Introduction
Temperature affects crop seed production in numerous ways (e.g. Porter and Gawith, Reference Porter and Gawith1999; Wheeler et al., Reference Wheeler, Craufurd, Ellis, Porter and Vara Prasad2000; Sanchez et al., Reference Sanchez, Rasmussen and Porter2014). It also affects the quality of seeds produced, including post-harvest survival (Ellis et al., Reference Ellis, Hong and Jackson1993; Sanhewe et al., Reference Sanhewe, Ellis, Hong, Wheeler, Batts, Hadley and Morison1996). It is becoming clear that the effect of temperature during the development of seed quality from fertilization to maturity in planta and its effect on seed quality thereafter ex planta may well vary over time. There is an overall positive effect of higher mean temperature (within limits) throughout wheat (Triticum aestivum L.) seed development and maturation on the development of seed quality (Sanhewe et al., Reference Sanhewe, Ellis, Hong, Wheeler, Batts, Hadley and Morison1996). However, the relationship is initially negative during early seed development but becomes positive during later development and throughout seed maturation in planta (Nasehzadeh and Ellis, Reference Nasehzadeh and Ellis2017). It then alters again with a negative relationship between air-dried seed survival ex planta and storage temperature (Ellis and Hong, Reference Ellis and Hong2007).
High temperature (32/24°C) throughout seed production is damaging to seed quality in Japonica cultivars of rice (Oryza sativa L.) (Ellis et al., Reference Ellis, Hong and Jackson1993; Ellis and Hong, Reference Ellis and Hong1994), but not if exposure to higher temperatures is delayed to the seed maturation phase (Ellis, Reference Ellis2011). On the other hand, the development of Japonica rice seed quality is damaged by exposure to either cool (18/14°C) or hot temperatures (38/34°C) during histodifferentiation and early seed filling (Martínez-Eixarch and Ellis, Reference Martínez-Eixarch and Ellis2015). After harvest, the relationship between rice air-dried seed survival and storage temperature is negative and quantitatively similar to that in wheat (Ellis and Hong, Reference Ellis and Hong2007).
Post-harvest seed drying takes place following late seed maturation and before subsequent storage. Advice on suitable temperatures for the operation of heated-air seed dryers implies a negative relationship between seed survival and temperature above a ‘safe’ threshold value, with cooler temperatures recommended with greater initial seed moisture content in crop seeds (Nellist, Reference Nellist and Hebblethwaite1980), including rice (Lewis, Reference Lewis1950). Safe drying temperatures were calibrated for different designs of heated-air seed dryers using standard procedures with mature, dry seeds rewetted to different ‘initial’ higher moisture contents (Nellist, Reference Nellist and Hebblethwaite1980).
Genebanks are recommended to dry seeds before storage to moisture contents lower than commercial seed practice and, currently, to use low humidity drying environments combined with cool temperatures rather than heated-air seed dryers. These recommendations are neither species specific nor dependent upon initial seed moisture content (FAO, 2014). This advice to genebanks has altered since the International Board for Plant Genetic Resources (now Bioversity International) was created in 1974. No singular recommendation was initially made for seed drying procedures in genebanks: two-stage procedures were suggested with advice to avoid using heated-air seed dryers above 40°C ‘until moisture content falls below 11% in which case 60°C is safe for most species’ as the first stage, with 15% RH as the second (IBPGR, 1976). The latter report cautioned that genebanks should investigate to confirm whether their drying procedures are reliable and safe. Subsequent advice evolved from the ‘suggestion’ of thin-layer drying at about 15°C with 10–15% RH with good air recirculation (Cromarty et al., Reference Cromarty, Ellis and Roberts1982), to ‘acceptable’ warm-air drying of certain crops, but ‘preferred’ conditions of 15°C with 10–15% RH (IBPGR, 1985). Then, there were ‘recommendations’ of 10–25°C with 10–15% RH (FAO/IPGRI, 1994); and, more recently, the cooler more moist range of 5–20°C with 10–25% RH (FAO, 2014).
The International Rice Research Institute's (IRRI) genebank (T.T. Chang Genetic Resources Center [GRC]) started in the 1960s and now conserves >127,000 accessions of cultivated rice and wild relatives. Initially, rice seeds were dried at high temperatures of 45–50°C. That environment was compatible with recommended safe drying temperatures for rice of 48.9°C at 20% moisture content or above, 51.7°C at 15–20%, and 60°C at <15% (Lewis, Reference Lewis1950). However, in accordance with the publication of the preferred or recommended conditions for genebanks (IBPGR, 1985; FAO/IPGRI, 1994), a drying room (DR) was built in the early 1990s to dry the seeds at 15 ± 2°C with 15 ± 5% RH.
Critical re-examination of procedures at IRRI's GRC, however, has now provided evidence that in many cases initial high-temperature (45°C) seed drying immediately after harvest, before further drying at 15°C with 15% RH in the drying room, improves seed quality (Crisostomo et al., Reference Crisostomo, Hay, Reano and Borromeo2011) and seed storage longevity considerably (Whitehouse et al., Reference Whitehouse, Hay and Ellis2015, Reference Whitehouse, Hay and Ellis2017). The greater longevity from initial high-temperature drying was associated with harvest moisture contents above a critical value close to 16% (Whitehouse et al., Reference Whitehouse, Hay and Ellis2015, Reference Whitehouse, Hay and Ellis2017). This benefit to air-dried seed survival may result from protection mechanisms being stimulated by high-temperature stress in high moisture content, and so still metabolically active, seeds (Whitehouse et al., Reference Whitehouse, Hay and Ellis2017).
We test here the hypotheses that: (i) there is a critical harvest moisture content below which there is little or no benefit to subsequent rice seed longevity from high-temperature drying but above which the benefit is a function of harvest moisture content; and (ii) that this broken-stick relationship does not differ amongst genetically diverse accessions of rice from different variety groups. Data from Whitehouse et al. (Reference Whitehouse, Hay and Ellis2015, Reference Whitehouse, Hay and Ellis2017) are included within the research reported here to provide a wide test of the second hypothesis.
Materials and methods
Plant material
Twenty O. sativa accessions (IRGC 117264–117283) from five variety groups (McNally et al., Reference McNally, Childs, Bohnert, Davidson, Zhao, Ulat, Zeller, Clark, Hoen, Bureau, Stokowski, Ballinger, Frazer, Cox, Padhukasahasram, Bustamante, Weigel, Mackill, Bruskiewich, Rätsch, Buell, Leung and Leach2009) were selected for different experiments carried out between 2013 and 2015 (Table 1). The accession(s) planted in the dry season (DS) and/or wet season (WS) each year differed amongst experiments, but the standard rice growing protocol at IRRI including production practices and plant protection measures (Reaño et al., Reference Reaño, Sackville Hamilton, Romero, Dulloo, Thormann, Jorge and Hanson2008) was followed in each case. Prior to sowing, seeds were sampled from the active collection of the GRC and held at 50°C for 5 days to break dormancy. With the exception of the 2015 batch dryer (BD) experiment, in which seedlings were transplanted in a screen house (Table 1), all seedlings were raised in a seed bed and then transplanted in the field at IRRI's Ziegler Experiment Station (14°9′N, 121°16′W). Seeds were harvested by hand between March and May in the DS, or October and November in the WS. The number of seed lots harvested per accession and their stage of maturity at harvest differed between experiments.
Table 1. International Rice Genebank Collection (IRGC) accessions used for the various experiments to look at the effects of different seed drying treatments on subsequent air-dried seed longevity. Seeds were harvested in the crop year and season (dry [DS] or wet [WS]) shown, at different maturities (DAA; days after 50% anthesis).
Seeds were dried following the standard drying protocol (control; 14 days in the drying room [DR; 15°C/15% RH]), or for an initial period in the drying regime shown before final drying in the DR. *McNally et al. (Reference McNally, Childs, Bohnert, Davidson, Zhao, Ulat, Zeller, Clark, Hoen, Bureau, Stokowski, Ballinger, Frazer, Cox, Padhukasahasram, Bustamante, Weigel, Mackill, Bruskiewich, Rätsch, Buell, Leung and Leach2009). ¶The doi references associated with these accessions are given in the reference list.
Immediately after harvest, seed lots were threshed and blown to remove debris and half-filled seeds, then cleaned by hand to remove empty, damaged and/or diseased seeds. Seed moisture content (%, fresh weight basis) at harvest was determined using three 5 g samples and the high-constant-temperature-oven method (ISTA, 2013).
Seed drying
Seeds from each lot were divided into samples of equal weight (total number varying with number of different drying treatments; Table 1) and placed into 0.2 × 0.33 m (length × width) nylon mesh bags (1 mm diameter holes). One sample from each seed lot was immediately placed in the drying room (DR; 15°C/15% RH) for 14 days. The DR environment complies with that recommended for genebanks (FAO, 2014) and so provided a control treatment, the ‘baseline’ against which the effects of different drying treatments were compared. The remaining seeds within each lot were dried for an initial period in the experimental drying regimes at 45°C provided by different equipment (Table 1), as follows.
(1) A locally fabricated batch dryer (BD) that dried seeds in an open environment with a kerosene gas burner providing hot air at about 45°C on average. The RH could not be controlled or reduced below about 35% (estimate based on prevailing ambient conditions and air–water relations) because there was no additional dehumidification.
(2) Saturated salt solutions of MgCl2 provided approximately 30% RH with seeds placed above the solution in a hermetically sealed electrical enclosure box (ENSTO Finland Oy, Finland). There was no airflow with passive drying (relying on a limited seed bulk and exposure of all seeds to the atmosphere) at 45°C.
(3) A hermetically sealed climate chamber (VC3 0034-M, Vötch Industrietechnik, Germany) with in-built dehumidifier, heater and cooler which provides a controlled, stable environment at the programmed temperature (45°C) and humidity.
After the initial high-temperature drying treatment (Table 1), all seeds were further dried towards equilibrium in the DR. Once seed samples had equilibrated in the DR (which required a total drying period of 14 days), they were sealed inside 0.17 × 0.12 m (length × width) laminated aluminium foil packets (Moore and Buckle, Saint Helens, UK) and stored at 2–4°C prior to experimental storage.
Seed storage
Seed samples from each experiment were removed from storage and left at room temperature for approximately 1 h to equilibrate before opening. They were divided into 5 g subsamples and rehydrated to 10.9% moisture content in 30 mm diameter open Petri dishes either over a non-saturated LiCl solution (60% RH), in a hermetically sealed box for 7 days at room temperature; or in the climate chamber set at 60% RH and 21.5°C for 4–5 days. The moisture content of each sample was checked (using three subsamples) before estimating the initial ability to germinate (using one subsample). The remaining subsamples were sealed within individual 0.12 × 0.09 m (length × width) laminated aluminiums foil packets and stored hermetically in an incubator at 45°C (experimental storage). One packet from each seed sample was removed after different periods of storage at 1–3 day intervals and tested for ability to germinate.
Seed germination
The seeds from each packet were divided between four 90 mm diameter Petri dishes with two layers of Whatman No. 1 filter paper wetted with 7.5 ml distilled water (30 seeds in each dish). Seeds were incubated at 30°C (12 h light/12 h dark) and then assessed at regular intervals. Seeds were scored as germinated once the radicle had emerged by at least 2 mm. After 14 days in test, non-germinated fresh seeds were dehulled and tested for an additional 7 days before final scoring.
Statistical analysis
The improved seed viability equation:
where v is probit percentage viability (NED) of a seed lot after p days in hermetic storage at constant temperature t (°C) and moisture content m (%, fresh weight basis), K E, C W, C H and C Q are species-dependent constants, and K i is the seed lot constant (Ellis and Roberts, Reference Ellis and Roberts1980), provided the basis of our approach to the analyses. Probit analysis was carried out using GenStat for Windows, versions 15–17 (VSN International Ltd., Hemel Hempstead, UK) in accordance with the equation:
derived from Eqn (1) for a single storage environment where σ is the standard deviation of the frequency distribution of seed deaths in time (days) (Ellis and Roberts, Reference Ellis and Roberts1980). For seed lots showing loss in dormancy during early storage, the combined loss in dormancy and loss in viability model was fitted using probit analysis:
where g is ability to germinate (NED), p, K i and σ are as in Eqn (2), K d is the initial proportion of non-dormant seeds (NED) and β1 is the probit rate of loss in dormancy (Kebreab and Murdoch, Reference Kebreab and Murdoch1999). For those seed lots showing reduced initial viability when fitting Eqn (2), the ‘control mortality’ parameter was included to estimate the proportion of ‘non-responding’ seeds (dead or empty) within the population (Mead and Gray, Reference Mead and Gray1999; Hay et al., Reference Hay, Mead and Bloomberg2014). For each experiment, probit analysis was carried out for all seed lots within an accession simultaneously, first fitting full models (all parameters fitted to seed lots independently) and then reduced models (one or more parameters constrained to the same value for different seed lots within an accession). An approximate F-test was used to select the best model.
The period in days to 50% viability (p 50, the product of K i and σ) is the most accurately estimated period of longevity and it was used as the measure of longevity to compare the effects of different drying treatments on seed storage longevity. For each seed lot within each experiment, the difference in longevity between drying in the alternative drying regime (AR p 50) and the control regime (DR p 50) was calculated as a proportion of DR p 50 as follows:
In all comparisons, the estimate of p 50 in alternative drying regimes was never less than that in the control regime (DR p 50); and so all values derived from Eqn (4) were zero or (more often) positive.
Results
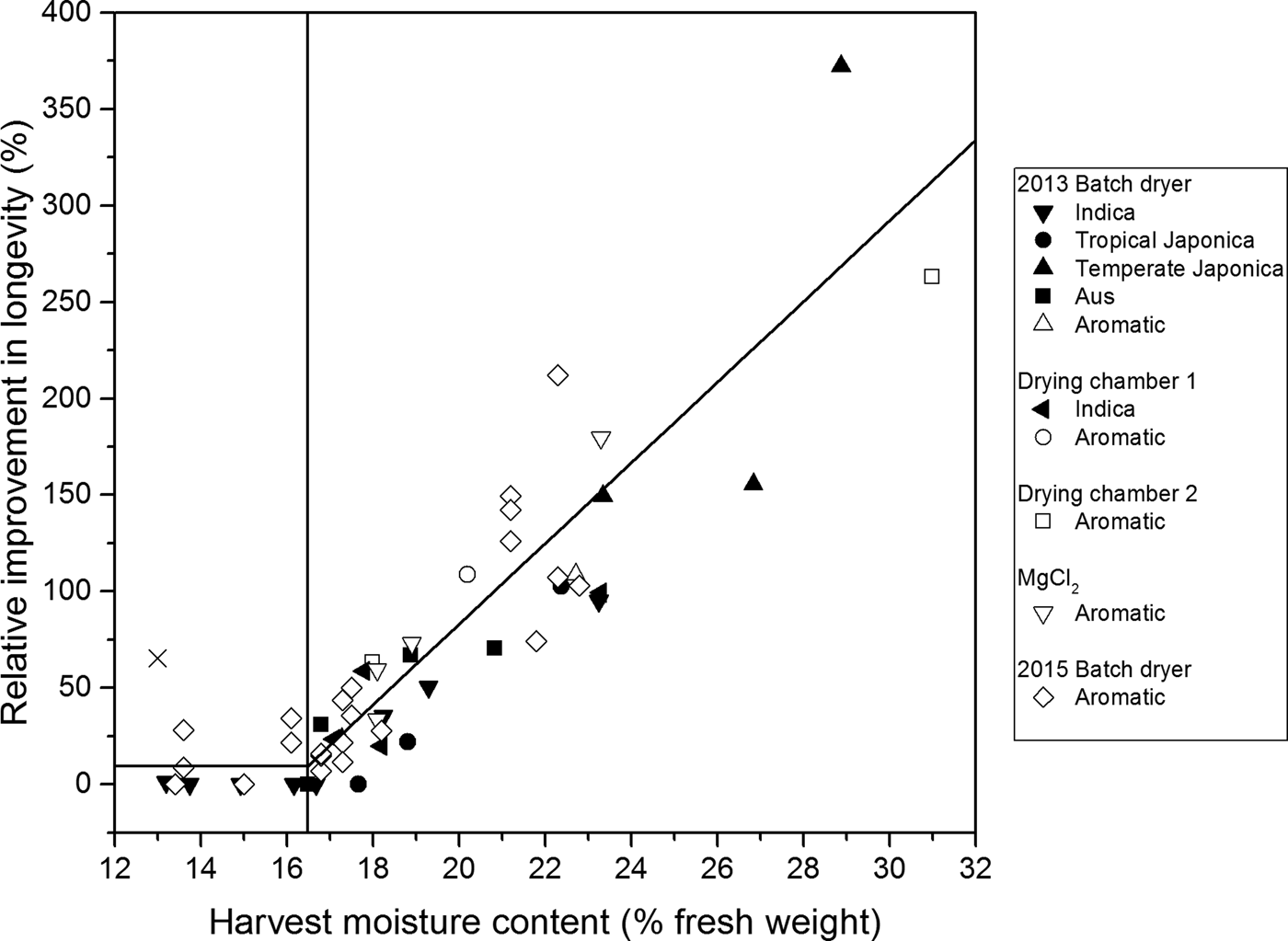
Seed lots varied considerably in the relative benefit from initial drying at high temperature to subsequent seed longevity (Fig. 1). The majority of initial treatments at 45°C provided greater longevity compared with drying in the DR throughout. Beneficial treatments were detected in each variety type and in every experiment.
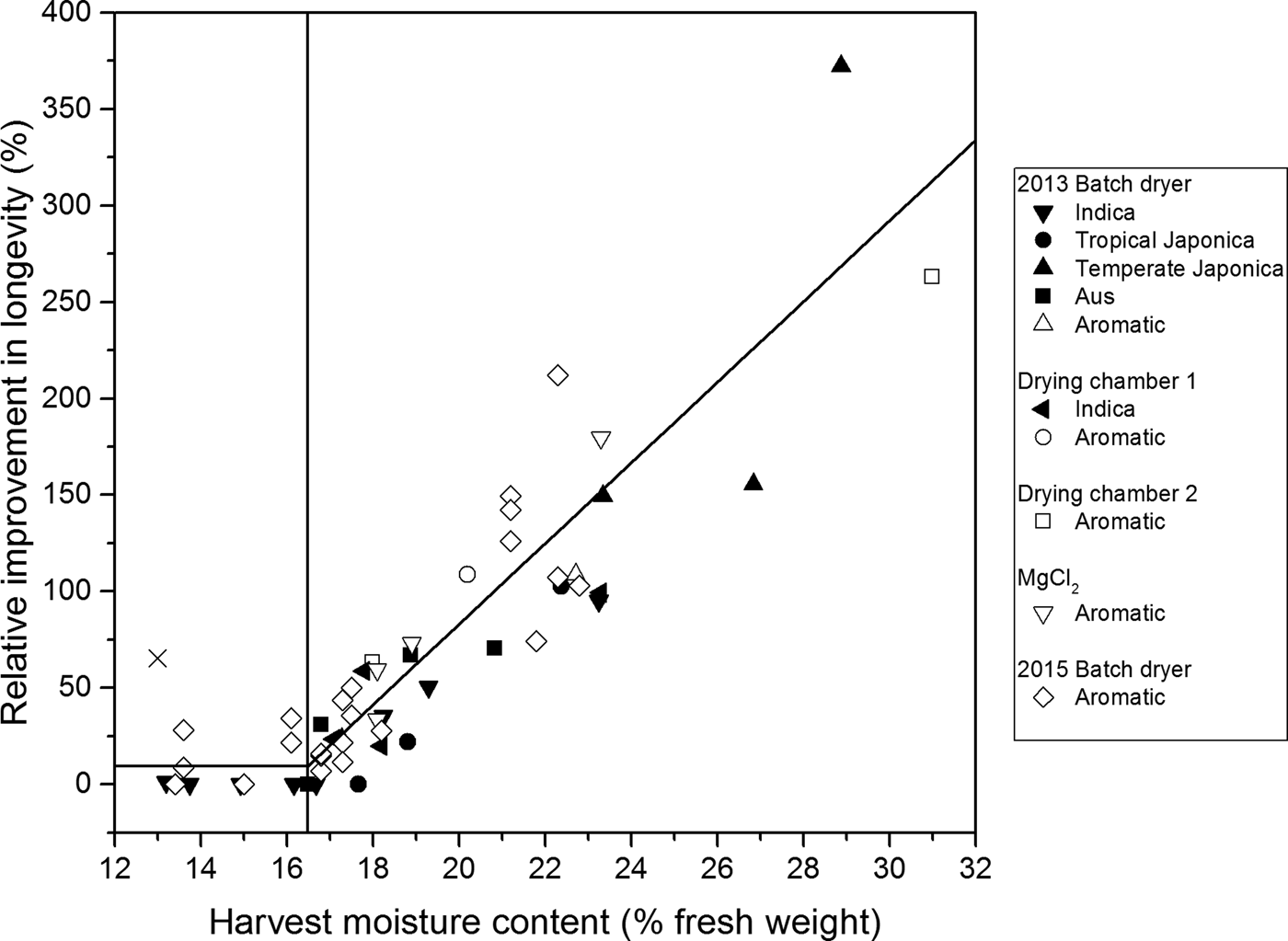
Figure 1. Relationship between the improvement in longevity (p 50) of rice seeds dried initially at 45°C under different conditions (Table 1) relative to that (p 50) when dried in the dry room (DR) throughout [%, Eqn (4)] and harvest moisture content (%, fresh weight). The results are for 20 accessions of different variety types (Indica, tropical Japonica, temperate Japonica, Aus and aromatic; McNally et al., Reference McNally, Childs, Bohnert, Davidson, Zhao, Ulat, Zeller, Clark, Hoen, Bureau, Stokowski, Ballinger, Frazer, Cox, Padhukasahasram, Bustamante, Weigel, Mackill, Bruskiewich, Rätsch, Buell, Leung and Leach2009) in 2013, 2014 and/or 2015 between 24 and 60 days after 50% anthesis (Table 1). A relative improvement of 100% is equivalent to a doubling in longevity compared with the control (DR). Some symbols represent multiple treatments (where longevity within an accession did not differ after different durations of high-temperature drying). The continuous vertical line is a split-line regression with a breakpoint at 16.5% harvest moisture content for all drying treatments; it accounted for 81.5% of the variance and provided no significant (P < 0.05) increase in residual deviance over different regressions for each separate experiment. One outlying observation at 13% moisture content (×) was excluded from analysis (see text).
The greatest benefit was a more than threefold increase in longevity. This was detected in a temperate Japonica cultivar harvested at 28.9% moisture content and initially dried intermittently (8 h day–1) for 1–6 days (i.e. all six treatment durations provided no difference in longevity, P > 0.05) in the BD until transfer to the DR. The lowest harvest moisture content at which benefit from initial drying at 45°C was detected was 13.0%, the driest sample at harvest in these studies (but an outlier – see later).
A minority of treatments at 45°C provided similar longevity to treatment in the DR throughout. These were largely confined to seeds harvested at moisture contents below 16.5% (Fig. 1). The greatest harvest moisture content at which no benefit from initial drying at 45°C was detected was 17.7%.
The magnitude of the improvement to subsequent seed longevity from drying at 45°C was associated positively with harvest moisture content (Fig. 1). The split-line regression shown in Fig. 1 accounted for 81.5% of the variance in relative longevity (once an outlier at 13.0% moisture content was omitted from analysis). Moreover, the common split-line regression did not differ amongst experiments: constraining all the observations to this relation did not increase residual deviance significantly compared with fitting split-line regressions to each experiment separately (F (5,75) = 0.96; P = 0.45). Seed longevity relative to the DR control was increased by initial drying at 45°C. The increase was greater the higher the harvest moisture content above a critical value of 16.5%. Seeds harvested at moisture contents below 16.5% showed limited or no improvement in relative longevity from initial high-temperature drying compared with seeds dried throughout in the cooler environment of the DR.
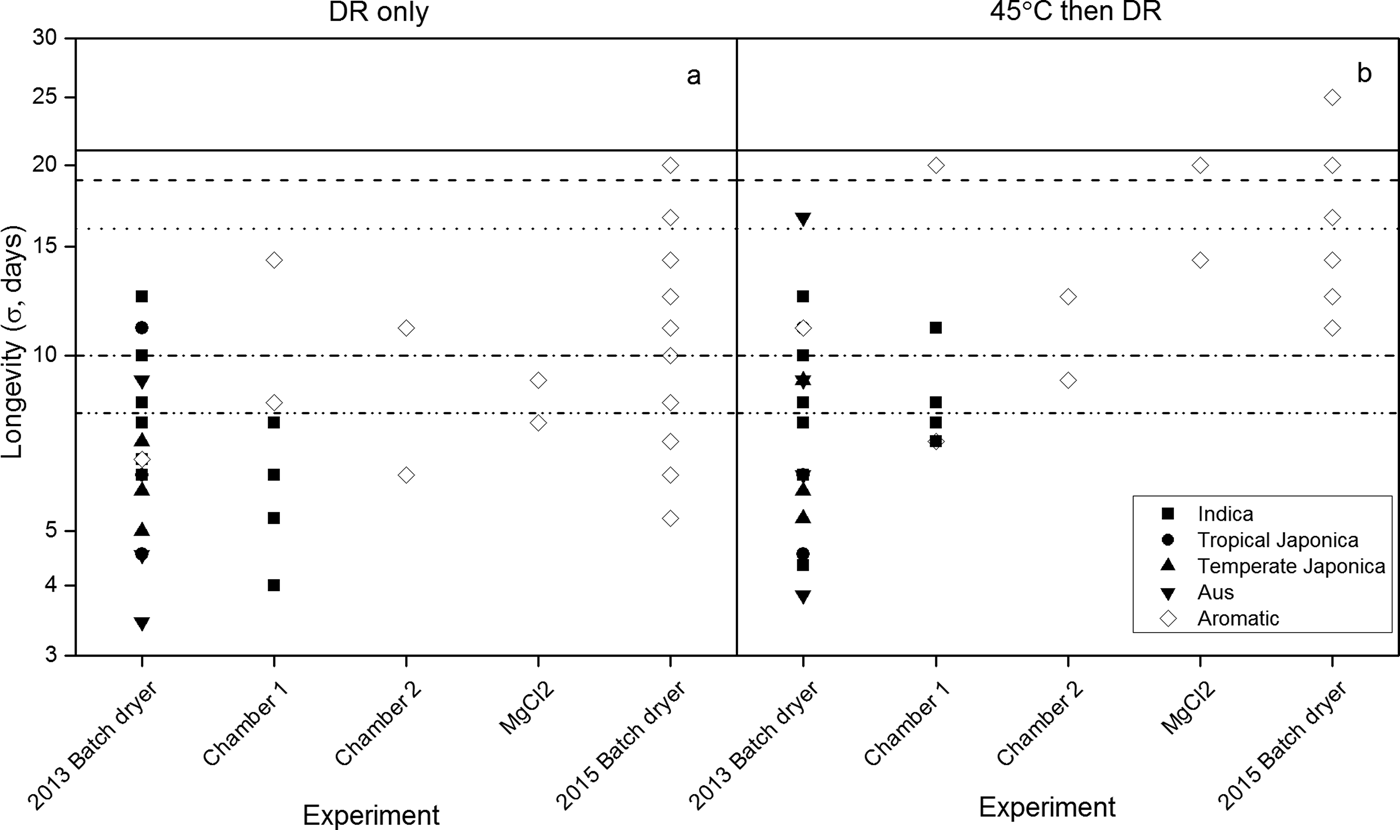
The separate estimates of σ (Eqn 2) for storage at 45°C with 10.9% moisture content varied considerably amongst and within both accessions and experiments (Fig. 2). For example, estimates of σ for Indica accessions dried solely in the DR ranged between 6.3 and 12.5 days and between 4 and 7.7 days in the 2013 BD and chamber 1 experiments, respectively. Seeds of the aromatic variety showed a σ value of 6.7 days in the 2013 BD experiment and ranged between 8.3 and 14.3 days in the chamber 1 experiment. This variation in σ was slightly greater after initial drying at 45°C (Fig. 2b) than for the DR alone (Fig. 2a), whilst the 2015 BD investigation with one aromatic accession provided the widest range of estimates of σ (5.3–20 in DR only, compared with 11.1–25 days when initially dried at 45°C) and the greatest mean value. The observed values, most notably when seeds were dried in the DR, were not consistent with independent estimates, with the observed values being considerably lower (Fig. 2).
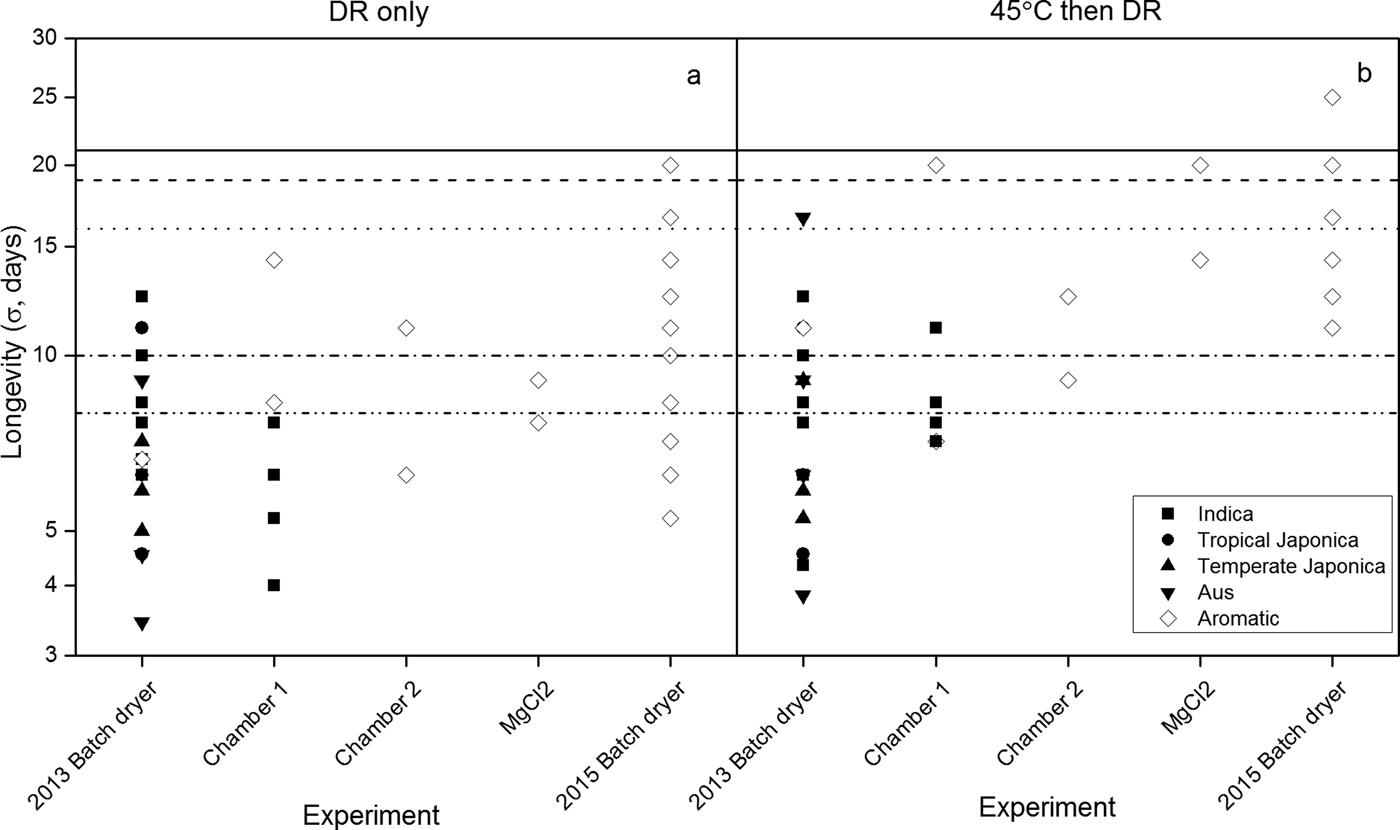
Figure 2. The standard deviation of the frequency of distribution of seed deaths in time, σ (days, logarithmic scale) for seed lots of different variety groups (Indica, tropical Japonica, temperate Japonica, Aus and aromatic; McNally et al., Reference McNally, Childs, Bohnert, Davidson, Zhao, Ulat, Zeller, Clark, Hoen, Bureau, Stokowski, Ballinger, Frazer, Cox, Padhukasahasram, Bustamante, Weigel, Mackill, Bruskiewich, Rätsch, Buell, Leung and Leach2009) from different experiments (Table 1) stored hermetically at 45°C with 10.9% moisture content either when first dried to equilibrium to 15% RH (a) in the dry room (DR) only, or (b) initially at 45°C and then in the DR. The symbols in b represent the longest-lived (p 50) seed lots of all treatments within each experiment. The open symbols represent the aromatic variety, accession IRGC 117265, common to all experiments. The horizontal lines represent independent estimates of σ at 45°C with 10.9% moisture content: from SID (Royal Botanic Gardens Kew, 2018) provided by extrapolations from observations for Indica (dashed line), tropical Japonica (dotted line), and temperate Japonica varieties of rice (dashed and dotted line) in different environments reported by Ellis et al. (Reference Ellis, Hong and Roberts1988) or Ellis et al. (Reference Ellis, Hong and Roberts1992); or from the seed viability constants for rice (continuous line) calculated from observations over a wide range of temperatures and moisture contents (Ellis and Hong, Reference Ellis and Hong2007) in accordance with Eqn (1).
Discussion
This analysis of results from several separate investigations builds upon recent research (Whitehouse et al., Reference Whitehouse, Hay and Ellis2015, Reference Whitehouse, Hay and Ellis2017) to confirm that the subsequent longevity of rice seeds harvested while still moist is improved by initial, high-temperature drying (Fig. 1). The critical seed moisture content at harvest of 16.5% below which little or no benefit from high-temperature drying was detected (Fig. 1) was similar to the previous estimate of 16.2% (Whitehouse et al., Reference Whitehouse, Hay and Ellis2015). Moreover, there was not one case of damage from drying moist seeds at 45°C (Fig. 1), although damage was detected previously in some treatment combinations at 60°C (Whitehouse et al., Reference Whitehouse, Hay and Ellis2017). It is also worth remembering that seeds losing moisture at 45°C, or at 60°C, will be cooler than the air temperature due to evaporative cooling (Nellist, Reference Nellist and Hebblethwaite1980). There was an outlying observation at 13.0% moisture content (Fig. 1) where relative longevity was 65% greater than the DR control and so well above the fitted line. The cause is not known. Nevertheless, this is a ‘right-side error’ that does not contradict the conclusion that initial, high-temperature drying of rice seeds harvested at a high moisture content benefits longevity.
The sequence of contrasting responses of rice seed quality post-harvest to temperature (approximately 15 to 45°C) from anthesis to post-harvest air-dry storage is therefore, negative early in seed development during histo-differentiation and early seed filling (Martínez-Eixarch and Ellis, Reference Martínez-Eixarch and Ellis2015); positive for brief durations late in the maturation phase whilst the seeds approach maturity but remain moist (16.5–31.0% moisture content at harvest, Fig. 1); neutral for brief periods as seed moisture content transitions to the air-dry range (13.4–16.5%, Fig. 1); and finally, negative in subsequent air-dry storage (Ellis and Hong, Reference Ellis and Hong2007).
There is a similarity between the critical harvest moisture content of 16.5% (Fig. 1) and discontinuities in the post-harvest relationships between seed survival and moisture content. The latter occurs at a water potential of around –14 MPa above which, provided oxygen is available, there is a positive relationship between seed longevity and moisture content in contrast to the negative relationship in conditions drier than –14 MPa (Roberts and Ellis, Reference Roberts and Ellis1989). In rice, the critical value of 16.5% moisture content (Fig. 1) is close to 90% equilibrium relative humidity at 25°C (Breese, Reference Breese1955; Whitehouse et al., Reference Whitehouse, Hay and Ellis2015). This provides a water potential close to –14 MPa (Roberts and Ellis, Reference Roberts and Ellis1989).
The improving quality of immature seeds may result from continued development ex planta mimicking maturation drying in planta (Hong et al., Reference Hong, Gedebo and Ellis2000), because such seeds remain metabolically active (Angelovici et al., Reference Angelovici, Galili, Fernie and Fait2010). The main driver of the improvement in quality detected here, however, may be a stress response caused by high-temperature exposure during this brief phase immediately ex planta, allowing enhanced metabolism of protectants and other metabolic pathways that aid the stabilization of the seed during desiccation and survival in air-dry storage (Whitehouse et al., Reference Whitehouse, Hay and Ellis2017). For example, the accumulation of sugars and heat-stable proteins during development is associated with desiccation tolerance and potential longevity, but the latter is more likely to account for differences in longevity between seed lots as they accumulate comparatively late in seed development (Sinniah et al., Reference Sinniah, Ellis and John1998). Moreover, and in accordance with the critical value estimated here, the results of proteomic studies suggest two stages of desiccation (Chatelain et al., Reference Chatelain, Hundertmark, Leprince, Le Gall, Satour, Deligny-Pennick, Rogniaux and Buitink2012): one with increasing seed longevity (equivalent to >16.5% moisture content in the current study); and a later one of no improvement because seeds are no longer metabolically active (≤16.5% moisture content).
Our results clearly show that two-stage drying of moist rice seeds at a high temperature (45°C, and sometimes warmer) before the cool (5–20°C) and dry (10–25% RH) conditions improves subsequent longevity greatly compared with immediate cool and dry conditions. As no high-temperature drying treatment reduced subsequent seed longevity compared with drying in the DR, and to avoid measuring the harvest MC of all seed lots, FAO might consider updating their advice so that all rice seeds intended for genebank storage are dried using this two-stage procedure. Rice grown in paddy conditions in the tropics is under conditions where ambient RH is rarely less than 80% and plant senescence and seed desiccation is delayed (if the paddy field is not drained), such that the maturing seeds may remain moist. Whilst many crop seeds mature under much drier conditions, we nonetheless suggest that they should be investigated for improvement in seed longevity if moist seeds are initially dried at high-temperature.
The seed viability equation assumes that σ is the same for all seed lots of a species in a constant, defined storage environment, with only K i varying in value amongst lots (Ellis and Roberts, Reference Ellis and Roberts1980). Considerable variation in σ in one environment (45°C with 10.9% moisture content) was found in these experiments (Fig. 2), but with most observations shorter than the independent estimate provided by the seed viability constants for rice (Ellis and Hong, Reference Ellis and Hong2007). Estimates of σ for Indica and temperate and tropical Japonica cultivars from the Seed Information Database (SID; Royal Botanic Gardens Kew, 2018) in this regime were closer to the experimental observations, but with temperate Japonica predicted to lose viability sooner than Indica cultivars (Fig. 2). Tejakhod and Ellis (Reference Tejakhod and Ellis2018) found that the seed viability constants from Ellis and Hong (Reference Ellis and Hong2007) overestimated longevity in a Japonica cultivar, but provided reasonable agreement for Indica cultivars. Chang (Reference Chang1991) and Ellis et al. (Reference Ellis, Hong and Roberts1992) also concluded that seeds of Japonica cultivars are the shorter lived. In contrast, in the current studies with less mature seeds, the variation within a variety group was greater than that between groups and the Indica cultivars tended to be the shorter lived (Fig. 2). The viability equations provide estimates of seed longevity in air-dry storage, but they are just estimates and each with their own significant error (see e.g. Hay et al., Reference Hay, Mead, Manger and Wilson2003). Once an indication of the seed longevity of a species in genebank storage has been established, whether from the viability equations or, for example, from historical routine viability monitoring data (Hay et al., Reference Hay, de Guzman, Ellis, Makahiya, Borromeo and Sackville Hamilton2013; van Treuren et al., Reference Van Treuren, de Groot and van Hintum2013; Ellis et al., Reference Ellis, Nasehzadeh, Hanson and Woldemariam2018), simple initial storage experiments may be useful. These can determine the relative longevity of different, new seed lots of a species prior to genebank storage in order that accession retest intervals can be customized from these rankings (Hay and Whitehouse, Reference Hay and Whitehouse2017). The variation in σ observed here also emphasizes the plasticity of seed longevity as a trait (Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017) and the possibility of developing greater understanding of the temporal effects of low moisture content for seed lots of the same species that differ in longevity (Walters, Reference Walters2015).
In conclusion, this summary analysis of results with diverse genotypes over five experiments in different seed production environments confirms that seed quality (subsequent seed longevity) in rice is increased substantially by high-temperature drying of seeds harvested before maturation drying ends. The temporal (immediately ex planta cf. subsequent storage) and water status discontinuities (above cf. below 16.5%) in the effect of temperature on subsequent air-dry longevity, suggest that moist rice seeds exposed to 45°C in late maturation are stimulated to synthesize protectants which stabilize seed during desiccation and aid survival in air-dry storage. The earlier during late maturation that rice seeds are harvested, the greater the relative benefit of high-temperature drying to longevity, with no benefit detected once seeds have dried in planta to 16.5% moisture content.
Acknowledgements
We thank the staff of the T.T. Chang Genetic Resources Center, especially R. Reanõ, F. de Guzman and Marlina Velasco-Punzalan, for technical assistance.
Financial support
This research was supported by funding from the German Federal Ministry for Economic Cooperation and Development (BMZ) through the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH.