwards, Reference Aiken, Dallwitz, McJannet and Consaul1997; Anderson, Reference Anderson2006). It grows on deep, well-drained soils, and is highly palatable to livestock, particularly during the winter. The fescue plant can propagate with seeds and rhizomes but seed production is erratic, perhaps dependent upon conditions in the previous growing season (Johnston and MacDonald, Reference Johnston and MacDonald1967; Romo et al., Reference Romo, Grilz, Bubar and Young1991). Remnant F. hallii stands have been found throughout the Canadian prairies. Efforts have been made to re-establish a network of fescue-dominated patches across its historic range. However, the effectiveness of such restoration efforts remains uncertain, as the biology of dominant fescue species is poorly understood (Romo, Reference Romo2003) and the germination of F. hallii seeds may affect the success of plant establishment in changing environments (Romo et al., Reference Romo, Grilz, Bubar and Young1991).
The objective of this study was to assess the comparative genetic variation of germinated seeds collected from three F. hallii populations in the Canadian prairie using the amplified restriction fragment polymorphism (AFLP) technique. The AFLP technique (Vos et al., Reference Vos, Hogers, Bleeker, Reijans, van De Lee, Hornes, Frijters, Peleman, Kuiper and Zabeau1995) is a robust, highly effective molecular tool that has been widely applied to assess genetic variability in grass species (e.g. Larson et al., Reference Larson, Waldron, Monsen, St John, Palazzo, McCracken and Harrison2001a, Reference Larson, Cartier, McCracken and Dyerb; Fu et al., Reference Fu, Coulman, Ferdinadez, Cayouette and Peterson2005a, Reference Fu, Thompson, Willms and Mackayb; Qiu et al., Reference Qiu, Fu, Bai and Wilmshurst2007). Three subpopulations of seeds with early, intermediate and late germination were identified based on the position of the seeds from each population in the time-profile of germination under a given temperature. Specifically, we strived to address the following questions. Is the germination timing of F. hallii seeds affected by germination temperature? Does the same germination subpopulation of seeds display a genetic difference under variable germination temperatures? Do different germination subpopulations of seeds show the same pattern of genetic variation under the same germination temperature? And, is there any genetic differentiation among various groups of seeds for surveyed populations?
Materials and methods
Seed collection, seed germination and subpopulation allocation
Three F. hallii populations were selected for this study: Prince Albert National Park (53°35′16″N, 106°02′26″W, located in Boreal Transition Ecoregion), Turtleford (53°27′38″N, 109°03′12″W, in Boreal Transition Ecoregion) in Saskatchewan, and Riding Mountain National Park (50°48′35″N, 100°14′42″W, in Mid-Boreal Upland Ecoregion) in Manitoba. These populations were abundant in seed production and represented the geographical range of all the surveyed populations. Seeds were collected within 1 week in late July. Collected seeds were kept in paper bags under room temperature for 2 months, then cleaned using a seed blower, and stored at − 18°C until use. Benomyl powder (0.05%) was applied to sterilize seeds at the beginning of the germination test.
Seed germination was investigated under 10, 15 and 20°C temperature regimes in growth chambers (Sanyo Versatile Environment Chamber MLR-350H, Sanyo Scientific, USA). A randomized complete block design was used with three replicates, and replicates were put into growth chambers at 1-week intervals. Seeds were randomly selected from each population and germinated on top of two layers of filter paper (Whatman No.1) in separate cells of 96-well plates. The filter paper was moistened with 40 μl of distilled water and one seed was placed on the top of the paper. In total, 288 seeds were germinated for each population and temperature. Seeds were checked and counted daily and the days to germinate for each seed were recorded. Germinated seeds were transplanted into pots and grown in the greenhouse. Seedlings were labelled with population, temperature, replicate and days to germination. Germination tests were terminated when no seeds germinated for 14 consecutive days. Non-germinated seeds were then tested for viability by finger pressing. Viable seeds were firm when pressed with finger tips. After finger pressing, all the soft seeds were cut longitudinally and checked for embryos. The numbers of viable, dead and empty seeds were recorded. Results showed that ungerminated seeds were either dead or empty.
Final germination percentage was calculated based on total seeds incubated minus dead and empty seeds. Since the final germination percentage of each population and temperature was not significantly different among replicates, transplanted seedlings were pooled among replicates for each population and temperature and ranked by the days to germination for subpopulation allocation. The first 0–10% of the seeds germinated were classified as early subpopulations, the middle range of seeds germinated (45–55%) were classified as intermediate subpopulations, and the last (range 90–100%) as late subpopulations (Fig. 1).

Figure 1 The time-course of seed germination at 10, 15 and 20°C for three F. hallii populations. Germination percentages, based on the total number of seeds incubated minus dead and empty seeds, were used to identify germination subpopulations. The first 0–10% of the seeds germinated were classified as early germination subpopulations, the middle range of the seeds germinated (45–55%) were classified as intermediate germination subpopulations, and the last (range 90–100%) as late germination subpopulations.
AFLP analysis
Twenty resulting seedlings were randomly selected from each germination subpopulation and a total of 540 seedling samples were obtained from 27 groups representing germination subpopulations, temperature regimes and seed-source populations. Young leaf tissue of selected seedlings was individually harvested after 45 d in the greenhouse from transplanting, freeze-dried and stored at − 80°C until use. Genomic DNA was extracted from the leaf samples using the DNeasy Plant Mini Kit (Qiagen Inc., Mississauga, Ontario, Canada) according to the manufacturer's instructions. Extracted DNA was quantified by fluorometry using Hoechst 33,258 stain (Sigma Chemical Co., St. Louis, Missouri, USA), and diluted to 25 ng μl− 1 for AFLP analysis.
The AFLP™ Analysis System 1 (Life Technologies, Burlington, Ontario, Canada) was applied following the protocol described by Vos et al. (Reference Vos, Hogers, Bleeker, Reijans, van De Lee, Hornes, Frijters, Peleman, Kuiper and Zabeau1995) with a modification of using γ33P to label EcoRI selective primers. After electrophoresis of amplified DNA fragments, the gel was transferred to Whatman 3MM paper, dried on a gel dryer for 2 h at 80°C, and exposed to Kodak BIOMAX film at − 80°C for 1 to 7 d, depending on the signal intensity. Three EcoRI:MseI primer pairs (E+AAC/M+CAG, E+ACG/M+CTA, and E+AGG/M+CTC) were used to screen all samples, based on the previous AFLP analysis of F. hallii (Qiu et al., Reference Qiu, Fu, Bai and Wilmshurst2007). To minimize technique-related and scoring errors, three duplicated samples of one individual were arranged across all gels as a control.
Automated analysis of banding patterns on 18 gels was conducted using GelComparII™ (Applied Maths, Belgium). The gel images were automatically scored as 1 (present) or 0 (absent). The duplicated samples were used to assess the consistency of AFLP reactions. Only the bands either present or absent in all the 18 control samples for each primer pair were output for data analysis.
Data analysis
A three-way analysis of variance (ANOVA) was conducted for the days to germinate (in log scale) with respect to source population, germination subpopulation and temperature. Since the population displayed significant interactions with the other two factors, a two-way ANOVA was then conducted for subpopulation and temperature. Significance of group means was assessed with post hoc multiple comparison tests.
The presence/absence data from the AFLP samples were analysed for the level of polymorphism by counting the total number of bands and the number of polymorphic bands and calculating their band frequencies with respect to source population, germination subpopulation and temperature. To identify the chromosome regions or segments associated with seed germination, a χ2-test was performed following Fu et al. (Reference Fu, Thompson, Willms and Mackay2005b) to determine the significance of the AFLP differences at each AFLP band between early and late germination subpopulations. This was repeated for all polymorphic AFLP bands for each temperature regime and each population.
Analysis of molecular variance (AMOVA) was performed with respect to source population, germination subpopulation and temperature regime. This analysis not only allows partitioning of the total AFLP variation into within- and among-group components, but also provides a measure of inter-group distances as a proportion of the total AFLP variation residing between any two groups (the Phi statistic; Excoffier et al., Reference Excoffier, Smouse and Quattro1992; Huff et al., Reference Huff, Quinn, Higgins and Palazzo1998). The significance of variance components and inter-group distances was tested with 10,098 random permutations. Three models of genetic structuring for germinated seeds were assessed: source population, subpopulation of germination and temperature group.
A group-specific AMOVA analysis was also performed to assess the differences in AFLP variation among 27 groups representing source population, germination subpopulation and temperature regime. The resulting group–distance matrix was analysed using NTSYS-pc 2.3 (Rohlf, Reference Rohlf1997) and unrooted trees were obtained from distance matrices using the neighbour-joining procedure (Saitou and Nei, Reference Saitou and Nei1987) to assess the genetic differentiation of the 27 representative groups of the fescue seeds.
Results
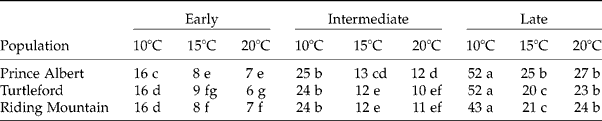
The germination timing of F. hallii seeds was affected by germination temperature. The interactions between germination timing and temperatures were significant for the days to germination (Table 1). Differences in germination time between early and intermediate subpopulations were smaller than those between intermediate and late germination subpopulations. Seeds took many more days to germinate at 10°C than at 15 and 20°C. No significant difference in the days to germinate was found between 15 and 20°C, except for seeds of late germination subpopulations from Riding Mountain and Turtleford populations.
Table 1 Days to completion of germination with respect to germination subpopulation and test temperature in three F. hallii populations. The numbers followed by different letters are statistically different within a population at P≤0.05

Three germination subpopulations in each population displayed variation in mean band frequency under three temperature regimes (Fig. 2). The same germination subpopulation of seeds displayed genetic differences under variable germination temperatures. Germinating seeds in all three populations had higher estimates of mean band frequency at 20°C than at 10°C. Different germination subpopulations of seeds showed different patterns of genetic variation under the same germination temperature. Early germination subpopulations had consistently lower estimates of mean band frequency than late germination subpopulations at 10°C but had consistently higher estimates of mean band frequency than late germination subpopulations at 15°C. At 20°C, the estimate of mean band frequency was higher in early germination subpopulations than the others from the Prince Albert population, but it was lower than the others from the Riding Mountain and Turtleford populations. None of the 188 polymorphic AFLP bands displayed a significant AFLP difference between the early and late germination subpopulations under any temperature in any population assayed, after Bonferroni correction.

Figure 2 Patterns of estimates in mean AFLP band frequency with respect to source population, germination subpopulation and temperature regime.
There was genetic differentiation among various groups of seeds for surveyed populations. Partitioning of the total AFLP variation showed that 92.4% of the total AFLP variation was held among seeds of any given population, 5.7% among seeds of different populations, and 1.9% among seeds grouped for germination timing and temperatures (Table 2). These variation fractions were significantly different from zero at P < 0.0001 based on the permutation test. Analysis of molecular variance with respect to germination timing and temperature regime revealed that only 0.3% AFLP variation resided among seeds grouped for germination timing and 0.5% AFLP variation among seeds grouped for temperature regime.
Table 2 Analysis of molecular variance (AMOVA) within and among groups of germinating seeds of the three F. hallii populations under variable test temperatures. Three models of genetic structuring were assessed with respect to source population, germination subpopulation and test temperature

All the variance components were statistically significant at P < 0.001 level, as calculated from 10,098 random permutations.
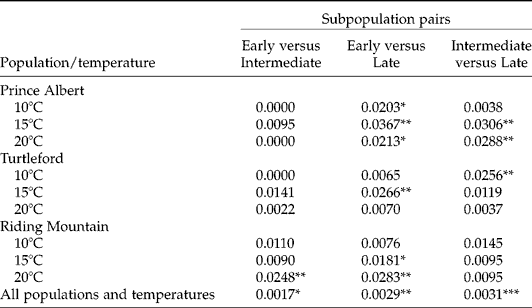
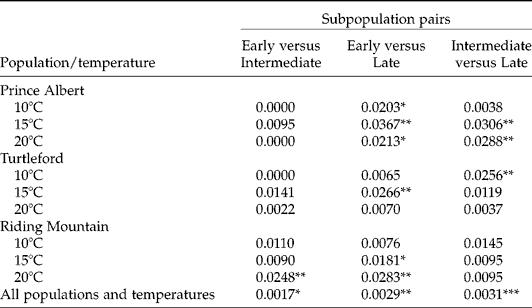
Assessment of group-wise genetic differences with the proportional AFLP variations showed significant genetic differences among three germination subpopulations (Table 3). The largest difference was present between intermediate and late germination subpopulations and the smallest between early and intermediate germination subpopulations. For specific populations and germination temperatures, the AFLP differences among germination subpopulations could be up to 3.7%. The differences between early and late germination subpopulations were found in all populations and temperatures, and those between early and intermediate germination subpopulations were found only in the Riding Mountain population under the germination temperature of 20°C.
Table 3 Genetic differentiation between germination subpopulations of F. hallii seeds with respect to source population and test temperature, as measured by the proportion of the total AFLP variation
*, ** and ***, significant at P ≤ 0.05, 0.01 and 0.001, respectively.
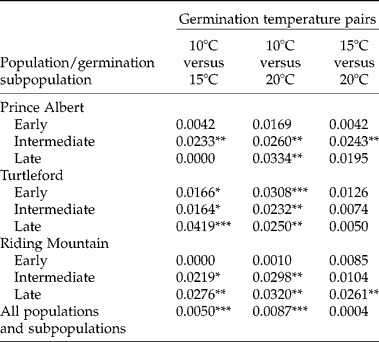
Early germination subpopulations from both Prince Albert and Riding Mountain populations did not display significant AFLP differences under variable germination temperatures (Table 4). Intermediate germination subpopulations from the Prince Albert population and late germination subpopulations from the Riding Mountain population showed significant AFLP variation under germination temperatures between 15 and 20°C. The largest AFLP difference was observed for germinating seeds under temperatures between 10 and 20°C and the least AFLP variation between 15 and 20°C.
Table 4 Genetic differentiation within the same group of F. hallii seeds under variable test temperatures with respect to source population and germination subpopulation, as measured by the proportion of the total AFLP variation
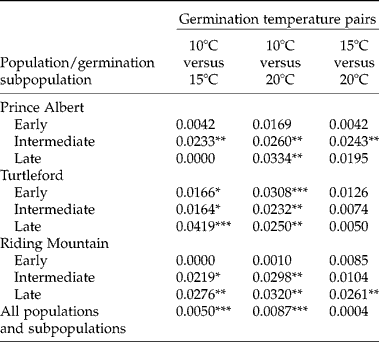
*, ** and ***, significant at P ≤ 0.05, 0.01 and 0.001, respectively.
Assessment of the genetic differentiation among 27 groups of germinating seeds revealed three distinct clusters with each population grouped together (Fig. 3). The most genetically distinct cluster consisted of various groups of germinating seeds from the Riding Mountain population. Within each distinct group, early and intermediate subpopulations tended to be clustered together.

Figure 3 A neighbour-joining tree of the 27 groups of germinating seeds representing source population, germination subpopulation and temperature regime, which was based on pairwise genetic distances (Phi statistics) derived from the analysis of AFLP variation. Each group is labelled with source population (PA, Prince Albert National Park; RM, Riding Mountain National Park; S77, Turtleford) and test temperature (10, 10°C; 15, 15°C; 20, 20°C) and germination subpopulation (Ear, Interm and Late for early, intermediate and late subpopulations, respectively).
Discussion
The present AFLP analysis of germinating fescue seeds revealed several interesting patterns of genetic variation with respect to seed germination. First, non-random patterns of mean band frequency were observed with variable germination temperature. Second, no chromosomal regions or segments were identified to be significantly associated with seed germination. Third, the proportions of the total AFLP variation explained by germination timing and due to test temperatures were 0.3% and 0.5%, respectively. Fourth, early and intermediate germination subpopulations shared similar genetic backgrounds and were genetically differentiated from late germination subpopulations. These findings clearly indicate a weak genetic basis of adaptive seed germination and provide the first molecular evidence for genetic differentiation in the germination of F. hallii seeds.
Significant changes detected in mean band frequency between early and late germination subpopulations under increased temperature indicate that F. hallii seed genotypes respond differently to environmental variation during the germination process. This finding accords well with those from related studies comparing allele frequencies using allozyme markers between seed-bank and above-ground populations under different soil water regimes (Cabin et al., Reference Cabin, Evans and Mitchell1997), in optimal and suboptimal conditions (Mandak et al., Reference Mandak, Bimova and Plackova2006) and across multiple populations (Cabin et al., Reference Cabin, Mitchell and Marshall1998). Patterns of change in mean band frequency in this study appear to imply that the germination timing of F. hallii seeds was involved with a genetic sorting of seed genotypes influenced by germination temperatures. If this is true, the AFLP variation observed in this study should reflect (or is genetically linked to) the variation residing among individual seeds in physiological, biochemical or molecular processes required for germination. First, variation among individual seeds in hormone sensitivity (Liptay and Davidson, Reference Liptay and Davidson1971) and enzyme activity such as endo-β-mannanase (Still and Bradford, Reference Still and Bradford1997; Still et al., Reference Still, Dahal and Bradford1997) was found to be associated with the variation in germination time among seeds within a population. The hormone sensitivity and enzyme activity can be affected by different temperatures. Second, seed germination potential is genetically controlled in dry seeds, while the germination rate is controlled by changes of gene expression initiated upon imbibition (Rajjou et al., Reference Rajjou, Gallardo, Debeaujon, Vandekerckhove, Job and Job2004; Holdsworth et al., Reference Holdsworth, Finch-Savage and Grappin2008). Zhang (Reference Zhang2008) found differences in gene expression pattern between 50% germinated and 50% ungerminated canola seeds imbibed in water, gibberellic acid (GA), saline and abscisic acid (ABA) solutions. These differences may be due to genetic sorting during imbibition in different solutions.
Assessing genetic differentiations among F. hallii seeds grouped for germination timing and test temperature further confirms that early germination subpopulations are genetically differentiated from late germination subpopulations. Thus, the germination of F. hallii seeds was under weak genetic control. A quantitative trait locus (QTL) has been identified in Arabidopsis for germination rate (Clerkx et al., Reference Clerkx, El-Lithy, Vierling, Ruys, Blankestijn-De Vries, Groot, Vreugdenhil and Koornneef2004), which ties in with the observed differences in genetic variation for early and late germination. A weak genetic differentiation of the germination of F. hallii seeds is not surprising. First, germination timing is a composite trait that results from mechanisms of dormancy induction and maintenance, dormancy breaking and germination after dormancy breaking (Bewley, Reference Bewley1997). Thus, many genes, probably of small effects each, are expected to be involved and stimulated to influence germination under variable environmental conditions. Second, the inability to detect significant chromosomal regions associated with germination may be due to the use of limited sample size (i.e. 20) per germination subpopulation and the limited genomic coverage of the assayed AFLP fragments. It might also reflect the true small effects of those genes involved with germination responses. Third, the smaller proportion of the total AFLP variation explained by germination timing than test temperature signals that environmental factors, or their interactions with genetic factors, may play a more important role in initiating germination. This reasoning also gains support from the finding of increased mean band frequencies with higher test temperatures, particularly for early subpopulations (Fig. 2).
Different patterns of seed germination in different temperature regimes observed here may enhance the ability of fescue plants to adapt to changing environments. The warmer and drier future climate as predicted for the Canadian prairie may favour earlier germination of subpopulations, as these groups of seeds could germinate in early spring. Different habitats with different environmental conditions could favour different alleles and select different seed genotypes during a certain favourable period of time for germination. Thus, the chance to succeed in restoration of remnant fescue stands with seeds may vary across the Canadian prairie. The patterns of seed germination revealed in the three populations should provide a rough guide to secure a higher germination rate in the restoration effort. However, the temperatures tested in this study may not realistically reflect real soil temperatures (Romo et al., Reference Romo, Grilz, Bubar and Young1991). Also, other environmental factors, such as moisture, may contribute more to the genetic sorting than temperature, as genetic and germination variation among F. halliii populations were more correlated with mean annual precipitation than mean annual temperature of sites (Qiu, Reference Qiu2009; Qiu et al., Reference Qiu, Fu, Bai and Wilmshurst2009). Moreover, maternal environmental effects on seed germination have been widely identified in many species (Roach and Wulff, Reference Roach and Wulff1987; Lacey, Reference Lacey1996; Pendleton and Meyer, Reference Pendleton and Meyer2004; Luzuriaga et al., Reference Luzuriaga, Escudero and Perez-Garcia2006; Boyd et al., Reference Boyd, Dorn, Weinig and Schmitt2007) and were not considered in this study.
This study has demonstrated the existence of subtle genetic control for seed germination and establishment of seed genotypes in response to environmental variation, which offers the potential for seeds to respond to spatial and temporal variation. This information should be helpful in explaining how fescue seeds germinate, how fescue plants adapt to a changing environment, and how substantial patterns of genetic variation are formed among fescue stands, which has been demonstrated in Fig. 3. However, this study provides only a preliminary glance at the genetics of seed germination in a native grass. Further research is needed to determine the number, effect and location of genes controlling germination timing. With the development of informative molecular markers, such research will offer many insights into the behaviours of germination genes in natural populations (Donohue, Reference Donohue2005). These insights will facilitate the development of reliable thermal time and hydrothermal time models to predict germination variability and quantify the impacts of temperature and water on germination (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982; Kebreab and Murdoch, Reference Kebreab and Murdoch1999a, Reference Kebreab and Murdochb; Wang et al., Reference Wang, Bai and Tanino2004; Qiu et al., Reference Qiu, Bai, Coulman and Romo2006) and consequently enhance our understanding of plant establishment in changing environments.
Acknowledgements
This research was financially supported by Parks Canada Ecological Integrity Innovation and Leadership Fund and a PostGraduate Scholarship from Natural Science and Engineering Research Council to J.Q. We thank M. Braun, J. Weir, N. Stolle, R. Fidler, C. Mckillop, W. Vanderschuit, E. Qualtiere, H. Liu and B. Xu for assisting in sample collection, and G.W. Peterson, C. Gilkinson and G. Wong for assisting in molecular screening.