Introduction
Many crop species can reproduce with their wild relatives (Ellstrand, Reference Ellstrand2003), yielding hybrid offspring that differ from their wild parents in ecologically important characteristics (Cummings et al., Reference Cummings, Alexander and Snow1999; Snow et al., Reference Snow, Uthus and Culley2001; Mercer et al., Reference Mercer, Shaw and Wyse2006a). In many cases, this gene flow from crop to wild populations (crop–wild gene flow) may present several significant evolutionary consequences, including extinction by assimilation (Wolf et al., Reference Wolf, Takebayashi and Rieseberg2001; Levin, Reference Levin, Ferriere, Dieckmann and Couvet2004), reductions of genetic diversity (Whitton et al., Reference Whitton, Wolf, Arias, Snow and Rieseberg1997) and increased weediness (Snow et al., Reference Snow, Uthus and Culley2001; Ellstrand, Reference Ellstrand2003), especially if the gene flow is recurrent. While these concerns previously existed, the widespread use of transgenic crops has increased their prominence and has prompted questions about whether transgene flow may present uniquely potent ecological dangers, especially with regard to the weediness of wild populations (Burke et al., Reference Burke, Gardner and Rieseberg2002; Ellstrand, Reference Ellstrand2003; Snow et al., Reference Snow, Pilson, Rieseberg, Paulsen, Pleskac, Reagon, Wolf and Selbo2003).
Crop–wild hybridization can affect various aspects of a plant's life cycle (Adler et al., Reference Adler, Wikler, Wyndham, Linder and Schmitt1993; Hauser et al., Reference Hauser, Shaw and Ostergard1998; Hooftman et al., Reference Hooftman, Oostermeijer, Jacobs and den Nijs2005; Mercer et al., Reference Mercer, Shaw and Wyse2006a). Crop plants are often differentiated from their wild counterparts by characteristics typical of domestication, such as increased seed size, decreased seed shattering, altered growth habit, self-compatibility and decreased seed dormancy. Thus, crop–wild hybrids may possess intermediate values for these traits. The fitness effects (positive, neutral or negative) on wild populations of crop alleles, including those responsible for domestication traits, largely determine whether crop alleles will persist in, or introgress into, wild populations (Ellstrand, Reference Ellstrand2003; Snow et al., Reference Snow, Culley, Campbell, Sweeney, Hegde and Ellstrand2010), though genetic linkage and hitchhiking may also affect crop gene introgression (Baack et al., Reference Baack, Sapir, Chapman, Burke and Rieseberg2007). Crop alleles that are detrimental to fitness under the selection pressures experienced by wild populations are much less likely to persist in wild populations than crop alleles that give crop–wild hybrids an advantage over their wild neighbours. For example, differences in patterns of seed dormancy between the crop and wild types could influence crop gene introgression. Increased germination rates in hybrids could hasten crop gene introgression as long as the hybrids germinate at the appropriate time (Mercer et al., Reference Mercer, Shaw and Wyse2006a). Conversely, reduced dormancy in crop–wild hybrids could result in increased autumn, or very early spring, germination, thereby reducing the chance of those individuals contributing to the next generation and hindering crop gene introgression.
As is the case in many crop–wild systems, crop and wild sunflowers (both Helianthus annuus, with the wild sunflower known as common sunflower) are differentiated for important traits (Presotto et al., Reference Presotto, Fernandez-Moroni, Poverene and Cantamutto2011), including branch number, inflorescence size and seed shattering (Mercer, Reference Mercer2005). Seed size and dormancy also differ, with wild achenes being smaller (wild ≥ 2 mm × 1 mm; crop ≤ 25 mm × 13 mm; Schneiter, Reference Schneiter1997) and more dormant (Mercer et al., Reference Mercer, Wyse and Shaw2006b; Brunick, Reference Brunick2007; Wills and Burke, Reference Wills and Burke2007; Presotto et al., Reference Presotto, Ureta, Cantamutto and Poverene2012). Furthermore, the sunflower is an ideal species for studying crop–wild gene flow because crop and wild types are often found in close proximity, are cross-compatible and share pollinators (Burke et al., Reference Burke, Gardner and Rieseberg2002). Thus, there are numerous naturally occurring crop–wild hybrid zones of this species across its native range of North America (Harter et al., Reference Harter, Gardner, Falush, Lentz, Bye and Rieseberg2004; Blackman et al., Reference Blackman, Scascitelli, Kane, Luton, Rasmussen, Bye, Lentz and Rieseberg2011), as well as in its introduced range (Presotto et al., Reference Presotto, Ureta, Cantamutto and Poverene2012). Sunflower has also been well studied. It is known that crop alleles persist in (Whitton et al., Reference Whitton, Wolf, Arias, Snow and Rieseberg1997; Burke et al., Reference Burke, Gardner and Rieseberg2002), and affect fitness in, wild populations (Snow et al., Reference Snow, Morgan-Palma, Rieseberg, Wszelaki and Seiler1998; Cummings et al., Reference Cummings, Alexander, Snow, Rieseberg, Kim and Culley2002; Mercer et al., Reference Mercer, Wyse and Shaw2006b, Reference Mercer, Andow, Wyse and Shaw2007). An experimentally introgressed Bt transgene has even been shown to increase seed production and decrease herbivory in wild sunflower (Snow et al., Reference Snow, Pilson, Rieseberg, Paulsen, Pleskac, Reagon, Wolf and Selbo2003).
Some foundational research conducted on sunflower provides a general understanding of causes of seed dormancy (Walters, Reference Walters, Black, Bewley and Halmer2006). Sunflower seeds are technically achenes; we will use ‘seeds’ when discussing general dormancy concepts and ‘achene’ when referring specifically to sunflower. Finch-Savage and Leubner-Metzger (Reference Finch-Savage and Leubner-Metzger2006) classify H. annuus as having non-deep physiological dormancy, which can be broken by treatment with gibberellins, and sometimes by scarification, after-ripening in dry storage or stratification in cold or warm storage. The fact that sunflower possesses physiological dormancy means that dormancy can arise from various sources, such as the embryo, the testa or the pericarp – all of which have been previously identified as sources of dormancy in sunflower (Baskin and Baskin, Reference Baskin and Baskin2001; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Brunick, Reference Brunick2007). In sunflower, the pericarp is composed of several layers of dead cells with a non-cellular phytomelanin layer in between (Vaughan, Reference Vaughan1970). Below the pericarp is the thin seed coat (i.e. testa), to which the thin endosperm is coalesced (Schneiter, Reference Schneiter1997). The innermost structure of the achene is the embryo, the micropylar (i.e. pointed) end of which forms the radicle and the chalazal (i.e. blunt) end of which consists of two cotyledons (Scheiter, Reference Schneiter1997). Similar seed coverings (i.e. testas and pericarps) in a variety of plant species have been shown to mechanically restrain the radicle, prevent water from reaching the embryo, filter the light that reaches the embryo, inhibit respiratory gas exchange, contain chemical inhibitors, block the escape of inhibitors from embryo or any combination of these (Baskin and Baskin, Reference Baskin and Baskin2001; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Gosling, Reference Gosling, Black, Bewley and Halmer2006; Hu et al., 2009; Rathjen et al., Reference Rathjen, Strounina and Mares2009). Yet research on how each of these anatomical structures affects dormancy in crop vs wild H. annuus is just beginning (Brunick, Reference Brunick2007).
Due to the disparity between the maternal and paternal influences on seed formation and their roles in controlling physiological dormancy, it can be useful to distinguish the contributions of two factors on dormancy: embryo nuclear genetics and maternal effects. The nuclear genetics of the embryo are a product of equal contributions from both parents. In reference to crop–wild hybrids, nuclear genetics of a given cross type or hybrid generation can be summarized by the average percentage of alleles of crop origin versus those of wild origin that an embryo is likely to possess. For example, an F1 hybrid seed produced on a crop inflorescence, pollinated by a wild plant, will possess alleles that are 50% of crop origin and 50% of wild origin. The resulting F1 hybrid, if then pollinated by a wild plant, will produce F1× W seeds with approximately 25% alleles of crop origin and 75% alleles of wild origin. Maternal effects, however, are those factors that depend only on the identity of the maternal parent (Roach and Wulff, Reference Roach and Wulff1987), i.e. is it crop-, F1- or wild-produced? Apart from the effects of the maternal environment, maternal effects include maternal inheritance of the cytoplasm (and organellar genetics), the double dose of maternal genes in the endosperm, and, possibly most importantly with respect to germination of species with physiological dormancy, the testa and pericarp (where present) that the maternal plant imparts. Because pericarp development in achenes initiates regardless of embryo fertilization, it is a completely maternal contribution. The testa is also a maternal contribution, as it develops directly from the ovule integuments. Due to the reduced nature of the endosperm in sunflower, we expect it to provide a minimal contribution to dormancy (Vaughn, Reference Vaughan1970; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). Both maternal effects (i.e. maternally inherited seed coverings) and the effects of maternally and paternally inherited embryo genetics on seed dormancy could influence crop allele introgression in crop–wild hybrid zones.
This study explores the effects of maternal parent and embryo genetics on germination and dormancy characteristics by testing 15 crop–wild hybrid sunflower cross types that could coexist in hybrid zones. We explore the influence on germination and dormancy with and without stratification, in order to delineate the consequences of achene characteristics at different times of year, i.e. in the autumn after seeds are produced and in the spring following the cold of winter, respectively. In addition, we examine the physical differences among achenes on the crop–wild hybrid spectrum that may be responsible for differences in germination and dormancy among different cross types.
We conducted three experiments to address the following objectives: (1) to determine the effects of stratification, cross type of maternal parent (i.e. maternal type) and embryonic nuclear genetics (i.e. percentage crop alleles) on the germination, dormancy and mortality of achenes from a range of crop–wild sunflower cross types; and (2) to investigate differences in the physical structures of sunflower achenes, especially those that are maternally inherited, that may be responsible for germination differences observed in objective 1. This study contributes to our understanding of the effects of seed dormancy in crop–wild hybrid zones by studying the germination and dormancy patterns of a large number of crop–wild cross types, while identifying physical mechanisms that may be responsible for the observed differences.
Materials and methods
Germplasm and crosses
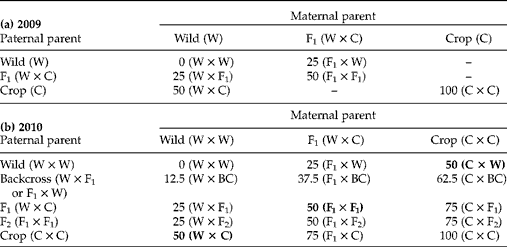
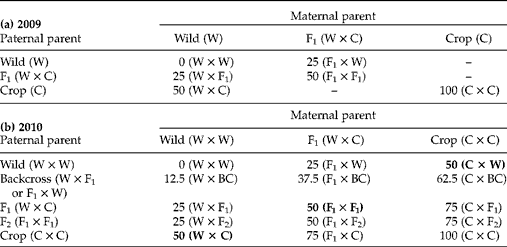
The sunflower achenes studied here were produced through hand-pollination at the Ohio State University Waterman Farm during the 2009 and 2010 growing seasons. Plants from USDA inbred line HA89 were used as the crop parent (C). Wild parents (W) came from collections from ten populations made around Lawrence, Kansas, USA – an area without crop sunflower cultivation, but proximal to potential crop–wild hybrid zones. Wild (W × W), crop (C × C), F1 (W × C), F2 (F1× F1), and backcross (BCw= W × F1 and BCF1= F1× W) cross types were produced in 2009 (Table 1a) (crosses are noted by maternal parent × pollen parent). These six cross types were used in the study of germination with and without stratification and to produce the 15 cross types used in the study of germination under simulated autumn conditions. The 15 cross types produced in 2010 (Table 1b), were produced on three maternal cross types (W, F1 and C) and represent eight levels of percentage crop alleles. Here a random mix of both BCW and BCF1 cross types were used as pollen donors for the W × BC, F1× BC and C × BC cross types. Three of these cross types – those with 50% crop alleles and produced on wild, F1 or crop maternal plants – were selected for the dissection microscopy study (in bold in Table 1b).
Table 1 Sunflower achenes of various crop–wild hybrid cross types produced in (a) 2009 and (b) 2010 through hand-pollinations. Cross types from 2009 were used to generate cross types in 2010. Numbers are expected average percentages of crop alleles in embryos for each cross type and they are followed by a description of the actual cross made, with maternal parent listed first. All six cross types produced in 2009 were used in the stratification experiment. All 15 cross types produced in 2010 were used in the autumn germination experiment. Only the bold cross types from 2010 were used in the dissection experiment
BC, backcross.
In both 2009 and 2010, we grouped plants of a given maternal type together in the field to facilitate hand-pollinations. Environmental variation across maternal environments can influence seed characteristics (Roach and Wulff, Reference Roach and Wulff1987), so if a strong environmental gradient with a large effect on dormancy were to parallel the axis along which we planted our maternal plants (crop, F1 and wild), it would be impossible to distinguish maternal environmental effects from maternal genetic effects. Although we can never know the size of maternal environmental effects, we feel confident that they were likely small for two reasons. First, we used a flat experiment station field where soil type remained consistent and we fertilized and irrigated our plants at the beginning of the season. There was no evidence of deprivation in any of the maternal cross types as we did not witness any obvious signs of stress during either season. All maternal types produced to a very high potential: the wild maternal plants produced hundreds or thousands of heads and the inbred crop plants were strong enough to produce large heads. (Often the latter are quite weak.) Thus, we feel that we provided more than sufficient nutrients and moisture to all maternal types which should have allowed for optimal provisioning of their seeds. Second, maternal effects were consistent across materials produced in the two different years despite the fact that their crosses were performed in different parts of the field station. Thus, here we discuss differences in cross types produced on different maternal types as resulting from maternally inherited genetic effects (e.g. from maternally inherited tissue, organelles, genomes or cytoplasm). Nevertheless, there may remain limited, unaccounted for effects of maternal environment on the achenes used.
Achenes were harvested at physiological maturity, cleaned and stored in opaque, dry envelopes in a laboratory at room temperature for 3–4 months prior to beginning experiments. Thus, they had begun to afterripen. To initiate each experiment we bulked achenes by cross type using achenes from a variety of maternal and paternal parent plants to approach the genetic diversity found in hybrid zones. For each of the six cross types tested with and without stratification, and for the cross types used in the microscopy study, we bulked each cross type from 15 maternal families. (Each maternal family was composed of seed from crosses with up to two individual pollen donors). For the autumn germination experiment, we bulked each cross type from eight maternal families (each having up to two pollen donors). These guidelines were followed except where we had seed limitations. Cross types with crop parents were expected to be inherently less diverse due to the inbred nature of the crop line used.
Germination experiments
We performed two germination experiments in a climate-controlled growth chamber (Conviron G30; Conviron, Winnipeg, Manitoba, Canada). The objective of the first was to determine the degree to which six crop–wild hybrid sunflower cross types germinated, remained dormant and died under favourable germination conditions, with and without stratification. The second experiment similarly quantified germination, dormancy and death, but for achenes from 15 cross types under simulated autumn conditions (i.e. cool temperatures without prior stratification). For the first experiment, stratification consisted of placing achenes on moist blotter paper in the growth chamber without light at 4°C for 4 weeks prior to the start of the experiment. After the stratification period, both stratified and unstratified achenes were exposed to favourable germination conditions (12-h days/12-h nights, light/dark, 25°C/12°C). No achenes were stratified prior to the autumn germination experiment and growth chamber settings were chosen to simulate average October climate data from Lawrence, Kansas (found at www.weather.com/weather/wxclimatology/monthly/graph/USKS0319; accessed 15 November 2010) where the wild germplasm originated (11-h days/13-h nights, light/dark, 21°C/9°C).
For both germination experiments, achenes were washed in a 10% bleach solution and rinsed well. Twenty achenes were then placed in each Petri dish lined with moistened blotter paper. In the stratification experiment, we had high levels of germination during stratification for some cross types, which required reducing the number of achenes per Petri dish for two cross types – W × C and F1× F1 – and excluding the C × C achenes entirely. In the autumn germination experiment, F1(C × W) Petri dishes contained 15 achenes due to seed limitations. For both germination experiments, Petri dishes were arranged in a randomized complete block design with growth chamber shelves as blocks. The stratification experiment and the fall germination experiment ran for 14 d and 27 d, respectively. Every day, the growth chamber temperature was verified, and the blotter paper was moistened as necessary. Every other day, all germinated achenes were recorded and removed. At the end of the germination periods, the remaining achenes were removed from the growth chamber and subjected to a standard tetrazolium viability test to determine whether achenes were dead or had remained dormant (Delouche et al., Reference Delouche, Still, Rapset and Lienhard1962).
For all analyses reported here, we used Proc Glimmix in SAS (version 9.2; SAS Institute Inc., Cary, North Carolina, USA) unless otherwise specified. We analysed the proportion of achenes that germinated, remained dormant and died in the stratification experiment using Proc Catmod (SAS Institute Inc.). The model tested effects of blocks, cross type, stratification, and the interaction between cross type and stratification on the weighted categorical responses variable, achene status, which included germinated, dormant and dead seeds. This multivariate treatment of the data precludes subsequent mean separation for particular seed responses. For the autumn germination experiment, we used Proc Lifetest (SAS Institute Inc.) to produce cumulative germination curves by maternal type. Then we performed multiple linear regression analyses to assess the effects of maternal type and percentage crop alleles, as well as their interactions, on percentage germination after 9, 19 and 27 d. We performed a similar analysis on percentage dormant at 27 d. Within each maternal type, we also determined the effect of percentage crop alleles on percentage germination or dormancy using a linear regression model, for the same time periods. Finally, we performed four additional analysis of variance (ANOVA) sub-analyses. We focused these sub-analyses on groups of four cross types: two of which shared the same percentage of crop alleles, but had different maternal parents, and two of which shared the same maternal parent, but had different percentages of crop alleles. We evaluated the effects of maternal type, percentage crop alleles, and interactions between maternal type and percentage crop alleles on percentage germination on days 9 and 19.
Dissection microscopy of imbibition and germination
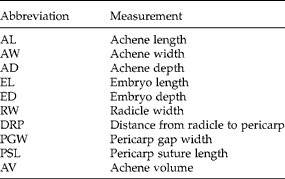
We used dissection light microscopy and digital image analysis to identify maternally inherited morphological characteristics of sunflower seeds that may have influenced differential germination patterns observed in the germination experiments. The dissection experiment focused on three cross types of crop–wild hybrid seeds – C × W, F1 ×F1 (F1= W × C), and W × C – that had the same estimated percentage of crop alleles in the embryonic nuclei (50%), but different types of maternally inherited seed coverings. In each of three blocks (i.e. 11 cm × 11 cm germination boxes with 1 cm of water-saturated white silica sand), we arranged 10 achenes of each cross type in rows as main plots in a split-plot design. Within each row, we randomly assigned achenes to 1 of 10 observation times (0, 6, 12, 24, 36, 60, 84, 108, 132 and 156 h after the initiation of imbibition). We set the growth chamber to constant, favourable germination conditions (i.e. 20°C and dark). Sand was remoistened as needed. Overall, we took 385 images to assess nine measurements on each achene. We conducted all observations using a stereomicroscope (Omano SZMN Series), a microscope specific digital camera (OptixCam Summit Series 3.0), and image analysis software (OptixCam OCView), calibrated appropriately for the magnification levels used (all Microscope.com LLC, Roanoke, Virginia, USA). We measured achene width (AW), parallel to the suture, at its widest point, under 10 × magnification. For germinated achenes, we measured radicle width (RW) perpendicular to the sides of the radicle and directly below the tip of the pericarp or below the cotyledons when cotyledons were expanding, excluding seedlings that had completely shed their pericarps (see Table 2 for abbreviations of all seed measurements). We then bisected ungerminated achenes from the micropylar to the chalazal end, perpendicular to the suture (i.e. the seam that splits during germination) and imaged the cut surfaces of each half separately under 10 × magnification. These images were used to measure achene length (AL) and depth (AD) as well as embryo length (EL) and depth (ED). (Achene length and depth were later used to standardize other measures taken on bisected seeds.) Measuring achene length along its axis from the centre of the chalazal end to the lightly coloured scar at the micropylar end, allowed us to exclude any asymmetrical pericarp tissue protruding on either side of the scar, which would have inflated estimates of achene size. We measured achene depth and embryo depth perpendicular to the suture, at their widest points. We took additional images of the micropylar ends of both halves of each achene under 45 × magnification, in order to take two more measurements of ungerminated achenes. First, we measured the distance from the radicle to the outside of the pericarp (distance radicle to pericarp, DRP) – the distance that the radicle extends to germinate. When the suture connecting both faces of the pericarp was still intact, we also measured the length of the fused portion at the micropylar end (pericarp suture length, PSL). Alternatively, for achenes whose pericarp sutures were not fused (i.e. PSL = 0 mm), we measured the distance between the two halves of the pericarp at the micropylar end as the pericarp gap width (PGW). We standardized these micropylar end measurements by achene size by dividing by the most relevant metric of achene size (i.e. DRP/AL, PSL/AL and PGW/AD). We calculated final values for all measurements of each achene by averaging the measurements for each half. We approximated achene volume (AV) for each cross type as an ellipsoid:
using mean values.
Table 2 Abbreviations of measurements made on dissected sunflower achenes

We tested the effect of maternal type on achene and embryo size variables using only achenes observed at, or before, 24 h after initiation of imbibitions, to reduce the effect of time (and imbibition) on our results, while maintaining a sample size of approximately 12 achenes for mean calculations. [Additional analyses of each time period separately yielded similar results (data not shown).] The exception was the analysis of radicle width, which was carried out on all germinated seeds, regardless of time since imbibition was initiated. Block was included as a random factor in all analyses. Due to the split-plot design, for all analyses, we tested maternal type with the interaction between maternal type and block as the error. We also used multiple linear regressions to determine the relationship of three achene characteristics (DRP, PGW and PSL or their standardized versions) with time, the effect of maternal type on these traits, as well as any interactions between time and maternal type. We performed additional regression analyses to determine the direction and strength of the relationship between time and the response measures for each cross type separately.
Results
Achene size differences among maternal types
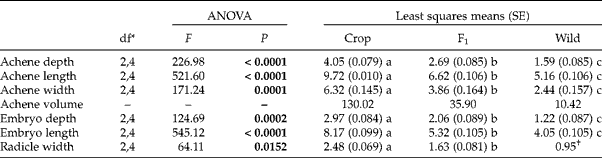
As we would have expected, for all achene size metrics studied in our microscopy study, crop-produced achenes (C × W) were about one and a half times larger than F1-produced achenes (F1× F1), which were about one and a half times larger than wild-produced achenes (W × C) (Table 3). This intermediate size of F1-produced achenes can be seen again in our estimates of achene volumes as ellipsoids. The estimated volume for a crop-produced achene was three and a half times larger than that of an F1-produced achene and an F1-produced achene was estimated to be about three and a half times larger than a wild-produced achene.
Table 3 ANOVA and least-squares means of the effects of maternal parent on crop–wild hybrid sunflower achene characteristics (within 24 h of the initiation of imbibition). Significant effects (P<0.05) are in bold. Standard errors are in parentheses. Letters reflect significant differences (P<0.05) among maternal types for each trait using a Tukey–Kramer adjustment for multiple comparisons. Achene volume was calculated from averages of achene depth, length and width, as described in the text
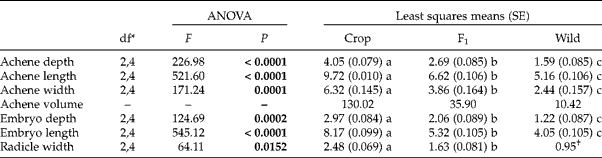
* Numerator, denominator degrees of freedom for each test of maternal parent.
† Wild radicle width reflects a single measurement and was thus excluded from the formal analysis.
Effects of stratification and cross type on germination, dormancy and mortality
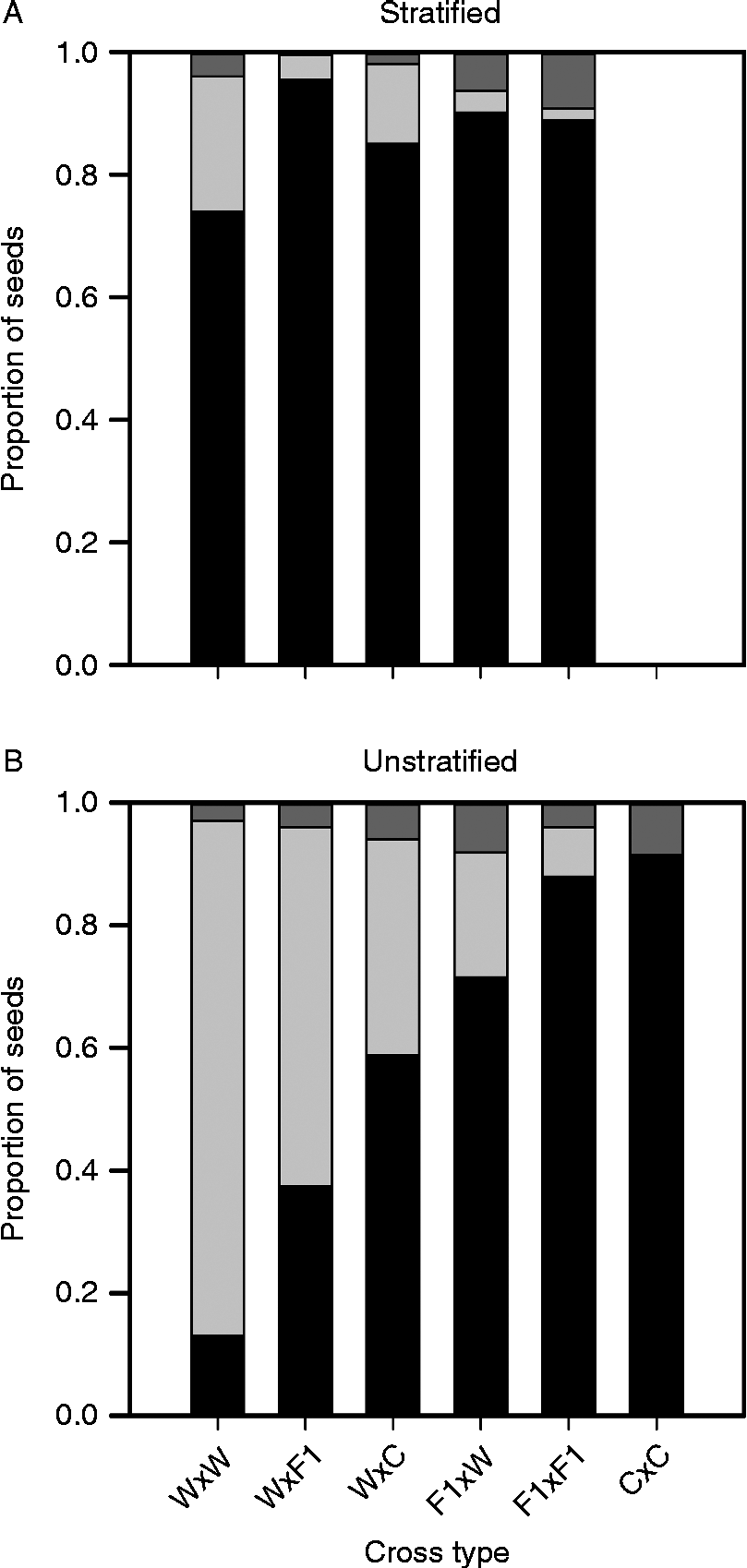
Stratification increased germination overall (P< 0.0001), but the amount of increase varied among cross types (stratification by cross type interaction: P< 0.0001; Fig. 1). Stratification had a large effect on the germination of W × W and W × F1 achenes, increasing their germination by 61 and 58%, respectively. Achenes of W × C and F1× W cross types had intermediate responses to stratification, with an increase in percentage germination of 26 and 19%, respectively. F1× F1 achenes had no response ( < 1%). Over 91% of C × C achenes germinated without stratification (Fig. 1B). Without stratification, the trend in germination was that the W × W achenes had the lowest germination followed by those from the W × F1, W × C and F1× W cross types (Fig. 1B). Achenes of the F1× F1 and C × C cross types had very high germination even without stratification. We may have expected even higher germination after stratification for the W × C and F1× F1 cross types had they not had such high germination during the stratification period (data not shown). Within a maternal type, increased percentage crop alleles increased germination. For stratified achenes, the W × W cross type had the least amount of germination, while all the other cross types had high and similar levels of germination (Fig. 1A) although, again, the W × C cross type had surprisingly low germination. Dormancy was inversely related to germination for both stratified and unstratified achenes, since mortality was similarly low ( < 10%) across all cross types. As expected, dormancy was higher in wild-produced achenes than F1-produced achenes. For the unstratified achenes, the W × W cross type had the highest dormancy, followed by achenes of the W × F1, W × C, F1× W, F1× F1 and C × C cross types (Fig. 1B). Dormancy was more variable in stratified achenes and was surprisingly low in the W × F1 cross type, in particular (Fig. 1A).

Figure 1 Proportions of stratified (A) and unstratified (B) sunflower achenes that were germinated (black), remained dormant (light grey) or died (dark grey) after 14 d in the germinator. Achenes were from six crop–wild hybrid cross types, which are each designated by the cross used to produce them (maternal parent first). Abbreviations for parents in the cross are as follows: W, wild; C, crop; F1, W × C. Standard errors cannot be shown, but ranged from 0 to 0.036. C × C crosses were not included in the stratified treatment because all seeds germinated during stratification, as discussed in the text.
Effects of maternal parent and percentage crop alleles under autumn-like conditions
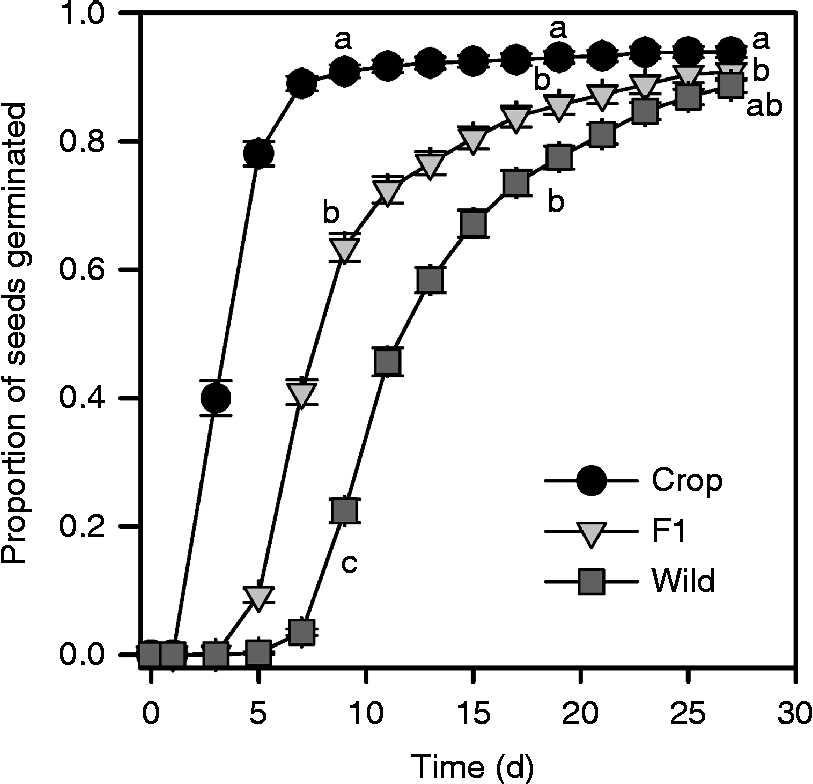
The three different maternal types differed in their patterns of germination over time (Fig. 2). Throughout the course of the experiment, germination was highest for crop-produced achenes, followed by that of F1-produced achenes, and then by that of wild-produced achenes. These differences imposed by maternal types were greatest early in the study and eventually decreased in magnitude as all maternal types approached high levels of germination (i.e. greater than 80%; Fig. 2). Germination of achenes of crop- and F1-produced achenes did not differ significantly from those of wild-produced achenes by day 27 (Fig. 2).

Figure 2 Cumulative proportion of germinated sunflower achenes produced on wild, F1 crop–wild hybrid and crop maternal types as calculated by Proc Lifetest. Error bars are standard errors. Letters denote significant differences (P< 0.05) among least squares means of maternal types (not shown) using Tukey–Kramer adjustments for multiple comparisons at 9, 19 and 27 d.
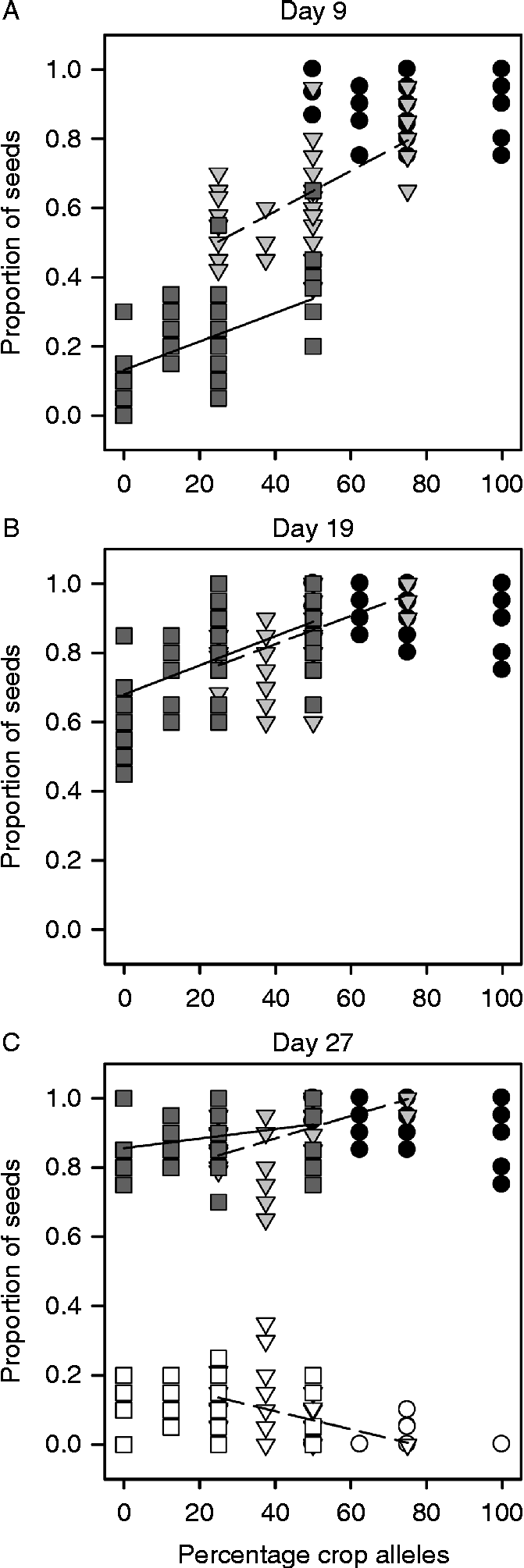
Percentage crop alleles significantly increased germination for the wild- and F1-produced cross types, but not for the crop-produced cross types (Fig. 3). At all three time periods tested, percentage crop alleles was positively related to percentage germination for wild- and F1-produced cross types, but was not significant for crop-produced cross types. The dormancy of F1-produced cross types was negatively affected by the percentage of crop alleles on day 27 (Fig. 3C), while that of wild- and crop-produced cross types was not. The probability that an achene remained dormant was relatively higher in the wild- and F1-produced cross types (each 0.076) than in the crop-produced ones (0.0047) due to the higher germination of crop-produced cross types (Fig. 3C).

Figure 3 The relationship between percentage crop alleles and the proportion of achenes that germinated for 15 crop–wild hybrid sunflower cross types on days 9 (A), 19 (B) and 27 (C). The relationship between percentage crop alleles and the proportion of achenes that remained dormant is also included on day 27 (C). Symbols denote ○ = crop-, ▿ = F1-, □ = wild-produced achenes. Shaded symbols = germinated achenes, unshaded symbols = dormant achenes. Significant linear regressions (P>0.05 for effect of hour) by maternal type are plotted. Dashed lines (- - - - -) denote regression lines for F1-produced achenes, while solid lines (—) denote regression lines for wild-produced achenes. Equations for all significant regressions lines are included here by day, maternal type and achene response (i.e. germinated vs dormant). Day 9: F1-produced and germinated achenes: y= 0.005849x+0.3569 (F 1,39= 34.27, P< 0.0001); wild-produced and germinated achenes: y= 0.004119x+0.1317 (F 1,39= 21.79, P< 0.0001). Day 19: F1-produced and germinated achenes: y= 0.004082x+0.6627 (F 1,39= 36.94, P< 0.0001); wild-produced and germinated achenes: y= 0.004211x+0.6801 (F 1,39= 21.83, P< 0.0001). Day 27: F1-produced and germinated achenes: y= 0.003254x+0.7536 (F 1,39= 29.06, P< 0.0001); wild-produced and germinated achenes: y= 0.001377x+0.8560 (F 1,39= 5.52, P= 0.0239); F1-produced and dormant achenes: y= − 0.00 261x+0.2006 (F 1,39= 17.92, P< 0.0001).
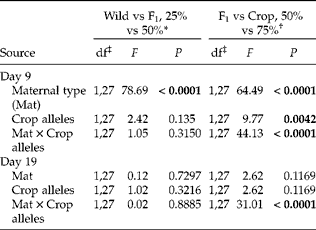
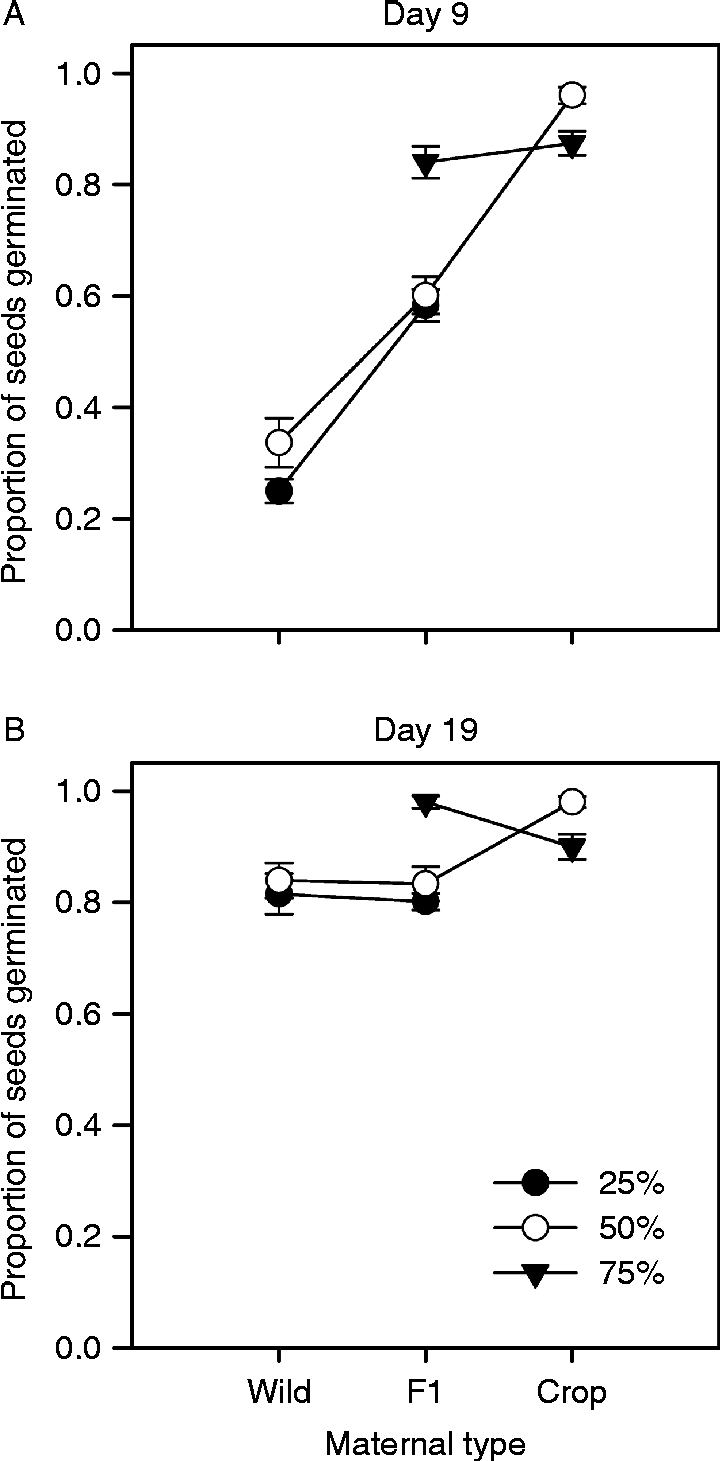
Analysis of data on cumulative germination for a subset of cross types (from days 9 and 19) highlights the impact on percentage germination of moving between maternal parent types with and without changing percentage crop alleles (Table 4, Fig. 4). In particular, early in the experiment (day 9), for cross types with 50% crop alleles, germination increased by 30% or more when produced on F1 rather than wild maternal types or on crop rather than F1 maternal types. A similar pattern was seen if achenes had 25% crop alleles, but the effect of changing maternal parent from F1 to crop was lessened if achenes had 75% crop alleles (Fig. 4A). By day 19, the effect of maternal parent was less substantial for achenes with 25% or 50% crop alleles, though changing the maternal parent from F1 to crop still increased germination by about 15% for achenes with 50% crop alleles (Fig. 4B). By contrast, achenes with 75% crop alleles saw a reduction in germination by day 19 if produced on a crop maternal parent (Fig. 4B) due to the higher dormancy of some achenes as tested on day 27 (Fig. 3C).
Table 4 ANOVAs employing SAS GLIMMIX performed on percentage seed germination at 2 days (9 and 19) during the autumn germination study. In these sub-analyses, we used groups of four crop–wild hybrid sunflower cross types that had one of two maternal parents (wild vs F1 or F1 vs crop) and one of two percentages of crop alleles (25 vs 50% or 50 vs 75%) to further elucidate their effects. Significant effects (P<0.05) are in bold
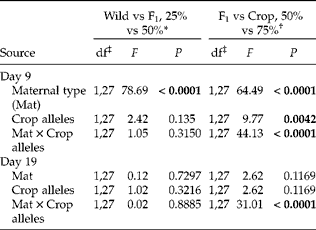
* Cross types tested: W × F1, W × C, F1× W and F1× F1.
† Cross types tested: F1× F1, F1× C, C × W and C × F1.
‡ Numerator, denominator degrees of freedom.

Figure 4 Percentage germination of sunflower achenes on days 9 (A) and 19 (B) for a subset of crop–wild hybrid cross types assayed in the autumn germination experiment. Some cross types share maternal type (wild, F1 crop–wild hybrid or crop) and some share percentage crop alleles (25, 50 or 75%). The figure illustrates the effects of maternal type on germination across levels of percentage crop alleles (i.e. maternal by percentage crop interactions). Least squares means from two separate ANOVAS (results in Table 4) were combined in each panel.
Dissection microscopy of imbibition and precursors to germination
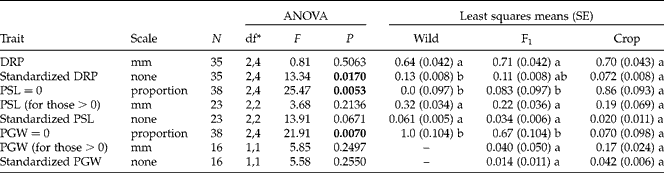
Achenes produced by different maternal types possessed some differences at their micropylar ends when measured at, or before, 24 h from initiation of imbibition (Table 5). In particular, wild-produced achenes had a greater value of standardized DRP than crop-produced achenes, indicating that a wild-produced achene's radicle has a proportionally greater distance to travel before germinating than a crop-produced achene's radicle. Additionally, the proportion of achenes that had a PGW of zero (i.e. fused suture) was greater for wild- and F1-produced achenes than for crop-produced achenes (100, 67 and 7%, respectively; Table 5). Neither the original or standardized version of PSL or PGW differed among maternal types (using only achenes with non-zero values), though trends identified followed the expected pattern of wild->F1>crop-produced for standardized PSL measures and crop->F1-produced for standardized PGW measures (no wild achenes had a measurable PGW) (Table 5). Interestingly, PSL and PGW values in the original scale also followed these trends, indicating that these relationships are not just relative to seed size, but absolute.
Table 5 Results from ANOVA employing SAS GLIMMIX testing for effects of maternal parent on characteristics of the micropylar end of crop–wild hybrid sunflower achenes within 24 h of the initiation of imbibition, as well as least squares means by maternal type (and standard errors in parentheses). P values<0.05 are in bold. Letters by least squares means reflect significant differences (P<0.05) between maternal types for a given trait using a Tukey–Kramer adjustment for multiple comparisons. Wild pericarp gap width (PGW) was zero for all achenes, so it was not included in these analyses
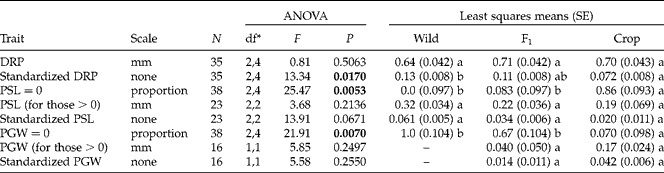
DRP, Distance from the radicle to the pericarp; PSL, pericarp seam length.
* Numerator, denominator degrees of freedom.
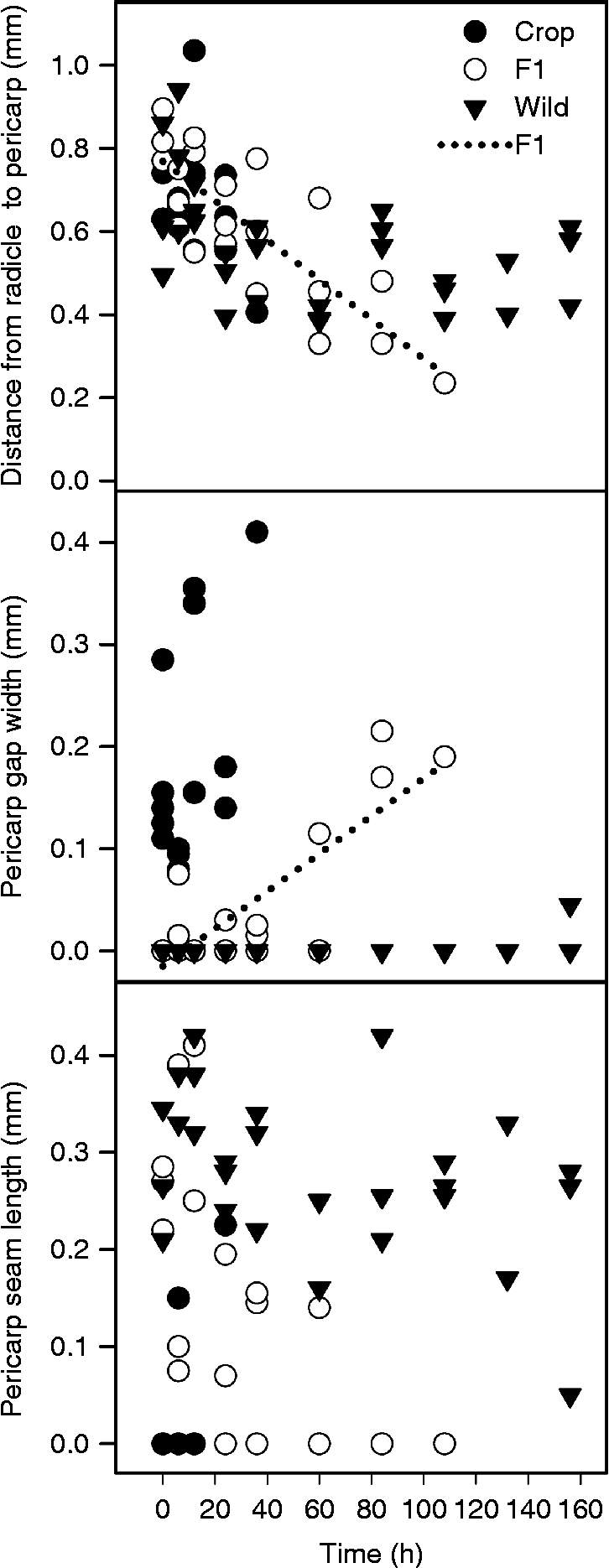
When analysing the responses of micropylar characteristics of achenes allowed to imbibe for up to 156 h with multiple regression, we found that the characteristics of achenes produced on different maternal types responded differently over time, as evidenced by significant interactions between maternal type and hour (all variables were significant at P< 0.05, except standardized PSL, with P= 0.054). Due to the lack of change of the micropylar morphology of the more dormant wild-produced achenes over the time course of the experiment and the exceedingly rapid response of crop-produced achenes (most of which actually germinated and became unmeasurable after 36 h of imbibition), we only saw significant linear relationships between micropylar end characteristics and hour for F1-produced achenes (Fig. 5). In particular, the original and standardized versions of DRP both had negative relationships with time (indicating elongation of the radicle), while PGW and standardized PGW had a positive responses to time (indicating a spreading of the suture in preparation for germination) (Fig. 5). PSL and standardized PSL both trended towards declining with time for F1-produced achenes (indicating an opening of the suture's seal), though the fact that these values became zero after 60 h or so points towards a possible non-linear relationship (Fig. 5). (Due to the similarity in graphs for original and standardized versions of these variables, we have only presented the original data in Fig. 5.)

Figure 5 The relationship between time and morphological features of the micropylar end of crop–wild hybrid sunflower achenes produced by three maternal types: wild, F1 crop–wild hybrid and crop. Regressions were only significant for the effect of time on the F1-produced cross type (not on the wild- or crop-produced one), so only significant F1 regressions are shown. (Relationships between time and standardized values of the measures are not shown in the figure, but significant regressions are included below). Distance from radicle to pericarp: y= 0.7691 − 0.00 475x (F 1,2= 38.86, P= 0.0248); pericarp gap width: y= − 0.01 522+0.001843x (F 1,2= 36.54, P= 0.0263); standardized distance from radicle to pericarp: y= 0.1160 − 0.00 077x (F 1,2= 67.58, P= 0.0145); standardized pericarp gap width: y= − 0.0051+0.000635x (F 1,2= 33.35, P= 0.0287).
Discussion
Our research suggests that both maternal parent and embryo genetics (percentage crop alleles) may play important roles in determining germination behaviour in crop–wild hybrid zones and that differences among cross types at the micropylar end of the pericarp could explain some of the maternal effects we observed. Maternal type affected achene germination, with crop-produced achenes germinating most readily; wild-produced achenes germinating least; and F1-produced achenes exhibiting intermediate germination. Germination also increased as the percentage of crop alleles in the embryo increased, especially for achenes produced by the wild and F1 maternal types. Further analyses indicated that variability seen in germination among maternal types may be related to morphological differences in the maternally inherited pericarp. In general, wild pericarps appear to provide the greatest and longest-lasting physical barrier to radicle emergence and wild radicles have the greatest relative distance to extend to leave the confines of the achene. These data provide a window into the kinds of variation in germination and dormancy behaviours found in crop–wild hybrid zones where many crop–wild hybrid generations coexist with their wild counterparts, while also proposing a physical mechanism responsible for those differences. As such, the results offer insight into the chances for crop gene introgression via various hybrid generations.
Maternal effects vs embryonic nuclear genetics
Maternal effects influencing germination and dormancy are well documented in a variety of species, and the effects of the seed coverings on seed germination and dormancy of physiologically dormant species have been well studied (e.g. Sung et al., Reference Sung, Cantliffe and Nagata1998; Tian et al., Reference Tian, Knapp, Moore, Brummer and Bailey2002; Brunick, Reference Brunick2007; Hu et al., Reference Hu, Wang and Wu2009). In this study, maternal type was an important determinant of percentage germination, especially without stratification or under simulated autumn conditions. Achenes produced on crop maternal plants exhibited the greatest germination, followed by F1-produced achenes, followed by wild-produced achenes, although the differences between these groups were reduced with time or eliminated with stratification. Such differences based on the identity of the maternal parent may be largely the result of differences in levels of dormancy imposed by the seed coverings. A study by Brunick (Reference Brunick2007) indicated that most dormancy in wild H. annuus originates in the seed coverings while, in the crop, the majority originates in the embryo. However, with afterripening, Brunick (Reference Brunick2007) found that the residual dormancy in crop HA 89 achenes tended to reside in the seed coat. Nevertheless, our wild, F1 and crop maternal types could be expected to differ in the degree of dormancy imposed by the seed covering, which may differ across maternal types in production of, or sensitivity to, gibberellic acid (GA), abscisic acid (ABA), reactive oxygen species or germination inhibitors (Lane, Reference Lane1965; Yamauchi et al., Reference Yamauchi, Ogawa, Kuwahara, Hanada, Kamiya and Yamaguchi2004; El-Maarouf-Bouteau and Bailly, Reference El-Maarouf-Bouteau and Bailly2008; Oracz et al., Reference Oracz, El-Maarouf-Bouteau, Kranner, Bogatek, Corbineau and Bailly2009) or in permeability to water and oxygen (see later discussion; Gay et al., Reference Gay, Corbineau and Come1991). While our study corroborates the importance of these maternal effects on germination and dormancy, we must clarify in future work whether these differences in germination and dormancy among maternal types endure under field conditions and thus have ecological relevance.
Although the differentiation by maternal parent dominated over differences imposed by the percentage crop alleles, embryo genetics remained an important secondary factor in determining germination. This importance of the allelic contributions of both parents supports the hypothesis that crop–wild hybridization can reduce dormancy (Snow et al., Reference Snow, Morgan-Palma, Rieseberg, Wszelaki and Seiler1998; Mercer et al., Reference Mercer, Shaw and Wyse2006a). Others have identified the presence of embryo-level dormancy in cultivated sunflower, but not in wild sunflower (Brunick, Reference Brunick2007), which runs contrary to our results. From our work, it appears that the wild parent does contribute to dormancy through genes encoded in the embryo. Nevertheless, responses to an increasing percentage of crop alleles were not consistent across maternal types. Germination increased significantly for the wild- and F1-produced achenes as their percentage crop alleles increased, but not within the crop maternal type. It may be that the relationship between embryo genetics and dormancy was masked (at least by day 9) for crop-produced achenes because the effect of extra crop alleles may be less in the higher range of percentage crop alleles. (Levels among the crop-produced cross types were 50–100%, as compared to 0–50% for wild-produced and 25–75% for F1-produced cross types.) Or, simply, the higher germination of crop-produced achenes driven by its seed coat and/or pericarp characteristics left little room for increased germination.
Other studies that have explored the ways that crop–wild hybridization affects germination in Helianthus, Brassica and Oryza systems corroborate our results of F1 seeds produced on wild parents having greater germination than wild seeds produced on wild parents (Adler et al., Reference Adler, Wikler, Wyndham, Linder and Schmitt1993; Snow et al., Reference Snow, Morgan-Palma, Rieseberg, Wszelaki and Seiler1998; Mercer et al., Reference Mercer, Shaw and Wyse2006a; Dong et al., Reference Dong, Xiao, Rong, Liao, Lu, Chen and Song2011). Although these studies did not examine hybridization with the range of cross types we discuss here, they did find that wild populations differed in levels of dormancy and that the effect of hybridization also differed across populations. Given our results, variation in dormancy among wild Helianthus populations seen in past studies may be related to allelic variants controlling characteristics of either embryo or pericarp dormancy. Given the large number of quantitative trait loci (QTLs) for achene- and dormancy-related traits, any number of loci could be involved (Burke et al., Reference Burke, Knapp and Rieseberg2005; Ghandi et al., Reference Ghandi, Heesacker, Freeman, Argyris, Bradford and Knapp2005; Brunick, Reference Brunick2007).
The effects of maternal type and nuclear genetics may have been dampened somewhat in our experiments by the effects of afterripening, which can induce germination under a wider range of temperatures and light conditions than normal, change the balance of germination-enhancing metabolites within the seed, and hasten germination (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). Between harvest and experimentation, the achenes may have afterripened by being stored dry for 3–4 months at room temperature. This afterripening likely reduced dormancy (Brunick, Reference Brunick2007; Presotto et al., Reference Presotto, Ureta, Cantamutto and Poverene2012) and may have obscured the differences between the cross types by increasing the germination of all of them. Brunick (Reference Brunick2007) determined that afterripening sunflower achenes for as little as 4 weeks significantly increased germination, particularly by diminishing embryo-level dormancy. The disproportionately large impact of afterripening on embryo dormancy may have diminished the effect of percentage crop alleles here. Presotto et al. (Reference Presotto, Ureta, Cantamutto and Poverene2012) also reported increased germination due to afterripening in an array of wild and crop–wild F1 hybrid sunflower cross types, but the effect of afterripening was not consistent across families. Therefore, there is no information in the literature that can help us predict exactly how afterripening should have affected our results other than by increasing germination in all cross types, thereby decreasing effects of maternal parent and percentage crop alleles. Our results followed expectations and the effects observed here may be conservative compared to what might have been seen with fresher achenes.
Achene structure and germination
This study elucidated several physical characteristics of sunflower achenes that were related to the process of germination. The suture, which holds the pericarp valves together and splits at the micropylar end to produce a gap through which the radicle passes, was present significantly more frequently early in the imbibition process in wild- and F1-produced achenes than in crop-produced achenes. When PSL was standardized, there were no significant differences among types, but the PSL in the wild-produced achenes trended towards being two times longer than in the F1-produced achenes and three times longer than in crop-produced achenes (Table 5). The suture was mostly non-existent on crop-produced achenes, whose pericarp gap was present before imbibition. This could possibly hasten the germination of crop-produced achenes because they are already more open to water infiltration for imbibition and air and water entry for active growth. (On some crop-produced achenes, loose tissue at their micropylar end appeared red or brown, suggesting that the open pericarp gap had allowed for oxidation.) By contrast, the initially fused (Table 5) and slow to give (Fig. 5) pericarp sutures of the F1- and wild-produced achenes may delay the germination process, which is consistent with the fact that achenes in wild pericarps take longer to germinate (Fig. 2). Similarly, the longer distance that wild radicles must travel to germinate, relative to the size of their achenes, could also slow germination. Yet many questions remain, including: what is required to initiate the loosening of that suture? Might there be an adhesive holding the valves of the achene together at the suture that must be biochemically degraded, or are the valves split open by biomechanical forces? There are some interesting possibilities, yet the genetic, biochemical or biomechanical processes involved are more complex than we can address here.
The obvious size differences between wild-, F1- and crop-produced achenes may also have implications for observed patterns of germination. These differences in size were largely driven by the maternal plant, probably due to an effect of testa or pericarp size on embryo development. In other species, variation in seed or embryo size caused by genetic or environmental factors can affect germination (Baskin and Baskin, Reference Baskin and Baskin2001). Yet most studies contrast seed size morphs within a plant, not across ecotypes. For instance, as seen in other Asteraceae species, position on the maternal plant or within an inflorescence can affect seed size (often termed heterocarpy) and resulting levels of seed dormancy (Esashi and Leopold, Reference Esashi and Leopold1968; Pitelka et al., Reference Pitelka, Thayer and Hansen1983; Tanowitz et al., Reference Tanowitz, Salopek and Mahall1987; McGinley, Reference McGinley1989). Studies performed with cultivated H. annuus have shown contradictory results (Radford, Reference Radford1977; Hernandez and Orioli, Reference Hernandez and Orioli1985). Mechanisms by which seed size can affect germination include influences on the rate of imbibition, the force exerted as an embryo swells to push apart (or through) seed coverings, and the force a radicle exerts when extending. For instance, Esashi and Leopold (Reference Esashi and Leopold1968) found that while larger and non-dormant embryos were able to produce sufficient force through imbibition and active growth to break through the testa, small and dormant Xanthium pensylvanicum embryos were not, indicating that these smaller embryos required breakdown of the testa prior to germination. Could genetic differences in growth potential of the embryo, or in the strength characteristics of the testa, be important determinants of the gradient we observed in germination of achenes produced on different maternal types? Might nuclear genetic composition (i.e. percentage crop alleles) also influence the growth potential of the embryos? Achenes produced on the same wild maternal plants with different percentage crop alleles in their embryos have been shown to have similar weights (average weight of 20 seeds from 19 or 20 maternal families (SE): W × W = 194.1 g (5.22), W × F1= 193.6 g (5.35), W × C = 189.1 g (5.21); Mercer and Alexander, unpublished data). However, variation in their growth potential would be worth investigating as it might explain differences found here in the relative germination rates of the various cross types produced by wild or F1 maternal types. Regardless of the effect of achene size on dormancy, differences in seed size have been shown to be important beyond germination for traits such as seedling size, adult plant size and competitive ability (Stanton, Reference Stanton1984; Roach and Wulff, Reference Roach and Wulff1987), as well as susceptibility to pre- (Cummings et al., Reference Cummings, Alexander and Snow1999) and post-dispersal (Alexander et al., Reference Alexander, Cummings, Kahn and Snow2001) seed predation.
Seed size polymorphisms can also be associated with significant differences in seed morphology, including different characteristics of seed coverings, which could, in turn, affect dormancy. In the Asteraceae, Hemizonia increscens, pericarps, pappuses and levels of dormancy differ radically between disc and ray positions within an inflorescence. Disruption of the pericarp of the more dormant ray achenes greatly increased their germination, indicating that the thicker ray pericarp could be responsible for higher dormancy (Tanowitz et al., Reference Tanowitz, Salopek and Mahall1987). In our system, the wild-produced pericarps look thicker and more fibrous and the crop-produced pericarps look more papery (K.L.M., personal observation). Thus, the more open crop-produced pericarps might not only increase water and gas exchange and reduce the physical barrier to germination (as well as influencing overwintering ability). Their different make-up could further influence these characteristics in addition to affecting the presence of chemical germination inhibitors, which have been preliminarily identified by others (e.g. Lane, Reference Lane1965). Distinguishing among these factors as causative for observed dormancy differences in this system requires further study.
Implications for introgression of crop alleles into wild populations
As the first stage in a plant's life cycle, germination behaviour can have important implications for plant fitness (e.g. Donohue et al., Reference Donohue, Dorn, Griffith, Kim, Aguilera, Polisetty and Schmitt2005). In crop–wild hybrid zones, variation in the fitness of different cross types could promote or hinder crop allele introgression into wild populations. In this study, nuclear alleles and maternally inherited factors conferred by the crop and F1 types appear to affect levels of dormancy in sunflower achenes in ways that could negatively affect fitness under field conditions. In particular, the nature of the dormancy of various cross types noted here could have implications for the chances that they die from fatal autumn germination and possibly the timing of their germination in the spring. Thus, these biological processes have relevance to the chances for crop gene introgression (or possibly transgene introgression within a biotechnology risk assessment framework). Wild-produced achenes and those cross types with lower percentages of crop alleles would seem least likely to germinate in the autumn and, thus, might be the most probable avenue for crop allele (or transgene) introgression. Yet, this study produces many more questions than it answers. These answers can only come from further research under field conditions, from which we can better understand the ecological relevance of these phenomena. This must be coupled with further investigation into the mechanisms producing ecologically relevant differences.
Thus, we end with four major hypotheses stemming from this work. (1) Yet to be described differences in growth potential, testa strength and pericarp morphology will further explain the differences in germination and dormancy noted here. (2) Certain cross types (F1- and crop-produced and those with a higher percentage of crop alleles) will experience some autumn germination, resulting in greater overwinter mortality. Crop-produced achenes will also have high overwintering mortality (Mercer et al., Reference Mercer, Shaw and Wyse2006a) due to the lack of protection afforded by their more open pericarp. (3) The greater degree of stratification achieved by overwintering could produce fewer differences between cross types in post-stratification germination than those noted in the lab. (4) Some cross types (i.e. those produced on wild maternal plants) may have the greatest chance of providing successful avenues for crop gene introgression due to reduced mortality and increased contributions to the seed bank. Yet, larger sizes of F1-produced achenes could also provide fitness benefits to resulting seedlings. Thus, studies of the entire life cycle of these cross types would prove useful. Finally, further studies should also explore the broad applicability of the effects of maternal parent and embryo genetics identified here in other systems with similar or different dormancy types, since these concepts may be applied to hybrid zones where the ranges of cross-compatible wild species with divergent germination characteristics overlap.
Acknowledgements
The authors would like to thank E. Grassbaugh, G. Mills and the staff of the Ornamental Plant Germplasm Center, especially E. Renze and S. Stieve, for technical support, as well as H. Alexander, A. Snow, M. Bennett, E. Regnier, M. Kost, M. Hanzlik, L. McHale and two anonymous reviewers for insightful discussions and/or reviewing earlier versions of the manuscript. We acknowledge financial support from: Wilhelm and Eleanor Beckert Scholarship to S.B.P., A.N.W. and K.L.M.; OARDC SEEDS Undergraduate Research Grant to A.N.W. and K.L.M.; USDA Biotechnology Risk Assessment Grant (CSREES 2006-03681) to K.L.M., A. Snow and H. Alexander.