Introduction
The demand for medicinal plants is increasing in both developing and developed countries because they are non-narcotic, generally free of side-effects, and easily available at affordable prices (Tewari, Reference Tewari2000). There is a large number of medicinal species in Iran, and some of the most important ones belong to the genus Ferula (Apiaceae), which includes about 213 species based on The Plant List (2013). In Iran, Ferula is represented by 30 species, of which 15 are endemic (Sattar and Iranshahi, Reference Sattar and Iranshahi2017), and almost all of them are perennials. Ferula ovina is an Iranian Ferula, and is spread across vast areas in the eastern and central parts of the country at altitudes of 2000‒4000 m with an average annual precipitation of 350‒700 mm (Safaian and Shokri, Reference Safaian and Shokri1993). Moreover, it is a source of aromatic resins used in traditional medication (Kurzyna-Młynik et al., Reference Kurzyna-Młynik, Oskolski, Downie, Kopacz, Wojewódzka and Spalik2008).
There are some common problems in growing medicinal plants. First, they usually germinate better within their native environments, and they often fail to show good germination under laboratory conditions or when cultivation is attempted (Nadjafi et al., Reference Nadjafi, Bannayan, Tabrizi and Rastgoo2006). Moreover, seeds often take a long time to germinate, which is not favourable for propagation. Seed dormancy and the long germination period are major problems for Ferula species, and the seeds germinate poorly or not at all in warm conditions (Nikolaeva, Reference Nikolaeva1969). Therefore, despite their economic significance, there are still problems with their cultivation (Nadjafi et al., Reference Nadjafi, Bannayan, Tabrizi and Rastgoo2006 and Rahnama-Ghahfarokhi and Tavakkol-Afshari, Reference Rahnama-Ghahfarokhi and Tavakkol-Afshari2007; Keshtkar et al., Reference Keshtkar, Azarnivand, Etemad and Moosavi2008, Reference Keshtkar, Azarnivand and Atashi2009).
Previous studies have classified F. ovina as having physiological dormancy, since cold stratification had been suggested as the main dormancy-breaking treatment (Amooaghaie, Reference Amooaghaie2004; Keshtkar et al., Reference Keshtkar, Azarnivand, Etemad and Moosavi2008). However, we believe that these studies have not shown a complete picture of dormancy in F. ovina. The existence of morphophysiological dormancy (MPD) is very frequent in the Apiaceae (Baskin et al., Reference Baskin, Chester and Baskin1992, Reference Baskin, Meyer and Baskin1995, Reference Baskin, Milberg, Andersson and Baskin2000; Phartyal et al., Reference Phartyal, Kondo, Baskin and Baskin2009; Vandelook et al., Reference Vandelook, Bolle and Van Assche2008, Reference Vandelook, Bolle and Van Assche2009; Scholten et al., Reference Scholten, Donahue, Shaw and Serpe2009; Yaqoob and Nawchoo, Reference Yaqoob and Nawchoo2015). Also in the case of closely related species of F. gummosa and F. asafoetida, there are reports of MPD (Otroshi et al., Reference Otroshi, Zamani, Khodambashi, Ebrahimi and Struik2009; Rouhi et al., Reference Rouhi, Rahmati, Saman, Shahbodaghloo, Karimi, Moosavi, Rezaei and Karimi2012). Additionally, there are contradictions concerning the effect of gibberellic acid (GA3) on dormancy break. Even though previous studies on F. ovina have reported its positive effect (Amooaghaie, Reference Amooaghaie2004; Keshtkar et al., Reference Keshtkar, Azarnivand, Etemad and Moosavi2008), some studies on other species of this genus (F. gummosa and F. asafoetida) have reported otherwise (Hassani et al., Reference Hassani, Saboora, Radjabian and Fallah-husseini2009; Otroshi et al., Reference Otroshi, Zamani, Khodambashi, Ebrahimi and Struik2009).
According to Baskin and Baskin (Reference Baskin and Baskin2014), linear embryos in the Apiaceae are under-developed, and seeds have morphological dormancy (MD) or MPD. Normally, seeds with MD only need suitable temperature, moisture, oxygen, and of course time to germinate (Baskin and Baskin, Reference Baskin and Baskin2014). However, in many cases, the fully differentiated under-developed embryos also have physiological dormancy (PD), which imposes an additional constraint to germination; such embryos do not germinate in less than one month in suitable germination conditions. In this case, the dormancy is not just morphological but morphophysiological (MPD), and the embryos require additional treatment, such as cold, to complete their growth. Therefore, if the fresh seeds of F. ovina or any other seeds, with under-developed embryos, do not germinate in about 4 weeks, they have MPD, which means they possess both MD and PD simultaneously (Baskin and Baskin, Reference Baskin and Baskin2014).
After defining the class of dormancy, the level of dormancy needs to be specified. MPD includes nine different levels, depending on the temperatures to break PD and MD and the effect of GA3 on dormancy break (Baskin and Baskin, Reference Baskin and Baskin2014). In addition, the timing of breaking both PD and MD could be different as well; they might occur at the same time or each one could follow or precede the other. These nine levels are as follows:
-
(1) Non-deep simple ‒ embryos grow at high temperatures, whether during autumn or spring (Baskin et al., Reference Baskin, Milberg, Andersson and Baskin2002);
-
(2) Non-deep complex ‒ with a PD that is released during autumn or both autumn and winter, and the embryos grow in winter;
-
(3 and 4) Deep simple and intermediate simple ‒ both include a PD with two parts: one is non-deep and is broken by warm, and the other is deep and is broken by cold temperatures. Once the PD is broken, embryos germinate during spring (Chien et al., Reference Chien, Chen, Baskin and Baskin2011). However, they are distinguished based on their responses to GA3;
-
(5 and 6) Deep complex and intermediate complex ‒ both only require cold temperatures to break both MD and PD. GA3 could substitute for cold in intermediate complex, but not in deep complex;
-
(7 and 8) Deep simple epicotyl and non-deep simple epicotyl ‒ other than the embryos, the shoots (epicotyl) also have dormancy (PD). In deep simple epicotyl dormancy, the embryos elongate within the seed and the radicle emerges in autumn, but cotyledons emerge in spring only if seeds with emerged radicles receive enough cold during winter to break dormancy (Kondo et al., Reference Kondo, Miura, Okubo, Shimada, Baskin and Baskin2004). However, in non-deep simple epicotyl, a period of warm stratification is required for embryo growth and root emergence, which is followed by another period of warm needed for breaking epicotyl dormancy (Baskin et al., Reference Baskin, Chien, Chen and Baskin2008);
-
(9) Deep simple double ‒ in the first winter, cold stratification breaks one part of the PD and the embryo initiates growth and the radicle emerges in the first spring. Moreover, in the second winter, the PD of the epicotyl is broken by cold, and the shoot emerges in the second spring.
Out of these nine MPDs, only three levels have been reported in Apiaceae: non-deep simple, non-deep complex, and deep complex. To determine the level of dormancy in F. ovina, we must answer three questions: (1) what is the temperature requirement for breaking dormancy?; (2) can GA3 can substitute for cold and break the dormancy?; and (3) can warm stratification and dry after-ripening reduce dormancy?
Different levels of MPD, like other dormancy types, have evolved in the habitat where the species grows, and only in one direction: synchronizing seedling establishment with the most favourable period for survival, and to survive conditions that are not suitable for seedling establishment. For example, the formation of deep complex MPD seems to be an adaptation to regions with a very cold winter and a dry, cool summer. In these areas, temporary sporadic favourable temperatures (elevated temperature) in winter or too early in winter are threatening for seedling establishment. Therefore, the dormancy helps seeds to remain ungerminated throughout the winter. Moreover, the low temperatures alleviate dormancy and once dormancy breaks, two possible scenarios might occur: non-dormant seeds either wait for a mild and moist spring to germinate (Baskin et al., Reference Baskin, Chester and Baskin1992), or they germinate at low temperatures in the middle of winter in cold soil, even covered with heavy snow, until late winter; while the shoots grow and emerge above the soil surface with the increase in temperature (Baskin et al., Reference Baskin, Meyer and Baskin1995).
Other than identifying the class and the level of dormancy, the study of the physiological processes involved in dormancy break is important as well. Recent findings have proved that reactive oxygen species (ROS) play important roles in dormancy break. El-Maarouf-Bouteau and Bailly (Reference El-Maarouf-Bouteau and Bailly2008) argued that GA3’s ability to alleviate dormancy is controlled by nitric oxide (NO) and that the elevation of ROS compounds like H2O2 in the embryos could induce the production of NO. Moreover, ROS radicals could induce embryo growth and break morphological dormancy. For example, ROS are suggested to mediate the breakdown of the seed reserves during cold stratification. In the course of cold stratification in apple seeds (cold is necessary for both embryo growth and release of PD), the amount of hydrogen cyanide (HCN) increases. HCN strongly affects the catabolism of reserves and supplies the embryo with energy throughout cold stratification (Lewak, Reference Lewak2011). The dormancy-breaking role of cyanide has been demonstrated to be under the control of ROS (Oracz et al., Reference Oracz, El-Maarouf Bouteau, Kranner, Bogatek, Corbineau and Bailly2009). Additionally, ROS compounds have been suggested as substances contributing to cell elongation during seed germination by loosening the cell walls (El-Maarouf-Bouteau and Bailly, Reference El-Maarouf-Bouteau and Bailly2008; Müller et al., Reference Müller, Carstens, Linkies, Torres and Leubner-Metzger2009). In lettuce, ROS is directly responsible for the endosperm cap weakening and embryo elongation and treatments with exogenous H2O2 significantly improved embryo growth potential and germination (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014). Therefore, the involvement of these compounds in both embryo growth and release of physiological dormancy suggest that they are promising factors to overcome different levels of morphophysiological dormancy.
The first part of this study had three specific goals: (1) to determine the exact level of dormancy, (2) to investigate the optimum temperatures for both dormancy break and germination, and (3) to assess the phenology and the ecological pattern of seed germination in the local environment. In addition, we investigated the physiological processes underlying the effect of low temperature on dormancy break. Accordingly, we studied the effect of both H2O2 and diphenylene iodonium (DPI), an NADPH oxidase inhibitor that suppresses the production of ROS radicals, on the alleviation of dormancy. We also examined the endogenous level of H2O2 and localized the superoxide (O2 −) production in embryos during stratification treatments, using the nitro blue tetrazolium (NBT) histochemical staining technique.
Materials and methods
Seed collection and preparation
Fresh seeds of Ferula ovina were purchased in early August 2014 from the Pakan Seed Company in Isfahan. According to the company, seeds were collected during mid-July from rangelands of Fereydunshahr County, Isfahan Province, Iran (32° 37′ N, 51° 40′ E), and after collection seeds were dried and stored at room temperature (20°C). After the purchase, seeds were kept under similar conditions for one month prior to the experiments. Altogether, 45 days passed from collection until the experiments commenced. In the laboratory, seeds were rinsed with running water, surface sterilized by soaking in 2.5% sodium hypochlorite for 5 min, and rinsed again with deionized water. The experiments were conducted in germinators with constant temperature and with white fluorescent lights (intensity of 100 µM m‒2 s‒1).
Phenology of embryo growth and germination
In early October 2014, 24 nylon bags were filled with farm soil and buried at the research farm of the University of Tehran: twenty bags, each containing 50 seeds, were buried at a depth of 5 cm to study embryo growth and germination, whereas the four remaining bags, each containing 200 seeds, were buried at a depth of 2 cm to study seedling emergence. The experiments were conducted near the farm's meteorological station. Daily minimum and maximum temperatures at soil depth of 5 cm were recorded three times a day using ground thermometers, and averaged on a monthly basis. Seeds were treated with Vitavax powder, to prevent damage by insects. Beginning in late October (20 days after sowing), the bags were exhumed at 10-day intervals, and other than counting the germination, the embryo to seed length ratio (E:S ratio) of 20 randomly selected non-germinated seeds was assessed. The embryos were excised from seeds using a surgical blade, and the lengths were measured under a dissecting microscope equipped with a micrometre. Germinated seeds were excluded from the calculations; instead, we applied the critical E:S ratio, which was calculated as the average E:S ratio of seeds with a split coat, but no radicle protrusion.
Temperature requirements for embryo growth and germination
The first aim of this experiment was to determine whether seeds were dormant, and if so, which kind (MD or MPD). The second aim was to determine the optimum temperatures for embryo growth and dormancy break. Twelve nylon bags each including 100 seeds in four replicates (25 moist seeds placed on moist filter paper in 9 cm diameter glass Petri dishes) were exposed to continuous light (cool white fluorescent tubes) at six temperatures: 0.5 ± 1, 3 ± 0.2, 5 ± 0.5, 10 ± 0.7, 15 and 25°C for 10 weeks. There were six bags for calculating the percentage of germination, and other bags each of which with 200 seeds, were used for measuring embryo and seed length. Germinated seeds were counted at weekly intervals and the total percentage of germination was calculated. Additionally, the embryo length to seed length (E:S) ratio was determined for 20 non-germinated seeds. To determine the optimal incubation temperature for germination, ten bags of 200 seeds were placed at 3°C for 2, 3 and 4 weeks and then transferred to incubation temperatures of 3, 5, 10 and 15°C for about one month. Those seeds that had already germinated during stratification were left out during the transfer and the final germination was assessed on the basis of the remaining non-germinated seeds.
Effect of warm stratification, dry after-ripening, darkness and GA3 on breaking dormancy
Eight bags, each containing 100 seeds, were prepared for three separate treatments. Two bags were wrapped with two layers of aluminium foil as the treatment of darkness; two bags were stored at room temperature for one year as the treatment of dry after-ripening; four bags were warm stratified at 20°C for 60 and 90 days. Afterwards, all bags were transferred to germination condition (3°C) for 5 weeks. For the other experiment, fresh intact seeds of F. ovina were treated with three concentrations of 0.03, 0.3 and 3 mM of GA3 at two temperatures of 3 and 15°C for 5 weeks. The gibberellic acid powder was dissolved in deionized water and the pH was adjusted to 6.5 with 0.5 mM KOH.
H2O2 content and localization of O2 − production
Embryos of seeds stratified at 3 and 15°C were excised on a weekly basis for a period of 28 days. Isolated embryos were immediately frozen in liquid nitrogen and kept at ‒80°C. Because embryos were so small, many of them were extracted for each sample in order to reach an overall mass of 0.5 g. Endogenous H2O2 was determined according to Loreto and Velikova (Reference Loreto and Velikova2001). Embryos (0.35 g) were ground in liquid nitrogen with a pestle and mortar and then homogenized in an ice bath with 5 ml of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000 g for 15 min, and 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M potassium iodide. The absorbance of the supernatant was measured at 390 nm with a spectrophotometer (Shimadzu UV-160; Shimadzu Corporation, Kyoto, Japan). The content of H2O2 was calculated by comparing it with a standard calibration curve that was made using different concentrations of H2O2 and expressed in μmol g‒1 fresh mass (FM).
The generation of superoxide was detected in excised embryos after 1, 2, 3, 4, 5 and 6 days stratification at 3 and 15°C, by using a histochemical staining technique as modified by Jabs et al. (Reference Jabs, Dietrich and Dangl1996). We used nitroblue tetrazolium chloride (NBT) ‒ a reagent forming an insoluble blue formazan product upon reduction (Bielski et al., Reference Bielski, Shine and Bajuk1980) ‒ as a probe for O2 − . Seeds were first kept in staining solution (0.5 mM NBT in 50 mM phosphate buffer; pH 6.8) for 20 min (as NBT could not penetrate the endosperm layer, seeds were first scarified; Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014), and washed in buffer for 1 min and observed for intensity of staining using a stereomicroscope (Olympus).
Effect of exogenous H2O2 and DPI on embryo growth and germination
Fresh F. ovina seeds were treated with distilled water, two concentrations of H2O2 (10 and 100 mM) at 3 and 15°C, and 1 mM DPI at 3°C for about one month (all solutions were made up with distilled water). DPI is a common ROS scavenger. It limits the production of H2O2 by inhibiting the activity of NADPH oxidase (the key enzyme in plasma membrane for the production of superoxide radicals). For each treatment, two bags of 100 seeds were used: one bag to measure the percentage of germination and the other for measuring the length of embryos. Moreover, as germination was under aerobic conditions, fresh H2O2 solutions were used every week. At weekly intervals, 20 non-germinated seeds were taken from all Petri dishes in order to measure embryo length.
Statistical analysis
Experiments were conducted in a completely randomized design with four replications. For experiments on temperature requirement, the effect of warm stratification, dry after-ripening and darkness, data were analysed using one-way ANOVA. However, for experiments on gibberellic acid and cold stratification, two-way ANOVA was used. Moreover, wherever required, arcsine transformation was used to normalize values prior to analysis. To determine significant differences between each treatment, Duncan's multiple range procedure at P < 0.05 was used. All analyses were carried out using SAS statistical software package, version 9.2.
Results
Phenology of embryo growth and germination
Fresh F. ovina seeds had an initial average embryo to seed (E:S) ratio of 0.37 ± 0.02 (mean ± SE). In the period between 5 October (time of burial) and 5 November, the embryos exhibited a growth of 0.42 ± 0.02 and reached an average E:S of about 0.79 ± 0.02 (exactly on 5 November) (Fig. 1), even though in the period from 5 to 26 October, there was no rainfall and the only moisture accessible, was from the soil. From 5 until 25 November, the average E:S ratio remained constant. However, over the 10 days from 25 November, a second increase in the growth of embryos occurred (ratio change of about 0.09), probably due to the decrease in soil temperature. After the second growth in embryos, the average E:S ratio increased up to 0.91 ± 0.019 on 5 December, which coincided with the initiation of germination, and was likely to be its critical value. The growth of the embryo and germination were consistent with the changes in soil temperature; both first and second growth of embryos occurred with a decrease in soil maximum and minimum temperature. From 5 December onward, as the temperature continued to decrease, more and more seeds came out of dormancy. Seeds germinated extensively in the very cold winter soil, and by 3 February 92% of them had germinated (Fig. 1). From 23 February, seedlings started to emerge from the soil as the local temperature increased (data not shown).

Figure 1. Phenology of embryo growth and germination. Seeds of Ferula ovina were buried in the field in August 2014. Grey lines indicate the mean minimum and maximum temperatures of the soil at 5 cm depth. The continuous line represents cumulative germination percentage and the dotted line represents the average E:S ratio. Error bars indicate ± SE.
Temperature requirements for embryo growth and germination
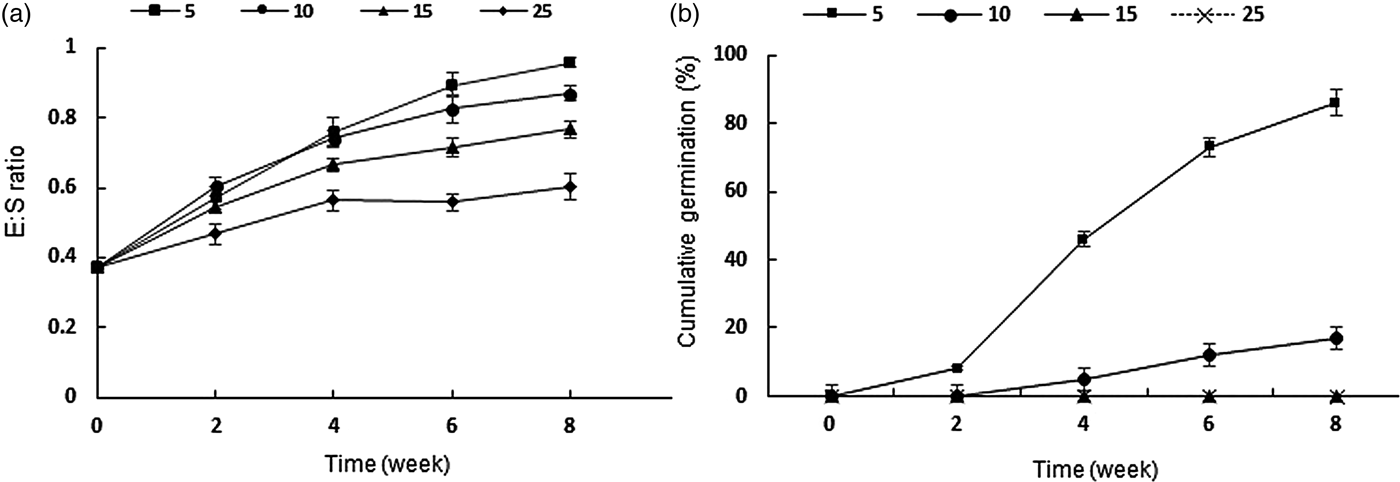
Embryos of F. ovina started growth at all incubation temperatures as soon as they imbibed water (Fig. 2), albeit at different rates. After 6 weeks, embryos at 5°C showed an average E:S ratio of 0.89 ± 0.03, which was significantly higher than at 10°C (0.82 ± 0.04) and 15°C (0.71 ± 0.02). Furthermore, the germination percentage was higher at lower temperatures; after 8 weeks, no seed had germinated at 15 and 25°C, while 17 and 86% of germinated seeds were observed at 10 and 5°C, respectively (Fig. 2). In addition, the slow-paced development of embryos at warm temperatures continued over the following weeks, and after 24 weeks, seeds incubated at 15°C attained 0.99 ± 0.03; however, they showed a very low percentage of germination (7%) (data not shown).

Figure 2. (a) Embryo to seed length ratio. (b) Cumulative germination percentage of Ferula ovina seeds kept at 5°C (squares), 10°C (circles), 15°C (triangles) and 25°C (diamonds) for 8 weeks. Error bars indicate ± SE.
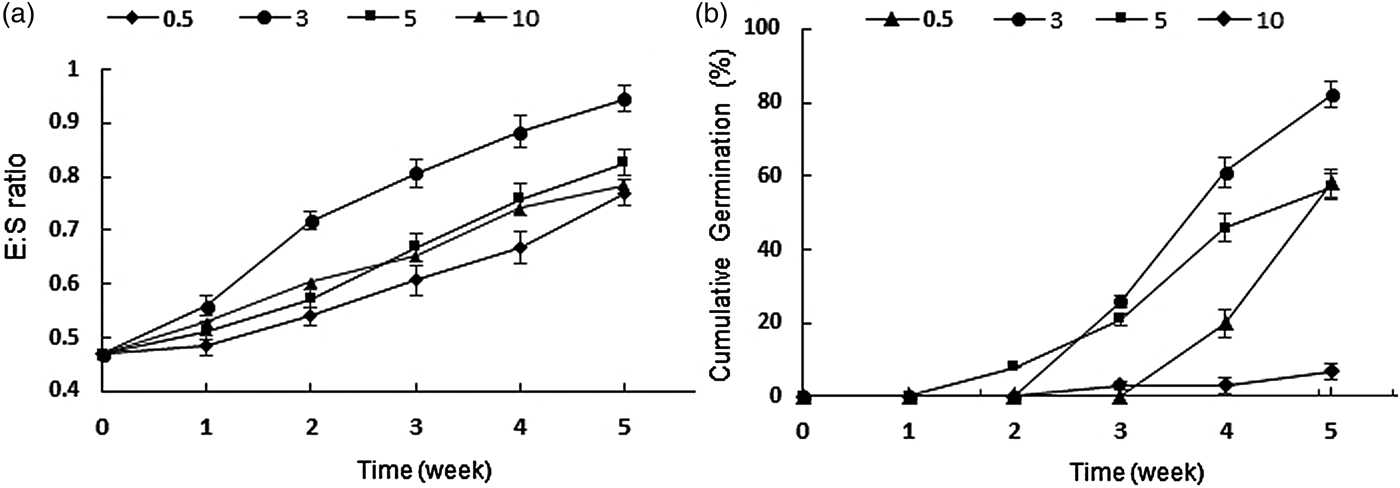
Results of comparing the embryo growth and germination under different cold temperatures identified 3°C as the optimum temperature for both embryo growth and germination. In 5 weeks, the embryos at 3°C grew to an average E:S ratio of 0.77 ± 0.02 and germinated to 82%, which was significantly higher than at other temperatures (Fig. 3). Seeds incubated at 0.5 and 5°C showed a similar percentage of germination over the period of 5 weeks. However, the rate of dormancy break was higher at 5°C; the first germination at 5°C occurred in the second week, but at 0.5°C no seed germinated before the third week.

Figure 3. (a). Mean embryo to seed length ratio. (b) Germination percentage of Ferula ovina seeds in response to the range of cold temperatures: 3°C (circles), 5°C (squares), 0.5°C (triangles) and 10°C (diamonds) over a period of 5 weeks. To avoid confusion, the figures for 5°C and 10°C in Fig. 2b are given here again. Error bars indicate ± SE.
Seeds germinated to 37, 42.2 and 61% at 15°C following respectively 2, 3 and 4 weeks of stratification at 3°C (Fig. 4), whereas, without cold, only 7% of the seeds germinated after 18 weeks at 15°C. Moreover, keeping seeds at cold temperatures (3 and 5°C) instead of moving them to 15°C led to the significantly higher percentages of germination, which identified cold temperatures as more optimum temperatures for seed germination in F. ovina.

Figure 4. Germination percentage of the seeds moved to four incubation temperatures of 3, 5, 10 and 15°C after 2, 3 and 4 weeks of cold stratification at 3°C. Values with the same letter are not significantly different at the P<0.05 level (Duncan's multiple range procedure). Values are means of four replicates ± SE.
Effects of warm stratification, dry after-ripening, darkness and GA3 on breaking dormancy
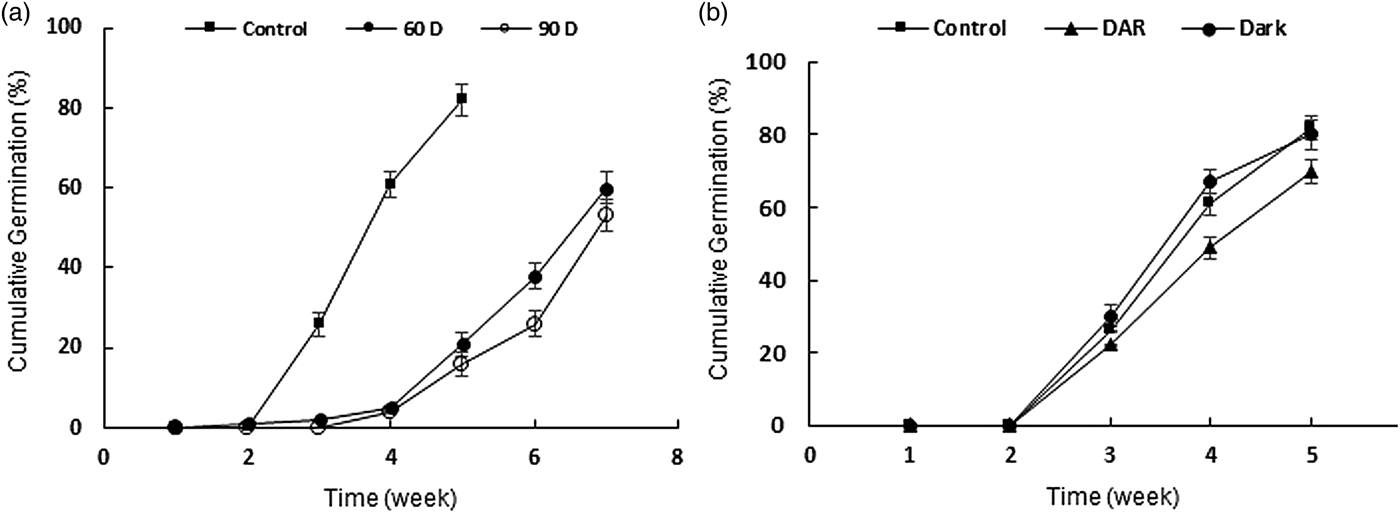
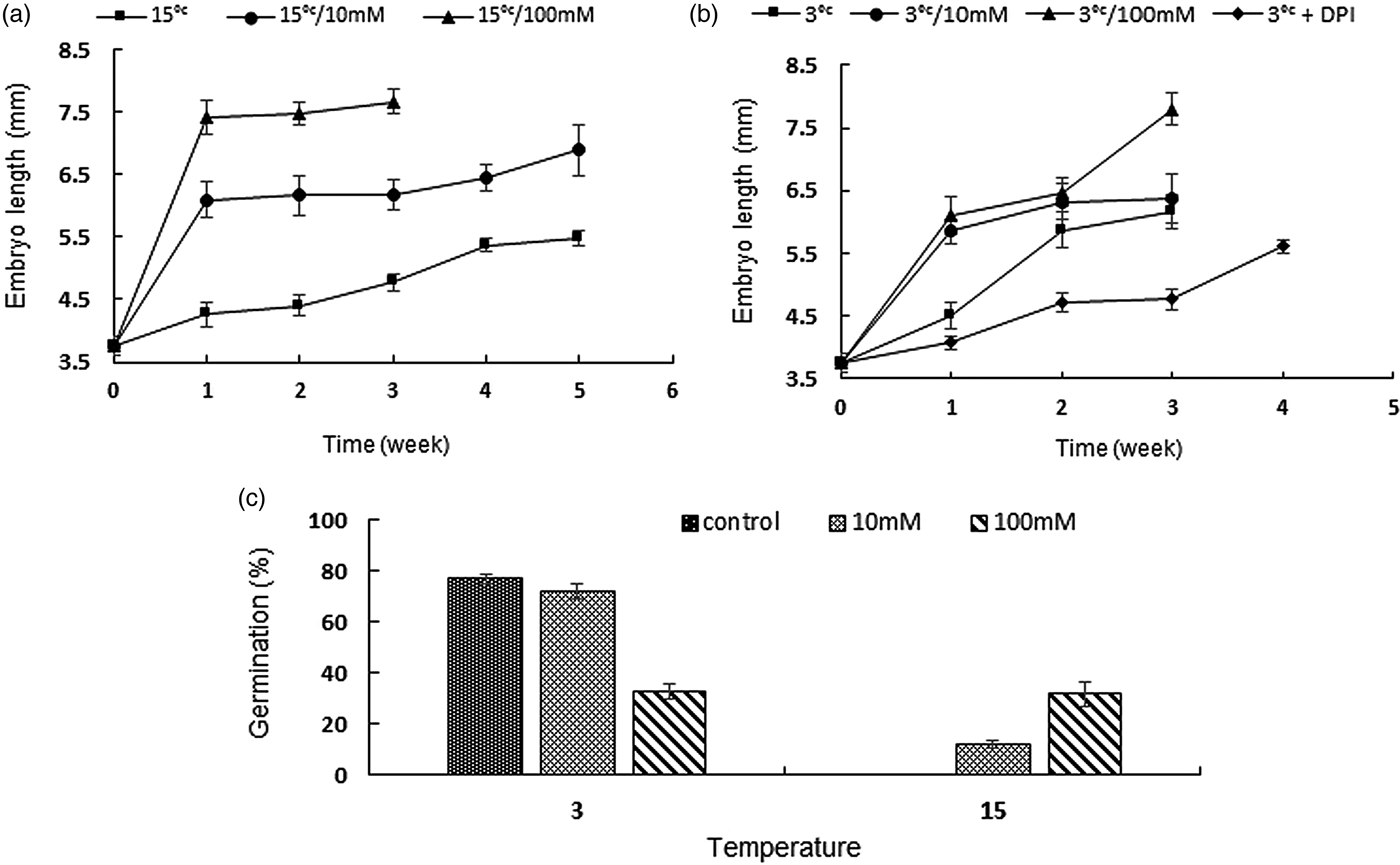
Treatments of seeds at moist warm conditions before placing them at 3°C reduced the rate and percentage of germination. Seeds germinated to 21 and 16% at 3°C following 60 and 90 days at 20°C, while, by cold stratification alone, 82% of the seeds germinated after 5 weeks. Extending the period of warm stratification adversely affected the seeds, as the percentage of germination was lower at 90 days compared with 60 days (Fig. 5a).

Figure 5. (a) Effect of warm stratification at 20°C for 60 days (filled circles) and 90 days (open circles) before cold stratification on the break of dormancy. (b) Effect of dry after-ripening pre-treatment at 20°C for one year (triangles) before cold stratification on the break of dormancy, and effect of darkness during cold stratification (circles). Control: 3°C solely (squares). Error bars indicate ± SE.
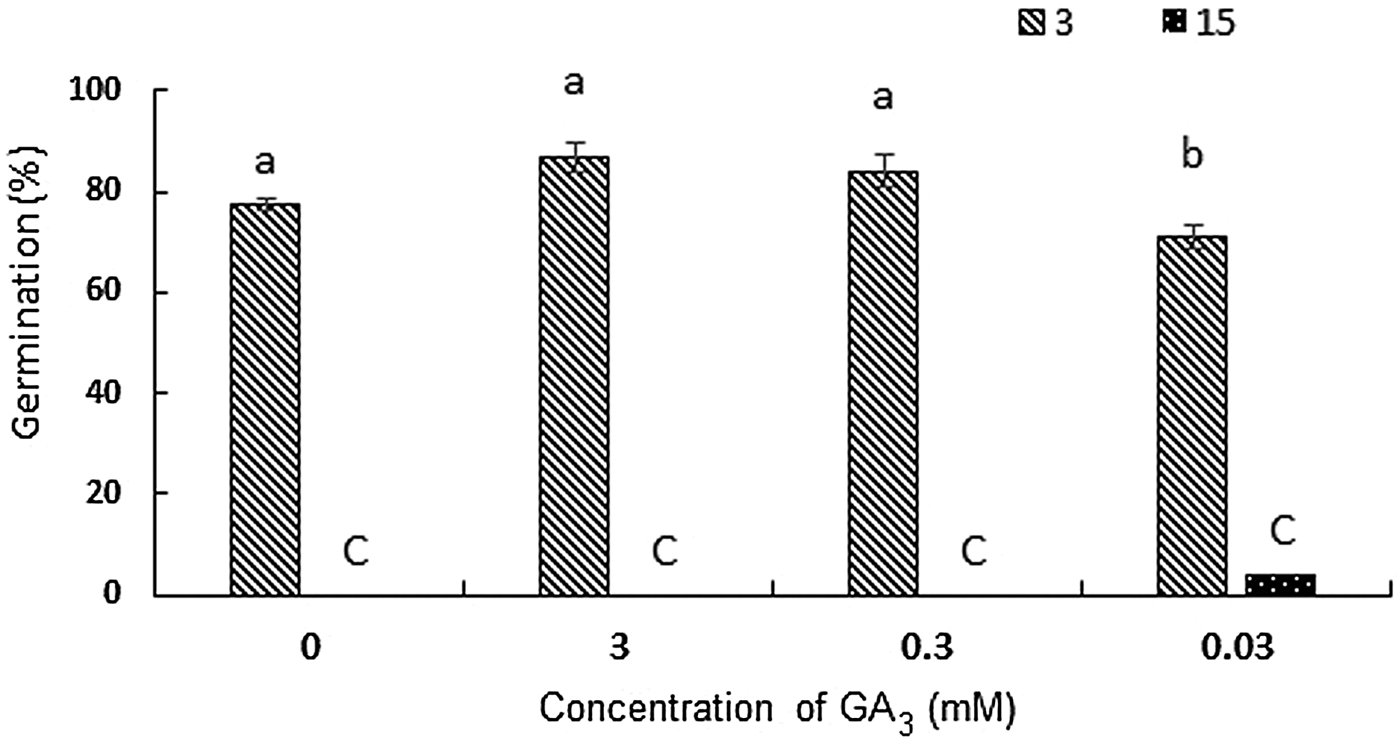
Our results showed that dry after-ripening not only could not reduce the amount of cold required for breaking dormancy, but it also decreased germination compared with the control treatment (70% compared with 82% of the control treatment) (Fig. 5b). Moreover, seeds did not show any light or dark requirement for dormancy break. Incubating seeds at 3°C in darkness led to 80% germination after 5 weeks, which was not significantly different from 82% germination in light (Fig. 5b). Application of different concentrations of GA3 showed no significant effect on the status of dormancy, neither at 3°C nor at 15°C (Fig. 6).

Figure 6. Germination percentages of the seeds after 5 weeks of treatment with three different concentrations of gibberellic acid at 3 and 15°C. Values with the same letter are not significantly different at the P<0.05 level (Duncan's multiple range procedure). Values are means of three replicates ± SE.
H2O2 content and localization of O2 − production
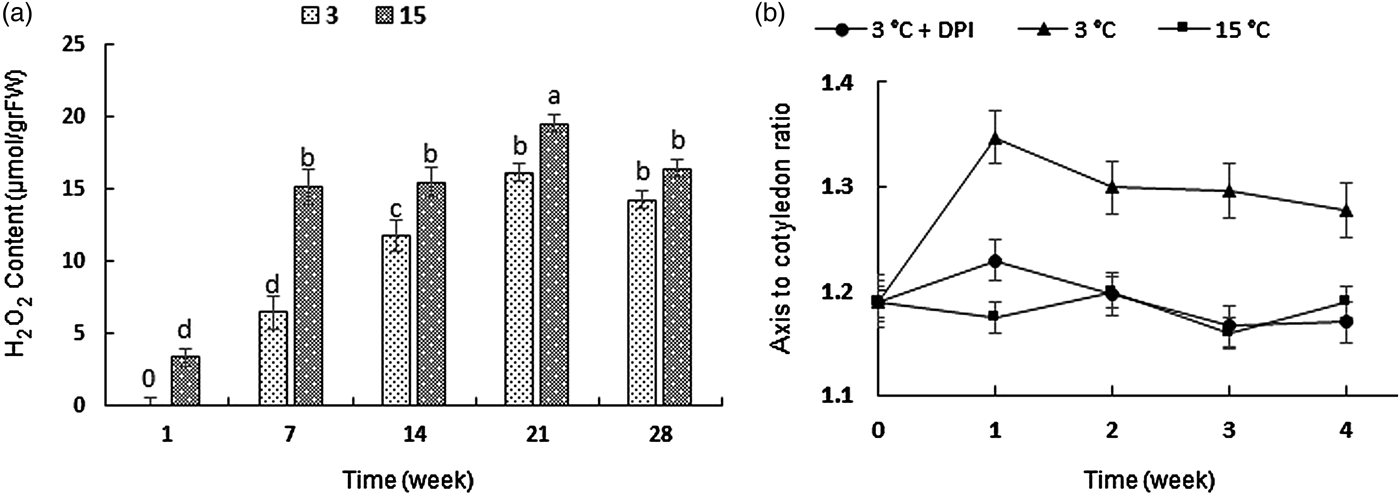
The internal level of H2O2 content increased during both warm and cold stratification in F. ovina but with different patterns. At 15°C, the content showed a sharp increase over the first week and reached 15.18 μmol g‒1 FW. The amount remained constant for the next 7 days and peaked after 3 weeks. On the other hand, at 3°C, H2O2 increased gradually over the first 2 weeks, and the maximum production was observed after the third week (Fig. 7a).

Figure 7. (a) Endogenous hydrogen peroxide contents (μmol g‒1 FW) during stratification at 3 and 15°C. Values with the same letter are not significantly different (P < 0.05) using Duncan's multiple range test. Values are the means of four replicates ± SE. (b) Axis: cotyledon ratio of embryos at 3°C (triangles), 15°C (squares) and 3°C + 1 mM DPI (circles). Error bars indicate ± SE.
The superoxide localization technique by NBT indicated a specific pattern of O2 − accumulation in embryo axes and cotyledons at both 3 and 15°C. The staining time-course of embryos is presented in Fig. 8A‒F. At cold temperatures, the blue stains, which are signs of O2 – generation, first became visible in the hypocotyl and radicle after approximately 2 days, and after a 24 h delay in cotyledons. Thereafter, the spots intensified gradually until the sixth day, when only radicles and cotyledons were blue and the central parts of embryos had no sign of O2 – accumulation (Fig. 8G). A similar pattern of staining was observed for warm-stratified seeds. Blue spots first appeared in the embryonic axes and then in cotyledons. However, the rate of O2 − generation was higher at warm than cool temperatures, and the strains were visible all over the embryos on the second day.

Figure 8. (A‒F) Histochemical detection of superoxide in embryos of Ferula ovina. Localization of O2 − was visualized in excised embryos after 1, 2, 3, 4, 5 and 6 days of stratification at 3°C by placing them in NBT for 20 min. (G) Close-up image of the stained embryos by NBT, after 2 weeks of cold stratification. (H and I) Growth of the embryos, treated with exogenous H2O2 after 2 and 3 weeks at 15°C, compared with control.
Effect of exogenous H2O2 and DPI on embryo growth and germination
Our data indicated that both concentrations of H2O2 were effective for the growth of the embryos in a dose-dependent manner. However, their impact on breaking dormancy was temperature dependent. At 3°C, both 10 and 100 mM H2O2 increased embryo growth dramatically in the first week (Fig. 9b). However, treatment with 100 mM H2O2 significantly decreased the number of germinated seeds at 3°C (Fig. 9c). On the other hand, at 15°C, both 10 and 100 mM H2O2 treatment had a significant positive effect on both the growth of the embryos and germination. For instance, with 100 mM H2O2, the average length of embryos reached twice the initial size in the first week (Fig. 9a). As observed (Fig. 8H and I), the higher growth of embryos was just due to the elongation of embryonic axes, and cotyledons did not grow during the treatment. In addition, exogenous concentrations of H2O2 significantly promoted germination at 15°C, and seeds of F. ovina germinated to 12 and 32% at 10 and 100 mM H2O2, respectively, after 5 weeks. First germination was observed by the third week. Treatment with 1 mM DPI reduced both germination and embryo growth at 3°C (Fig. 7b). After 4 weeks, the average length of DPI-treated embryos was 5.6 mm (compared with 7.2 mm of 3°C), and seeds germinated to 38.5% (compared with 61% of the control).

Figure 9. Embryo growth and germination of Ferula ovina seeds treated with hydrogen peroxide. Effect of H2O2 on embryo growth at 15°C (a) and 3°C (b) at 100 mM (triangles) and 10 mM (circles); controls (squares) and DPI (diamonds). Error bars indicate ± SE. (c) The effect of H2O2 on germination at both 3 and 15°C. The values sharing the same letter are not significantly different (P < 0.05) using Duncan's multiple range test. The values are means of three replicates ± SE.
Throughout the whole period, the average embryonic axis:cotyledon (A:C) ratio of the seeds at 3°C was higher than that at 15 or 3°C + 1 mM DPI, which indicated the overall greater length of embryonic axes compared with cotyledons during cold stratification (Fig. 7b). In the first week at 3°C, embryonic axes grew faster than cotyledons and attained an A:C ratio of 1.34 ± 0.03. However, in the following weeks, growth continued at an even rate in both tissues. On the other hand, the higher elongation of the embryonic axes was not observed at either 15 or 3°C + 1 mM DPI, and both axes and cotyledons grew at about the same rate (Fig. 7b).
Discussion
Fresh seeds of F. ovina contained embryos with an E:S ratio of 0.37 ± 0.02 that increased to 0.91 ± 0.019 before germination, indicating that each embryo grew almost 2.5 times its initial length within the seed. Germination did not occur at temperatures higher than 10°C within 8 weeks, which indicates that other than an underdeveloped embryo, a physiological block prevented seeds from germinating immediately after dispersal. Therefore, like many other Apiaceae species, seeds of F. ovina have MPD. The embryos of F. ovina initiated growth at all temperatures as soon as they imbibed water, and the rate of growth was greater at low than high temperatures. Moreover, seeds incubated at 0.5, 3 and 5°C germinated to high percentages over a period of 5 weeks. However, the seeds failed to germinate at temperatures above 10°C. Therefore, F. ovina seeds, like many other summer species, only germinate after being exposed long enough to cold temperatures over the winter. In Heracleum sphondylium, the seeds exhibited faster embryo growth at 15°C than at 2°C but the completion of growth only occurred at 2°C (Stokes, Reference Stokes1952b). Baskin et al. (Reference Baskin, Chester and Baskin1992) observed that embryos of Thaspium pinnatifidum (Apiaceae) started growth at all incubation temperatures, but growth was only completed at 5°C. Additionally, our laboratory results were consistent with the results obtained under the natural environment. In the field, embryos initiated growth as soon as they were buried. However, growth only continued as soil temperature decreased in late November. Subsequently, radicles started to emerge and up to 95% of the seeds germinated in the nylon bags during winter while the soil temperature was low. The emerged radicles tolerated the severe cold, and shoots emerged from the soil in spring. Similarly, in the laboratory, as we extended the incubation at 0.5°C, the germinated seeds survived 2 months of incubation, and by the time the experiment was over, the radicles were still alive and elongated (data not shown).
Effective temperatures for cold stratification range from 0 to 10°C, with about 5°C being optimal for many species (Crocker and Barton, Reference Crocker and Barton1957; Stokes, Reference Stokes and Ruhland1965). However, in Lomatium dissectum (Apiaceae), the alleviation of dormancy only occurred at temperatures between 3 and 6°C and was negligible at 0.5 and 9.1°C (Scholten et al., Reference Scholten, Donahue, Shaw and Serpe2009). In F. ovina, the highest rate and percentage of germination was achieved at 3°C, with 82% germination over 5 weeks. Therefore, 3°C was the optimum condition for both embryo growth and dormancy break, which was consistent with Nikolaeva (Reference Nikolaeva1969) who reported 0‒3°C to be the optimum stratification temperature for Ferula spp. Moreover, following cold stratification, seeds germinated to higher percentages at cold temperatures of 3 and 5°C than at warm temperatures of 10 and 15°C, indicating that low temperature also favoured germination (Fig. 4).
Baskin et al. (Reference Baskin, Chester and Baskin1992) investigated whether the seed of Thaspium pinnatifidum (Apiaceae), whose dormancy was alleviated within 16 weeks of stratification at 5°C, only had MD with an unusually low-temperature requirement for germination. Maybe we should ask the same question here for the seeds of F. ovina. Embryos grew and germinated to 82% when seeds were placed at 3°C for 4 weeks. Does this mean that seeds only have MD with an unusually low-temperature requirement for germination? We believe this is not true. Dormancy is an innate constraint that limits the germination to a narrow range of environmental conditions (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). However, as the layers of dormancy are gradually broken, the temperature range at which seeds can germinate widens (Benech-Arnold et al., Reference Benech-Arnold, Sanchez, Forcella, Kruk and Ghersa2000; Batlla et al., Reference Batlla, Kruk, Benech-Arnold, Benech-Arnold and Sanchez2004). Thus, whereas the fresh seeds of F. ovina germinated to 7% after 18 weeks incubation at 15°C, they germinated to 37, 42 and 60% at 15°C, after 2, 3 and 4 weeks of stratification at 3°C, respectively, indicating the ability to germinate at increased temperature as dormancy break continued.
So far, the dormancy in F. ovina is either deep complex or intermediate complex, as embryos only completed growth and germinated by cold stratification or at warm temperatures, after treatment with cold stratification. Our results for the effect of GA3 on dormancy were different from previous studies. Based on our data, the application of GA3 was not effective in breaking MPD, whereas Keshtkar et al. (Reference Keshtkar, Azarnivand, Etemad and Moosavi2008) reported a positive effect. Moreover, the results of Amooaghaie (Reference Amooaghaie2004) only demonstrated the positive effect of GA3 on the induction of germination (GA3 as a germination-stimulating factor) and not on breaking dormancy. A treatment with GA3 was only effective when accompanied by moist cold. On the other hand, Baskin and Baskin (Reference Baskin and Baskin2014) argued that in seeds with intermediate complex MPD, not only GA3 could substitute for cold stratification and break dormancy, but sometimes warm stratification and dry storage could have the same function and reduce the period of cold stratification required for dormancy break. Therefore, to specify the exact level of dormancy, we examined the effect of these treatments on dormancy as well. The results showed that not only did they not break dormancy, but they also reduced the rate and the percentage of germination. Therefore, as none of these treatments (GA3, moist warm and dry after-ripening) could replace cold stratification, the PD part of dormancy is deep.
The characteristics of deep complex MPD in F. ovina are part of the plant's adaptation to its environment. The seeds are dispersed at the end of the warm season in northern temperate climates with cold winters at elevations of more than 2500 m. In such regions, frost damage poses the greatest risk to seedlings, and plants do not experience warm moist temperatures before winter. Moreover, seeds of F. ovina could germinate to high percentages in the very cold soil and the radicles can withstand this extreme condition. Once the cold season ends, with the rise in temperature seedlings emerge from the soil. Seeds of Osmorhiza occidentalis, with deep complex MPD, share a similar phenological pattern of dormancy break and germination; the dormancy is broken by cold temperature and seeds germinate to over 80% in the middle of the winter, while they are under snow at temperatures of about 0‒1°C (Baskin et al., Reference Baskin, Meyer and Baskin1995).
Cold stratification is the main prerequisite for breaking deep complex dormancy in F. ovina, and the physiological reason behind this requirement is not yet fully understood. One possible explanation is that during cold stratification, nutrients in the seed become more accessible. Embryos initiate their growths at all temperature conditions upon imbibition using soluble food reserves in the endosperm until it is depleted. Thereafter, the growth stops at high temperatures but continues at low temperatures, because there are more nutrients available for embryos to continue growth (Stokes, Reference Stokes1952a, Reference Stokes1952b, Reference Stokes1953). Lewak (Reference Lewak2011) reported two lipid hydrolysing enzymes in apple seeds responsible for breaking down the reserves with an optimum activation temperature of 5°C. However, there is one other theory that was suggested by Scholten et al. (Reference Scholten, Donahue, Shaw and Serpe2009). Based on this theory, some parts of the embryo such as shoot and root apical meristems may be quiescent, and therefore require periods of cold stratification to get activated and start growth. For the case of F. ovina, the first theory seems to be more plausible; embryos started growth at all temperatures, but it only continued and completed in cold conditions. Moreover, Amooaghaie (Reference Amooaghaie2009) reported higher concentrations of soluble organic phosphorus and proteins in the course of cold stratification in F. ovina, which showed more available food for embryos.
As mentioned previously, ROS compounds are suggested to control the breakdown of food reserves within seeds through their control over cyanide. Thus, they could have a positive effect on the growth of the embryo, and in turn, affect both MD and MPD. Based on our data, the endogenous level of H2O2 increased upon stratification at both 15 and 3°C, with levels being higher in seeds kept at warm temperatures. This different pattern was consistent with the results of studies on apple and pomegranate seeds (Dębska et al., Reference Dębska, Krasuska, Budnicka, Bogatek and Gniazdowska2013; Shalimu et al., Reference Shalimu, Sun, Baskin, Baskin, Sun and Liu2016). Furthermore, other than cold stratification, the application of exogenous hydrogen peroxide was also effective in breaking dormancy. Treating seeds with H2O2 significantly improved growth and germination at 15°C, but not at 3°C. During 5 weeks of incubation at 15°C, embryos of seeds treated with 100 mM H2O2 elongated more than those at 3°C, and seeds germinated to 32%. The involvement of H2O2 in dormancy break is argued in many studies (Hendricks and Taylorson, Reference Hendricks and Taylorson1975; Oracz et al., Reference Oracz, El-Maarouf Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007; Shalimu et al., Reference Shalimu, Sun, Baskin, Baskin, Sun and Liu2016; Wojtyla et al., Reference Wojtyla, Lechowska, Kubala and Garnczarska2016), and it also has an effect on germination (Barba-Espin et al., Reference Barba-Espin, Diaz-Vivancos, Clemente-Moreno, Albacete, Faize, Faize, Pérez-Alfocea and Hernández2010; Ishibashi et al., Reference Ishibashi, Koda, Zheng, Yuasa and Iwaya-Inoue2012). The positive effect of hydrogen peroxide on both embryo growth and germination was further demonstrated by the application of DPI. DPI is reported to enhance seed dormancy by scavenging H2O2, and reversing its action (Liu et al., Reference Liu, Ye, Liu, Chen and Zhang2010). Here, in our study, we reached a similar conclusion: the concentration of 1 mM DPI significantly suppressed the growth and germination at 3°C (seeds germinated to 38.5% after 4 weeks). The negative effect of DPI on germination is also suggested by other studies. Ishibashi et al. (Reference Ishibashi, Tawaratsumida, Zheng, Yuasa and Iwaya-Inoue2010) reported that DPI (1 mM) reduces seedling growth during seed germination of barley.
After the first week, cold-stratified embryonic axes elongated more rapidly than warm-stratified and DPI-treated axes, which led to their significantly higher axis:cotyledon ratio (Fig. 7b). Similarly, by the application of exogenous H2O2, the embryos showed major growth, which was mostly due to elongation of embryonic axes. These results emphasize the possible significance of the early growth of the embryonic axes in alleviation of the dormancy in F. ovina. Meanwhile, our result of visualizing the localization of O2 − in embryos by using the histochemical staining technique indicated the earlier production of superoxide in the embryonic axes than in cotyledons during both cold and warm stratification. However, the time gap was longer during cold than warm conditions. All in all, the results suggest connections between the earlier accumulation of O2 − in the embryonic axes and their higher elongation at 3°C. Singh et al. (Reference Singh, Chaudhuri and Kar2014) reported that during seed germination in Vigna radiata, the elongation of the axis is associated with the generation and asymmetric accumulation of O2 − in embryo tissues. The growth-inducing effect of O2 − is also reported in other studies (Müller et al., Reference Müller, Carstens, Linkies, Torres and Leubner-Metzger2009; Libik-Konieczny et al., Reference Libik-Konieczny, Kozieradzka-Kiszkurno, Desel, Michalec-Warzecha, Miszalski and Konieczny2014). Accordingly, the dormancy-breaking effect of exogenous H2O2 in our study might be through increasing the amount of inner O2 − which in turn induces elongation of the axes. Roach et al. (Reference Roach, Beckett, Minibayeva, Whitaker, Chen, Bailly and Kranner2010) demonstrated that the increase in germination and seedling growth in desiccation-stressed seeds of Castanea sativa by exogenous H2O2 was through enhancing the internal level of O2 −.
In summary, fresh seeds of F. ovina have deep complex MPD. Cold stratification is the only natural treatment that causes embryos to complete growth and germinate, which is probably because more nutrient reserves become available for embryos during cold than warm stratification. However, we observed a differential pattern of growth between embryos at 3 and 15°C. Even though embryos at both temperatures grew to the same length during the first 3 weeks, cold-stratified seeds projected higher growth of the embryonic axis, and eventually these embryos could complete their growths and germinate. The same results were obtained during treatments with exogenous hydrogen peroxide and the elongation of the embryos was mostly because of the higher growth of axes during the first week, which finally led to the germination of the embryos. Moreover, as ROS could control the breakdown of the reserves and provide food for the tissues, the sooner production or accumulation of superoxide radicals might be the reason for the higher growth and elongation of the embryonic axes.
Moreover, in the case of F. ovina, cold temperatures favour seed germination as well. The maximum percentages of germination were achieved by incubating seeds at cold temperatures. Under environmental conditions, continuous embryo growth and dormancy break only coincide with the decrease in temperature, and seeds germinate to high percentages during winter and under low-temperature soil. The germinated radicles tolerate the cold and elongate with the rise of temperature.
This study was among the very first reports of MPD in Iran. The results will hopefully help improve and optimize the germination of dormant F. ovina seeds, which in turn helps promote its cultivation. Moreover, ROS experiments contribute to understanding MPD dormancy break. We believe, as there are many species of Apiaceae in Iran (especially the genus Ferula), providing significant economic and medicinal benefits, their dormancy and germination characteristics as well as their germination patterns in local habitats have not yet been properly characterized. Therefore, a great deal of research still needs to be undertaken.