Introduction
The turnover of seed storage proteins involves their synthesis during development and their degradation following germination. Globulins are the main storage proteins within the protein storage vacuoles (PSVs) of dicotyledonous seeds (Shewry and Casey, Reference Shewry and Casey1999), and several proteases responsible for their degradation are deposited along with them during seed development. However, degradation is negligible at this stage, indicating that storage proteins are protected against premature degradation (Shutov et al., Reference Shutov, Baumlein, Blattner and Muntz2003; He et al., Reference He, Huang, Wilson and Tan-Wilson2007).
Several mechanisms have been proposed to explain how storage proteins avoid hydrolysis in developing seed PSVs. Jiang and Rogers (Reference Jiang and Rogers2002) suggested a differential sub-compartmentation of stored proteins and proteases, whereas several other authors proposed the maintenance of proteases in an inactive form (for reviews, see Muntz et al., Reference Muntz, Belozersky, Dunaevsky, Schlereth and Tiedemann2001; Tan-Wilson and Wilson, Reference Tan-Wilson and Wilson2012). Protein structural changes were also proposed to prevent protease accessibility, as reported for the low specificity of papain-like proteases towards globulin crystals (Weber and Neumann, Reference Weber and Neumann1980). However, these structural alterations were not fully demonstrated. In vitro studies have shown that purified seed storage proteins from several legume species self-aggregate when incubated in the presence of Ca2+ and/or Mg2+, whereas Cd2+, Cu2+, Zn2+, Mn2+ and K+ are rather inefficient in promoting this (Ferreira et al., Reference Ferreira, Franco and Teixeira1999, Reference Ferreira, Freitas and Teixeira2003). The two alkaline earth cations, Ca2+ and Mg2+, present in the cotyledons of legume seeds (Trugo et al., Reference Trugo, Donangelo, Duarte and Tavares1993; Regvar et al., Reference Regvar, Eichert, Kaulich, Gianoncelli, Pongrac, Vogel-Mikus and Kreft2011), may be considered promising candidates to fulfil an important physiological role in globulin self-aggregation in vivo, either by promoting efficient protein packaging in relatively small volumes and/or by conferring protection against proteolytic attack.
During germination, at the onset of protein storage mobilization, when there is no storage protein synthesis and when amino acids are mobilized to nourish the embryo, the mechanisms that protect stored proteins from degradation must be overcome. In dicotyledonous seeds, proteases stored in PSVs during maturation are apparently responsible for the initial protein mobilization, with the de novo synthesized proteases mediating the bulk of storage protein degradation only at a later stage (Muntz, Reference Muntz2007; Tan-Wilson and Wilson, Reference Tan-Wilson and Wilson2012). The precise control of these proteolytic events during protein reserve deposition and mobilization remains largely unclear.
To help determine the physiological significance of alkaline earth cations on globulin mobilization, the in vivo concentrations of these elements were determined in both the cotyledons and PSVs isolated from Lupinus albus seeds. Globulin self-aggregation–disaggregation profiling, as a function of pH in the presence of those cations, was also evaluated. Selected protein structural data and physiological events reported in the literature were gathered and used to support a Ca2+/Mg2+-dependent model to explain how legume seed storage globulins are efficiently packed during seed formation, free from the action of neighbouring proteases, and subsequently dismantled and subjected to proteolytic digestion during seed germination and subsequent seedling growth.
Materials and methods
Plant material
Dry seeds of white lupin (Lupinus albus cv. Lublanc) were surface sterilised with 1% (v/v) sodium hypochlorite and germination was initiated by immersion of the seeds in running tap water for 2 d. The seed coats were removed and axes and intact cotyledons dissected from the embryos and stored at − 80°C until required.
Protein purification
Lupinus albus seed globulins were purified from cotyledons of seeds germinated for 2 d, following a methodology similar to that described by Franco et al. (Reference Franco, Ferreira and Teixeira1997). Briefly, the cotyledons were ground in cold water (adjusted to pH 8.0) containing 0.01 M CaCl2 and 0.01 M MgCl2 [13 ml g− 1 fresh weight (FW)] and stirred for 4 h. The suspension was filtered through a 20-μm mesh (Miracloth, CalBiochem, California, USA) prior to centrifugation for 1 h at 30,000 g. The resulting pellet was used for total globulin extraction by stirring it in a solution containing 10% (w/v) NaCl, 0.01 M EDTA and 0.01 M EGTA [13 ml (g FW)− 1], for 12 h. The suspension was centrifuged for 1 h at 30,000 g and the resulting globulin solution was concentrated by ammonium sulphate (561 g l− 1) precipitation. The precipitated globulins were centrifuged at 30,000 g for 20 min, resuspended in 0.02 M Tris-HCl at pH 7.5 and desalted on Econo-Pac 10 DG columns (BioRad, Hercules, California, USA), previously equilibrated in the same buffer. All operations were performed at 4°C.
Isolation of protein storage vacuoles
The protein storage vacuoles (PSVs) were isolated from imbibed L. albus seeds following a protocol based on that described by Einhoff et al. (Reference Einhoff, Fleischmann, Freier, Kummer and Rudiger1986). The seeds were gently homogenized with chilled double-distilled water [5 ml (g FW)− 1 of tissue] and the homogenate filtered through cheesecloth and centrifuged at 350 g for 10 min. The pellet was discarded and the supernatant centrifuged at 17,500 g for 10 min. The resulting pelleted intermediate layer containing the PSVs was collected and resuspended in water to 5 ml g− 1 of the initial seed FW. The crude PSV fraction was placed on to a 5% (w/v) Ficoll solution in water, which was layered on top of an equal volume of a 25% (w/v) Ficoll solution, and centrifuged at 500 g for 20 min. The isolated PSVs, forming a layer at the interface between the two different Ficoll concentrations, were removed with a micropipette. All operations were performed at 4°C. The PSVs were identified using a phase-contrast microscope (Leica DMRB, Wetzlar, Germany), after staining with 2% (w/v) potassium iodide in distilled water. From the mass of Lupinus cotyledons used as starting material, the purification yield and mass of purified PSVs, as well as the contribution of PSVs to the cotyledonary dry weight (DW) were calculated.
Measurement of enzyme activity
α-Mannosidase was used as a PSV marker and its activity was assayed essentially as described by Einhoff et al. (Reference Einhoff, Fleischmann, Freier, Kummer and Rudiger1986). A 0.005 M solution of p-nitrophenyl-α-d-mannopyranoside in 0.05 M sodium acetate buffer pH 5.0 was incubated with aliquots of enzyme preparations in a total volume of 150 μl for up to 15 min. The reaction was stopped by the addition of 0.2 M sodium carbonate buffer pH 9.0 (150 μl) and absorbance was measured at 405 nm in a Diagnostics Pasteur LP400 microplate reader (Sanofi, Marnes-la-Coquette, France). One enzyme unit (U) corresponds to the enzyme amount capable of releasing 1 μmol of 4-nitrophenol min− 1.
Turbidity measurements
The turbidity measurements of the globulins were made according to an adapted procedure based on the one described by Okubo et al. (Reference Okubo, Myers and Iacobucci1976): the globulin fraction obtained after desalting was quantified according to the Bradford method as modified by Ramagli (Reference Ramagli1999), and resolubilized in universal buffer (Britton and Robinson, Reference Britton and Robinson1931) diluted to one-third at pH 7.5, pH 6.5 or pH 5.5 for a final protein concentration of 2.2 mg ml− 1; 90 μl of the globulin solution was incubated with 10 μl of the different chloride salts (Be2+, Mg2+, Ca2+, Sr2+ and Ba2+) in concentrations ranging from 0 to 100 mM. Turbidity measurements were made spectrophotometrically at 600 nm in a PowerWave XS microplate reader (BioTek, Bad Friedrichshall, Germany) after an incubation period of 2 min; longer periods of incubation did not result in an increase in absorbance.
Alkaline earth element concentrations
Alkaline earth elements were extracted by digesting the dried residue of PSVs or of cotyledons in a 1:1 solution of 50% (v/v) HNO3 and 50% (v/v) HCl, after which the samples were filtered and the volumes adjusted to 20 ml (PSVs) or 50 ml (cotyledons). Their concentrations were then determined using inductively coupled plasma-optical emission spectroscopy (ICP-OES) Ultima (Jobin-Yvon Horiba, Stanmore, Middlesex, UK). The wavelengths used were 313.042 nm for Be2+, 285.213 nm for Mg2+, 422.673 nm for Ca2+, 346.446 nm for Sr2+ and 233.527 nm for Ba2+. All plasticware and glassware used in PSV isolation were previously soaked in 50% (v/v) HCl to minimize mineral contamination. All measurements were made in triplicate. Blank assays yielded element concentrations that varied between 4 and 7% of those obtained for samples. Although this methodology may underestimate cation concentrations, due to their leakage from the organelles during purification, alternative approaches described in previous studies do not provide an accurate quantification. Among these are cryofixation and cryosectioning (Lott and Buttrose, Reference Lott and Buttrose1978), which partially overcome cation leakage since the element distribution is not significantly affected by the sample preparation, but due to difficulties in standardizing the X-ray microanalytical technique, the measurements are only semi-quantitative or only allow a qualitative analysis of the radial distribution of elements (Bucking et al., Reference Bucking, Kuhn, Schroder and Heyser2002).
Protein data search
A protein data search for L. albus conglutins was performed in the UniProt database [http://www.uniprot.org; (accessed 2010); Jain et al., Reference Jain, Bairoch, Duvaud, Phan, Redaschi, Suzek, Martin, Mcgarvey and Gasteiger2009]. The sequences chosen for β-conglutin (Q6EBC1), α-conglutin (Q53I54) and γ-conglutin (Q9FSH9 and Q9FEX1), were used for sequence homology searches in the Protein Data Bank [http://www.pdb.org; (accessed 2010); Berman et al., Reference Berman, Westbrook, Feng, Gilliland, Bhat, Weissig, Shindyalov and Bourne2000] using an expectation value, E < 10− 20.
Results and discussion
On the basis of known cation electrostatic involvement in the macromolecular aggregation of legume seed storage proteins, a mechanism that ensures an efficient packing inside PSV, the alkaline earth cations were used to evaluate their effects not only on aggregation of L. albus globulins but also on disaggregation profiles.
Alkaline earth elements and in vitro globulin self-aggregation–disaggregation
To assess the ability that the alkaline earth elements have to promote L. albus globulin self-aggregation, turbidity measurements were used to follow its aggregation–disaggregation profiles. Purified globulins, at a time before hydrolysis was initiated (Ferreira et al., Reference Ferreira, Melo and Teixeira1995), were incubated in the presence of the various divalent cations: Be2+, Mg2+, Ca2+, Sr2+ and Ba2+ (Fig. 1). Not all cations were equally effective in promoting globulin self-aggregation and disaggregation. For instance, Ba2+, which caused the highest turbidity measurements, produced globulin aggregation at concentrations ranging from 5 to 20 mM. However, no globulin disaggregation was observed for higher Ba2+ concentrations. When compared with the other cations studied, Be2+ originated a distinct pattern of globulin aggregation–disaggregation; small changes in concentration in the range of 5–15 mM produced very high variations. Only Mg2+, Ca2+ and Sr2+ produced similar globulin patterns of aggregation–disaggregation. Among these, the highest turbidity measurement was recorded for Ca2+ at 20 mM, followed by Sr2+ and Mg2+ at 25 mM. Cation-dependent formation of high-order aggregates of globulin molecules were previously observed by turbidity measurements and validated by isopycnic density gradient centrifugation (see figure 4 in Ferreira et al., Reference Ferreira, Franco and Teixeira1999). Above 65 mM no globulin aggregation could be detected using Mg2+, Ca2+ or Sr2+. Under the conditions studied, Ba2+ promoted an irreversible globulin aggregation, whereas the remaining cations generated a reversible globulin aggregation profile, with Be2+ producing a narrower dose-mediated reversibility than Mg2+, Ca2+ or Sr2+. This profile of reversible globulin aggregation for Mg2+ and Ca2+ was previously shown for L. albus globulins as well as for other legume seeds (figure 3 in Ferreira et al., Reference Ferreira, Franco and Teixeira1999) and was tentatively explained as an electrostatic process (Ferreira et al., Reference Ferreira, Freitas and Teixeira2003).

Figure 1 Lupinus albus globulin aggregation–disaggregation profiles when incubated in the presence of increasing concentrations (0–100 mM) of Be2+(▲), Mg2+ (![]() ), Ca2+ (□), Sr2+(○) and Ba2+(♦) at pH 7.5. The values shown are the average of three determinations and the bars represent ± SD.
), Ca2+ (□), Sr2+(○) and Ba2+(♦) at pH 7.5. The values shown are the average of three determinations and the bars represent ± SD.
Alkaline earth element concentrations inside PSVs
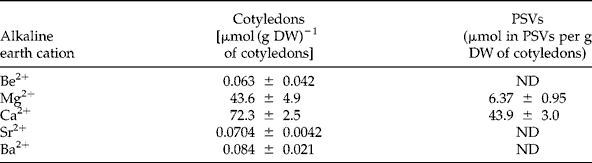
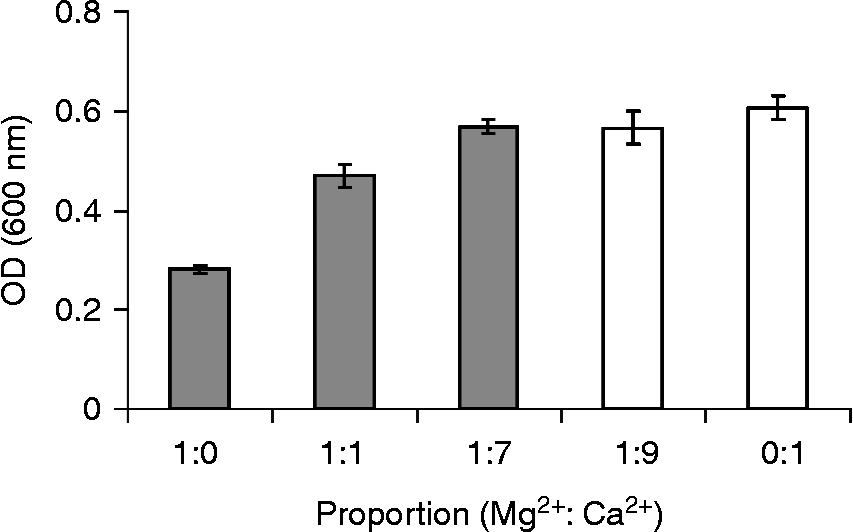
To determine which alkaline earth elements (Be2+, Mg2+, Ca2+, Sr2+ and Ba2+) can be effectively involved in the in vivo mobilization of legume storage proteins, their concentrations were measured in cotyledons and PSVs isolated from L. albus seeds (Table 1). As expected, Mg2+ and Ca2+ were by far the most abundant alkaline earth elements present, comprising 43.6 μmol and 72.3 μmol per g DW of cotyledons, respectively, whereas Be2+, Sr2+and Ba2+ were present in trace amounts. The cotyledonary contents of Mg2+ and Ca2+ are consistent with the values reported in the literature for the chemical composition of lupin seeds (Trugo et al., Reference Trugo, Donangelo, Duarte and Tavares1993); however, for the minor components, Be2+, Sr2+ and Ba2+, there are no reports. In purified PSVs Mg2+ and Ca2+ were the only alkaline earth elements detected, 6.37 μmol and 43.9 μmol per g DW of cotyledons, respectively; the other elements were below the detection limit of the method employed, and thus too little to participate in globulin mobilization. Because PSVs represent approximately 52% of L. albus cotyledonary DW (0.519 ± 0.034 g DW of PSVs per g DW of cotyledons), it is calculated that 14.6% and 60.7% of the seed Mg2+ and Ca2+, respectively, are located within PSVs. Considering that the Ca2+ concentration is approximately seven times higher than that of Mg2+ in PSVs, and almost twice that of Mg2+ in cotyledons (Table 1), the patterns of globulin aggregation–disaggregation were determined in vitro for different combinations of cation concentrations: 1:1, 1:7 and 1:9 (Fig. 2).
Table 1 Alkaline earth cation content in cotyledons and protein storage vacuoles (PSVs) isolated from Lupinus albus seeds. Values are averages of three determinations ± SD
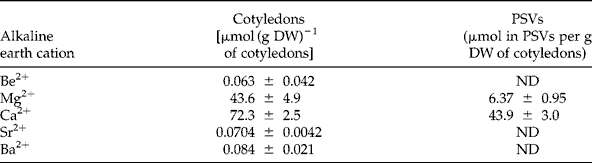
ND, not detected.

Figure 2 Lupinus albus globulin aggregation–disaggregation profiles when incubated in the presence of increasing concentrations (0–100 mM) of Mg2+ (![]() ), Ca2+ (□), or different combinations of Mg2+ and Ca2+: 1:1 (⋄), 1:7 (□) and 1:9 (●) at pH 7.5. Values shown are the average of three determinations and bars represent ± SD.
), Ca2+ (□), or different combinations of Mg2+ and Ca2+: 1:1 (⋄), 1:7 (□) and 1:9 (●) at pH 7.5. Values shown are the average of three determinations and bars represent ± SD.
The combined concentrations of Mg2+ and Ca2+ resulted in globulin aggregation–disaggregation profiles intermediate between the globulin profiles obtained for Mg2+ and Ca2+ alone, with the turbidity increasing as Ca2+ proportion increased. For each condition, the total cation concentration that is able to promote maximum globulin aggregation decreases as the Ca2+ contribution in the fractions increases (Fig. 3). The requirement of less Ca2+ than Mg2+ to promote higher turbidity indicates that these two cations may have distinct contributions to globulin aggregation and disaggregation, with the former being the most efficient.
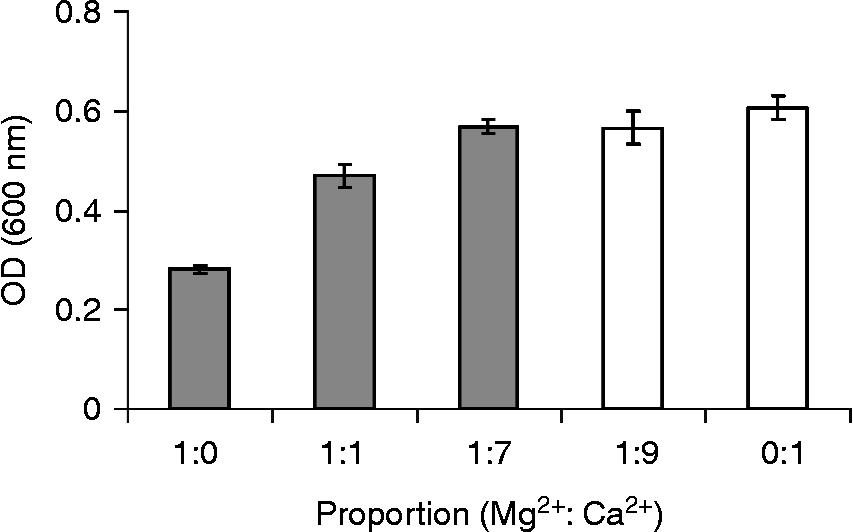
Figure 3 Maximum turbidity obtained for globulin aggregation in the presence of Mg2+, Ca2+ and combined fractions of Mg2+ and Ca2+(1:1, 1:7, 1:9). Columns represent the maximum turbidity obtained for 25 mM (grey columns) and 20 mM (white columns). Values shown are the average of three determinations and bars represent ± SD.
Proposed dual role for Ca2+ and Mg2+ from a structural standpoint
According to the globulin aggregation–disaggregation profile observed, both Ca2+ and Mg2+ are expected to be involved in vivo in globulin structural changes. A hypothesis is formulated which underlies their proposed role in the way storage globulins are efficiently packed during legume seed formation, free from the action of neighbouring proteases, and subsequently dismantled and subjected to proteolytic digestion during seed germination and seedling growth. This relates to the in vitro response of the globulins to Ca2+ or Mg2+ and the amount of these cations inside PSVs. The annotated protein structures were scrutinized to verify if either Ca2+ or Mg2+ promote crystallization, by aiding the interaction between these protein molecules. Although crystallization is not aggregation, but rather a salting out of protein molecules in an ordered and repetitive manner, it is still possible to infer the position of Ca2+ and Mg2+ in the protein structure.
The sequences annotated in the UniProt database (Jain et al., Reference Jain, Bairoch, Duvaud, Phan, Redaschi, Suzek, Martin, Mcgarvey and Gasteiger2009) for β-conglutin (Q6EBC1), α-conglutin (Q53I54) and γ-conglutin (Q9FSH9 and Q9FEX1) from L. albus were chosen for sequence homology searches in the Protein Data Bank (Berman et al., Reference Berman, Westbrook, Feng, Gilliland, Bhat, Weissig, Shindyalov and Bourne2000). Several hits that are mainly from other legume crops were observed for all three proteins (Table 2). Some were reported for β- and α-conglutins that showed the presence of either Ca2+ or Mg2+ in their crystal structure. Only three of the β-conglutin homologues showed the presence of Ca2+ or Mg2+, namely β-conglycinin from Glycine max (1UIK) which is reported to contain Mg2+ (Maruyama et al., Reference Maruyama, Maruyama, Mikami and Utsumi2004), and globulin-3 and globulin-1 from Vigna angularis (2EAA and 2EA7, respectively) which are reported to contain Ca2+ (Fukuda et al., Reference Fukuda, Maruyama, Salleh, Mikami and Utsumi2008) (Fig. 4a). These elements are positioned at the protein surface and are co-ordinated by protein residues and water molecules or by just water molecules, as observed for β-conglycinin from Glycine max. For α-conglutin (Q53I54), there is a single homologous protein, prunin-1 from Prunus dulcis (3FZ3), where the presence of Ca2+ is reported (Jin et al., Reference Jin, Albillos, Guo, Howard, Fu, Kothary and Zhang2009). In this case, Ca2+ is at the surface of each subunit, allowing the transition between two trimers in the hexameric structure of the native protein (Fig. 4b). From all the hits reported for γ-conglutin, none shows the presence of either Ca2+ or Mg2+, in agreement with the reduced aggregation reported for γ-conglutin in the presence of Ca2+ or Mg2+; this protein preferentially interacts with Zn2+ (Ferreira et al., Reference Ferreira, Franco and Teixeira1999, Duranti et al., Reference Duranti, Scarafoni, Di Cataldo and Sessa2001).
Table 2 Proteins annotated in the Protein Data Bank (Berman et al., Reference Berman, Westbrook, Feng, Gilliland, Bhat, Weissig, Shindyalov and Bourne2000) that have sequence similarities, for an expectation value E < 10−20, with β-(Q6EBC1), α-(Q53I54) and γ-(Q9FSH9 and Q9FEX1) conglutin sequences annotated in the UniProt database (Jain et al., Reference Jain, Bairoch, Duvaud, Phan, Redaschi, Suzek, Martin, Mcgarvey and Gasteiger2009) for L. albus. The ligands described to be at the surface of the proteins are indicated

NAG, N-Acetyl-D-glucosaminidase; CIT, citric acid; GOL, glycerol.

Figure 4 Three-dimensional structures representative of β-conglutin homologues (a) and α-conglutin homologues (b), with Mg2+ represented by pink spheres and Ca2+ by yellow spheres (see online at http://www.journals.cambridge.org/ssr for a colour version of this figure). The precise location of the spheres is highlighted by arrows. The structures presented were prepared using Pymol (DeLano, Reference DeLano2002).
β-Conglutin was previously reported to have the highest total globulin fraction aggregation, not only because it is the most abundant lupin storage protein, but also because a higher turbidity was observed in the presence of Mg2+ and Ca2+(1:1) when compared to α-conglutin (Ferreira et al., Reference Ferreira, Franco and Teixeira1999). Despite the reported lectin-like properties exhibited by β-conglutin (Sharon and Lis, Reference Sharon and Lis1990), its self-aggregation was previously proposed to be electrostatic rather than lectin-mediated (Ferreira et al., Reference Ferreira, Freitas and Teixeira2003). Nevertheless, either mechanism requires Ca2+ and Mg2+ to promote β-globulin self-aggregation. The fact that for the same amount of protein, β-conglutin produces higher turbidity measurements than α-conglutin (see figure 1 in Ferreira et al., Reference Ferreira, Franco and Teixeira1999), suggests that β-conglutin is more prone to bind Ca2+ and/or Mg2+.
Proposed dual physiological role of Ca2+ and Mg2+
PSVs are acidified following seed germination (Otegui et al., Reference Otegui, Herder, Schulze, Jung and Staehelin2006; He et al., Reference He, Huang, Wilson and Tan-Wilson2007). Given the electrostatic nature of the globulin supramolecular aggregates, a decrease in pH will alter the net charge of the protein aggregates, influencing globulin interaction with Ca2+ and Mg2+; however, globulin aggregation–disaggregation profiles were screened at a neutral pH (Ferreira et al., Reference Ferreira, Franco and Teixeira1999, Reference Ferreira, Freitas and Teixeira2003). To assess the alterations induced by a mildly acidic pH, the globulin aggregation–disaggregation profiles were evaluated at pH 6.5 and pH 5.5 in the presence of Mg2+ (Fig. 5a), Ca2+ (Fig. 5b) and also Ca2+ and Mg2+ combined (1:1, 1:7, 1:9) (data not shown). The values obtained were compared with those achieved at pH 7.5. The globulin profile differs with pH, with the maximum values for turbidity gradually increasing and shifting towards lower Ca2+ and Mg2+ concentrations as the pH drops from 7.5, through 6.5, to 5.5. A similar behaviour was observed for the combined Mg2+ and Ca2+(1:1, 1:7, 1:9) fractions (data not shown), once again with profiles that are intermediate between those of Mg2+ and Ca2+ alone. This decrease in globulin solubility due to acidification is in accordance with the previous observation that a decrease in pH (5.5–6.0) promotes an increase in the aggregation of legumin-type globulins from Arabidopsis thaliana (Gruis et al., Reference Gruis, Schulze and Jung2004).

Figure 5 Lupinus albus globulin aggregation–disaggregation profiles obtained in vitro at different pH values. The isolated globulins were incubated in the presence of increasing concentrations (0–100 mM) of (a) Mg2+ and (b) Ca2+ at pH 7.5 (![]() ), pH 6.5 (▲) or pH 5.5 (⋄). Values shown are the average of three determinations and bars represent ± SD.
), pH 6.5 (▲) or pH 5.5 (⋄). Values shown are the average of three determinations and bars represent ± SD.
A decrease in pH will exert two effects on legume globulins: (1) as it gradually approaches globulin pI values, which are mainly acidic (Crouch and Sussex, Reference Crouch and Sussex1981; García et al., Reference García, Torre, Marina, Laborda and Rodriquez1997; Magni et al., Reference Magni, Scarafoni, Herndl, Sessa, Prinsi, Espen and Duranti2007), electrostatic interaction changes will decrease protein solubility to a minimum value; (2) as the number of negative charges on the globulin surface is reduced, so will the Ca2+ and/or Mg2+ binding sites, shifting the equivalence point of maximum turbidity to lower cation concentrations. The slightly different globulin aggregation–disaggregation profile observed for Ca2+ at pH 5.5, when compared either with pH 6.5 or pH 7.5 profiles (Fig. 5b), may result from these different contributions by causing altered conglutin interactions with this cation (figure 1 in Ferreira et al., Reference Ferreira, Franco and Teixeira1999). The data in Fig. 5 can have physiological significance, following germination, if an increase in free Ca2+ and Mg2+ concentrations is concomitant with a drop in pH inside the PSVs.
In addition to insoluble protein deposits and soluble storage proteins, PSVs also contain a matrix of globoids of phytic acid and oxalate crystals (Jiang et al., Reference Jiang, Phillips, Rogers and Rogers2000). Phytate, a fully phosphorylated form of inositol, chelates most metal ions, such as Mg2+ and Ca2+ which, in addition to their participation in crystal oxalate formation (mainly calcium oxalate), makes the active participation of these two cations in metabolic processes unlikely (Rendle, Reference Rendle1888; Clarkson, Reference Clarkson1980; Ilarslan et al., Reference Ilarslan, Palmer and Horner2001; Franceschi and Nakata, Reference Franceschi and Nakata2005). This PSV storage function is in agreement with the high amounts of Ca2+, and to a lesser extent Mg2+, detected inside L. albus PSVs (Table 1).
Upon seed germination and seedling growth, the low free cation content can be reversed by the combined action of several events that arise from PSV acidification, namely phytase activation and oxalate crystal dissolution (Franceschi, Reference Franceschi1989). In L. albus, phytase has an optimal pH of 5.0 (Greiner, Reference Greiner2002) and a decrease in phytate content, and also oxalate crystal dissolution, will cause a substantial increase in the free cation content inside PSVs. In lupin, phytase maximum activity was reached 4 d after seed imbibition (Greiner, Reference Greiner2002), which is coincident with the beginning of globulin degradation (Ferreira et al., Reference Ferreira, Melo and Teixeira1995). Acidification inside PSVs also mediates the activation of several other enzymes, such as oxalate oxidase, an enzyme proposed to participate in the degradation of the oxalate liberated from the crystals (Dumas et al., Reference Dumas, Freyssinet and Pallett1995, Kanauchi et al., Reference Kanauchi, Milet and Bamforth2009), and subtilisin-like serine proteases, proposed to be involved in storage protein mobilization in soybean seeds (He et al., Reference He, Huang, Wilson and Tan-Wilson2007). Furthermore, a protease from Vigna radiata seeds involved in storage protein mobilization was recently reported to be activated by Ca2+ (Khan et al., Reference Khan, Verma and Sharma2010). These physiological events can be related to the in vitro profile observed for globulin aggregation and disaggregation with decreasing pH and in the presence of increasing Mg2+ and Ca2+ concentrations (Fig. 6).

Figure 6 Schematic representation of the main physiological events that occur during seed maturation, seed germination and seedling growth, correlated with in vitro globulin aggregation and disaggregation profile in the presence of increasing free Mg2+ and Ca2+ concentrations. References: 1Jiang et al., Reference Jiang, Phillips, Rogers and Rogers2000; 2Clarkson, Reference Clarkson1980; 3Franceschi and Nakata, Reference Franceschi and Nakata2005; 4Ilarslan et al., Reference Ilarslan, Palmer and Horner2001; 5Rendle, Reference Rendle1888; 6Franceschi, Reference Franceschi1989; 7Greiner, Reference Greiner2002; 8Dumas et al., Reference Dumas, Freyssinet and Pallett1995; 9Kanauchi et al., Reference Kanauchi, Milet and Bamforth2009; 10He et al., Reference He, Huang, Wilson and Tan-Wilson2007; 11Khan et al., Reference Khan, Verma and Sharma2010; 12Otegui et al., Reference Otegui, Herder, Schulze, Jung and Staehelin2006; 13Ferreira et al., Reference Ferreira, Franco and Teixeira1999; 14Ferreira et al., Reference Ferreira, Freitas and Teixeira2003.
Although there is no doubt that dry seed PSVs contain proteases, their mechanisms of action in storage protein mobilization remains unclear. Our results suggest that relatively low free Mg2+ and Ca2+ concentrations that occur during seed maturation contribute to globulin aggregation by promoting protein–divalent cation–protein interactions, thus explaining the tight storage protein packaging inside PSVs. An increase in free cation concentration, during and following seed germination, could contribute to globulin disaggregation by oversaturation of the protein–divalent cation binding sites, causing the positively charged globulin molecules to repel each other (Ferreira et al., Reference Ferreira, Freitas and Teixeira2003). These globulin aggregation events could modulate PSV protease accessibility to their substrates. Globulin aggregation could block the access of proteases to the substrate, whereas aggregate dismantling could contribute to protease accessibility, allowing globulin mobilization. To further investigate this, the environment inside PSVs has to be fully characterized.
In conclusion, the coexistence of storage proteins with proteases responsible for their degradation is well documented, although globulin packaging and mobilization inside legume PSVs is still poorly understood. By integrating the in vitro data for globulin reversible aggregation in the presence of Ca2+ and Mg2+ with the physiological events that occur during legume seed development, we suggest that the low free Ca2+ content inside PSVs, and to a lesser extent Mg2+, can mediate globulin packaging and therefore contribute to protection against proteolytic attack. Following seed germination and seedling growth, an increase in free cation levels could reverse the process by promoting globulin disaggregation and protease accessibility to the substrate.
Acknowledgements
This work was supported financially by the Fundação para a Ciência e a Tecnologia through grant PEst-OE/EQB/LA0004/2011 and also by financial support of C.N.S. (SRFH/BPD/26,562/2006) as well as M.M.A. (SFRH / BPD / 76,646 / 2011).