Introduction
Climate has a significant effect on plant recruitment and population dynamics (Adler and HilleRisLambers, Reference Adler and HilleRisLambers2008; Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011). Temperature and precipitation are critical to release of seed dormancy, germination and plant distribution, three main drivers of regeneration (Woodward and Williams, Reference Woodward and Williams1987; Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011). Natural regeneration is a site-specific ecological process. In arid and semi-arid environments, poor regeneration coincides with prolonged drought and high temperatures (Parker, Reference Parker1993). Limited water resources have a profound effect on seed longevity, dormancy length and the structure of desert plants (Tompsett and Pritchard, Reference Tompsett and Pritchard1998; C.Z. Liu et al., Reference Liu, Murch, EL-Demerdash and Saxena2003). Seed dormancy and dormancy release mechanisms are, therefore, of great ecological importance for modulating the distribution and abundance of plant species, with the breaking of physical dormancy being particularly significant in this respect (Baskin and Baskin, Reference Baskin and Baskin1998; Handley and Davy, Reference Handley and Davy2005; Venier et al., Reference Venier, Funes and García2012).
Physical dormancy can be found in most temperate Fabaceae species (Baskin and Baskin, Reference Baskin and Baskin1998, Reference Baskin and Baskin2005; Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003; Venier et al., Reference Venier, Funes and García2012) and is commonly very long lasting, even at high temperatures (Taylor, Reference Taylor2005). Dormancy controls germination and indirectly maximizes timing for successful seedling establishment (Liebst and Schneller, Reference Liebst and Schneller2008); it also affects plant recruitment and population dynamics, especially for native species in harsh environments (Baskin and Baskin, Reference Baskin and Baskin1998; Walck and Dixon, Reference Walck and Dixon2009; Baeten et al., Reference Baeten, De Frenne, Verheyen, Graae and Hermy2010).
Fabaceae species have specialized adaptations for breaking physical dormancy (Baskin et al., Reference Baskin, Baskin and Li2000). Exposure to high alternating and constant temperatures in wet and dry conditions breaks physical dormancy in Fabaceae (Taylor, Reference Taylor1981; Williams et al., Reference Williams, Congdon, Grice and Clarke2003; Van-Klinken, Reference Van-Klinken2005; Patanè and Gresta, Reference Patanè and Gresta2006; Hu et al., Reference Hu, Wu and Wang2009). Exposure to a wet-heat treatment of 70°C for 15 min causes 63% of Astragalus hamosus (Fabaceae) seeds from the Mediterranean basin to break dormancy, and 70% break dormancy after exposure to wet-heat at 80°C for 10 min (Patanè and Gresta, Reference Patanè and Gresta2006). Seeds of Trifolium subterraneum (Fabaceae) from western Australia, break dormancy when exposed to fluctuating temperatures (15–75°C) but not under a constant temperature of 15°C (Quinlivan, Reference Quinlivan1966). More seeds of Fabaceae species from tropical eucalypt savannas germinated when exposed to higher temperatures than those not treated (80°C, 100°C, 120°C; Williams et al., Reference Williams, Congdon, Grice and Clarke2003). Thus, high temperatures can break physical dormancy in Fabaceae seeds, but the effects differ by species and habitat. Less information is available regarding the effect of high temperature on the releasing of physical dormancy in Fabaceae seeds from cold desert regions.
Plants in the Fabaceae family are some of the most dominant pioneer species in the Taklimakan, Kumtag, Gurbantunggut and Takeermohuer deserts. These deserts are in the central Tarim Basin, eastern Tarim Basin, central Junggar Basin and western Junggar Basin, respectively, of Xinjiang province, China; they are among the world's most characteristic cold deserts. The Gurbantunggut desert has unreliable precipitation; soil temperatures up to 80°C and maximum summer air temperatures of approximately 40°C have been recorded (Zhang and Hu, Reference Zhang and Hu1983; Yin et al., Reference Yin, Tan and Wang2006; Zhang et al., Reference Zhang, Ma, Shi, Wang and Wang2008, Reference Zhang, Liu, Shi, Wang and Zhang2011). The maximum recorded air temperatures in the Taklimakan and Kumtag deserts are 45.16°C and 47.6°C, respectively (Yin, Reference Yin1987; Li et al., Reference Li, Lei, Xu, Wang, Wang and Li2006); the mean monthly maximum temperature in the Takeermohuer desert has exceeded 22.4°C (Cao et al., Reference Cao, Li, Zhang, Zhuang, Feng and Li2011). Thus, the four deserts have high temperatures in the summer. Few seedlings from the Fabaceae species Eremosparton songoricum from the Gurbantunggut desert and Ammodendron bifolium from the Takeermohuer desert have been observed in the field (Yin et al., Reference Yin, Pan, Wang, Zhao and Yan1991; Yang, Reference Yang2005; Liu et al., Reference Liu, Shi, Wang, Yin, Huang and Zhang2011). Therefore, we inferred that high summer temperatures and low precipitation may affect dormancy release and seedling establishment in these deserts. The aim of this study was to examine whether simulating typical high summer temperatures in conjunction with dry or wet treatments affect dormancy release in seeds of the Fabaceae species native to the four deserts. To answer this question, we hypothesized that: (1) seeds of most Fabaceae species from the four deserts have physical dormancy; (2) simulating typical high summer temperatures under wet and dry conditions breaks dormancy; and (3) seeds of individual species respond differently to simulated high summer temperatures under wet and dry conditions. The results may contribute to an understanding of physical dormancy in seeds from cold deserts and provide guidance for desert restoration.
Materials and methods
Species
Nineteen Fabaceae species were used in this study (see Table 1). Caragana korshinskii, C. intermedia, C. microphylla, Eremosparton songoricum (Litv.) Vass. and Astragalus lehmannianus are drought-tolerant shrubs or semi-shrubs. They are found in the fixed and semi-fixed desert dunes and in the sandy lands of the Gurbantunggut desert (Chen et al., Reference Chen, Zhang and Hu1983; Zhao, Reference Zhao2005; Yin et al., Reference Yin, Tan and Wang2006; Alamusa and Jiang, Reference Alamusa and Jiang2009; Shen et al., Reference Shen, Li and Yan2011). Ammodendron bifolium is a rare psammophyte that lives only in the semi-fixed and fixed sand dunes of the Takeermohuer desert (Chen et al., Reference Chen, Zhang and Hu1983; Pan, Reference Pan1989; Yin et al., Reference Yin, Tan and Wang2006). Halimodendron halodendron is a drought-tolerant shrub that grows on sandy and saline soil (J.Z. Liu et al., Reference Liu, Murch, EL-Demerdash and Saxena2003). Sophora alopecuroides, Sphaerophysa salsula, Glycyrrhiza glabra, G. uralensis, Thermopsis lanceolata, Cassia tora, Amorpha fruticosa, Vicia costata, Melilotus suaveolens, Onobrychis taneitica, Astragalus nanjiangianus and Oxytropis sp. are more broadly adapted and are found in desert, grassland, riverside and farmland environments (Chen et al., Reference Chen, Zhang and Hu1983; Cock and Evans, Reference Cock and Evans1984; Wen, Reference Wen1995; Shen et al., Reference Shen, Li and Yan2011; Musa et al., Reference Musa, Zong and Niu2013).
Table 1 A list of the Fabaceae species studied
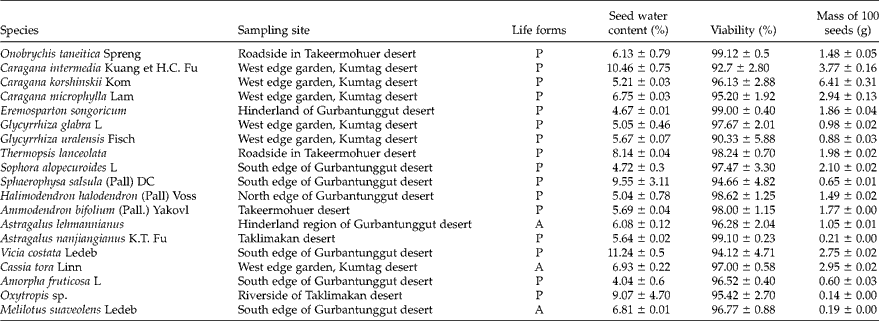
A, annual; P, perennial.
Ripe fruits of the 19 Fabaceae species were harvested from the four deserts from June to September, 2011 and 2012, dried in the laboratory and seeds were manually removed from the fruits. Undamaged seeds were stored in paper bags under ambient laboratory conditions until the start of the experiment.
Seed moisture
Seed moisture content was calculated by weighing seeds (N= 100 seeds for each species) before and after drying in an oven at 105°C for 12 h (International Seed Testing Association, 2005). Seed moisture content was then calculated as follows (Hong and Ellis, Reference Hong and Ellis1996):
The 100 fully formed seeds (three replicates for each species) were weighed to the nearest 0.0001 g using a digital analytical balance, and the mean mass and standard error were calculated. Seed viability (N= 100 seeds) was tested by immersing the scarified seeds in 1% TTC (2,3,5-triphenyl tetrazolium chloride) at 20°C for 24 h in the dark (Baskin and Baskin, Reference Baskin and Baskin1998). A seed was considered viable when the embryo was coloured red after the TTC test.
Imbibition of seeds
The imbibition experiment was conducted to test whether the seeds possessed water-impermeable seed coats. Seeds were placed in 9 cm diameter Petri dishes on filter paper moistened with distilled water at room temperature; three replicates of 50 seeds were prepared for each species. The imbibition of each seed was measured after 0, 1, 2, 4, 8, 12 and 24 h, and then at 24-h intervals until 8 d, depending on the species. Each time a seed was weighed it was blotted dry and weighed to the nearest 0.0001 g using a digital analytical balance. The seed was then returned to moist filter paper in the Petri dishes (Funes and Venier, Reference Funes and Venier2006). Measurements continued until there was no further increase in the mass, or until all seeds within a replicate had begun to germinate. The amount of absorbed water was determined as the actual increase in seed mass, and converted to a percentage. The percentage increase in seed mass was calculated using the following formula (Hidayati et al., Reference Hidayati, Baskin and Baskin2000):
where W i and W d are the mass of imbibed and dry seeds, respectively.
At the end of the imbibition experiment, the numbers of imbibed and non-imbibed seeds of each species were recorded. The seeds that did not have physical dormancy (PY), based on observations in this study as well as from previous reports, were not used in further studies.
Effects of scarification on dormancy breaking
An experiment was conducted to determine the effects of mechanical scarification on germination. Three replicates of 15 intact seeds for each of the 10 species that exhibited physical dormancy were manually scarified with a razor blade, and the same number of non-scarified seeds of each species was used as a control. Also, an experiment was conducted to determine the effects of acid scarification on germination. Three replicates of 15 intact seeds for each of the 10 species that exhibited physical dormancy were scarified with 98% concentrated sulphuric acid for periods of time ranging from 10 min to 6 h, depending on the species. The seeds were washed with tap water for 10 min.
Effects of simulating summer high temperatures under wet and dry conditions on dormancy breaking
Experiments under dry and wet conditions were conducted to test the effects of simulated summer high temperatures on the release of physical dormancy. The simulated maximum air temperature was 40°C, the mean high soil temperature was 65°C, and the maximum soil temperature was simulated as 80°C. To test the relationship between exposure time and breaking of dormancy in wet and dry conditions, seeds of each species were subjected to 1, 3 and 7 consecutive daily cycles of 3 h at each temperature under wet or dry conditions. Seeds were sealed in nylon mesh bags and dipped into hot water in a thermostatic water bath (wet heat) or were placed in paper bags in an oven (dry heat). The seeds were allowed to cool at room temperature. The germination percentages in each treatment were compared with those of the control (non-scarified seeds incubated at 25/10°C) to determine the effectiveness of the dormancy-breaking treatment.
For the germination test, the seeds were placed in 9 cm diameter Petri dishes on filter paper moistened with distilled water and incubated in a climate chamber at 25/10°C, representing the maximum and minimum temperature of the natural habitat during the spring germination period. The seeds were exposed to 12 h of dark and 12 h of light each day for 20 d; the seeds of A. bifolium were placed in a light-tight box and kept in continuous darkness. Seeds were considered to have germinated when the radicle broke the seed coat. Germinated seeds were counted every 24 h and removed at each counting. Any mouldy seeds were classified as non-viable. If intact seeds did not germinate, viability was tested by using a 1% TTC solution (Baskin and Baskin, Reference Baskin and Baskin1998).
Statistical analysis
Data were analysed using SPSS v.16.0 (SPSS Inc., Chicago, Illinois, USA). Values were expressed as means ± SE. Germination data were arcsine transformed before statistical analysis to ensure homogeneity of variance. One-way analysis of variance (ANOVA) (P < 0.05) was used to test for significant treatment effects of manual scarification and acid scarification on breaking of dormancy and germination. Three-way ANOVA (P < 0.05) was used to test for significant treatment effects of temperature (40°C, 65°C, 80°C), treatment duration (1, 3 and 7 consecutive daily cycles of 3 h exposure for each day), dry–wet condition (dry and wet) and their interactions on the germination percentages of the ten species. If the ANOVA indicated significant treatment effects, the Duncan post-hoc test was used to test for differences among treatments.
Results
Seed moisture content
The moisture contents of the seeds from each species were low, ranging from 4.04 ± 0.6% to 11.24 ± 0.5%. Each species had high seed viability (>90%; Table 1). The results suggest that all the species had orthodox seeds (moisture content < 15%). The range of seed mass was very broad, for example, the masses of 100 seeds of Oxytropis sp. and C. korshinskii were 0.14 ± 0.0 and 6.41 ± 0.31 g, respectively (Table 1).
Imbibition percentage
At room temperature, the non-scarified seeds of C. korshinskii, C. intermedia and C. microphylla showed the same imbibition pattern. The imbibition rate increased rapidly during the first day. It then slowly increased to a plateau, reaching 145.27 ± 8.20%, 111.63 ± 7.10% and 100.27 ± 6.57%, respectively. The seed mass of O. taneitica increased 33.01 ± 4.67%, and many seeds germinated within less than 24 h (Fig. 1, Table 2). Therefore, the seeds of these four species were not physically dormant.
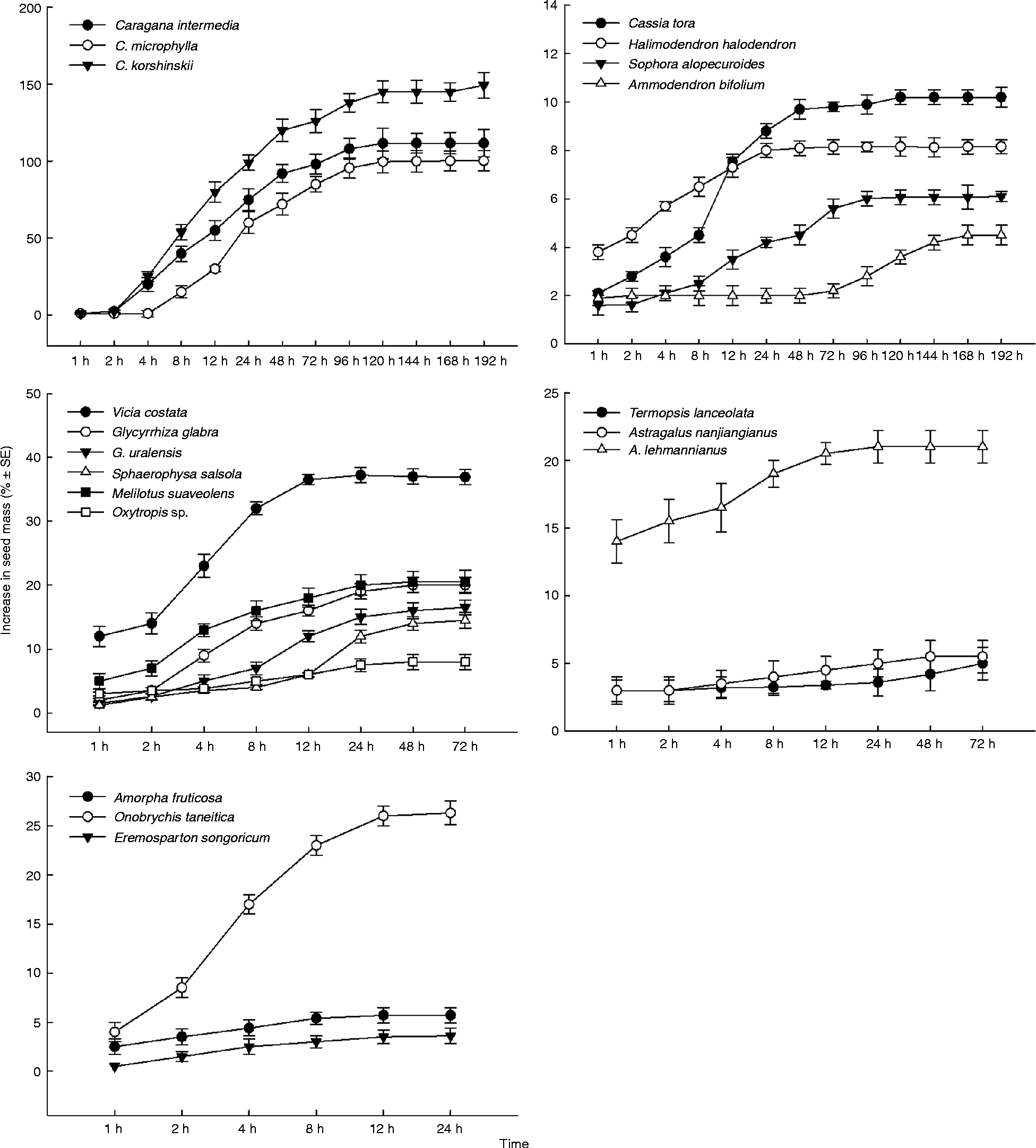
Figure 1 Mean percentage of seed mass increase of non-scarified seeds of 19 species during imbibition at room temperature.
Table 2 Mean germination percentage (±SE) after 20 d of incubation at 25/10°C, 12 h/12 h light/dark, for scarified and non-scarified seeds of the 19 species. Asterisk (*) indicates significances (P < 0.05) in mean germination percentage between scarified and non-scarified seeds
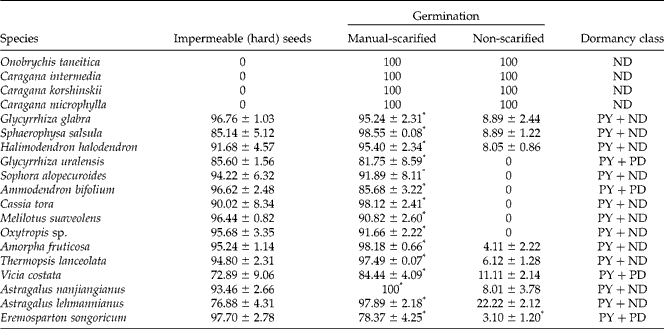
ND, non-dormancy; PY, physical dormancy; PD, physiological dormancy.
However, the other 15 species displayed different patterns of increase in seed mass. The mass of A. fruticosa and E. songoricum seeds stopped increasing after 1 d, and that of T. lanceolata and A. nanjiangianus seeds stabilized after 3 d; the increase in mass was less than 6%. Seed mass of C. tora, H. halodendron, S. alopecuroides, A. bifolium, G. glabra, G. uralensis, S. salsula, M. suaveolens and Oxytropis sp. gradually increased to 6.15–22.48%. Seed mass did not change after 1 d for G. glabra, G. uralensis, S. salsula, M. suaveolens and Oxytropis sp. and not after 3 d for C. tora, H. halodendron and S. alopecuroides. However, the seed mass of A. bifolium began to increase after 3 d, levelling off after 7 d. Seed mass of A. lehmannianus and V. costata increased to 8.22 ± 1.28% and 14.36 ± 1.06% within 1 h of imbibition; it then increased slowly, stabilizing after 1 d. Seed mass of A. lehmannianus and V. costata increased to 22.36 ± 2.78% and 37.48 ± 1.04%, respectively, after 3 d (Fig. 1). Therefore, the seeds of most of these 15 species possessed water-impermeable coats and were thus considered physically dormant.
Effects of scarification on dormancy breaking
The percentage of impermeable seeds of the 15 species ranged from 72.89 ± 9.06% to 97.70 ± 2.78%, depending on the species (Table 2). The germination percentage of non-scarified seeds was < 23% at 25/10°C. The non-scarified seeds of G. uralensis, S. alopecuroides, A. bifolium, C. tora, M. suaveolens and Oxytropis sp. did not germinate (Table 2). However, mechanical scarification broke seed dormancy and significantly increased germination (P < 0.001). The total germination was 78.37% and more, with most species exceeding 95%. Based on patterns of seed storage behaviour and dormancy type (Jayasuriya et al., 2013), seeds of the 19 species could be grouped into three categories: (1) orthodox and non-dormant (ND); (2) orthodox with a high proportion of physical dormancy and low proportion of non-dormancy (PY+ND); (3) orthodox with a high proportion of physical dormancy and low proportion of physiological dormancy (PY+PD).
The effect of sulphuric acid scarification in breaking physical dormancy was statistically significant (Fig. 2, Table 3). The one-way ANOVA showed significant differences in the percentage of seeds breaking dormancy across treatment times. The germination of H. halodendron greatly improved after a 15 min treatment, reaching 88.87%. For the seeds of E. songoricum, A. lehmannianus and S. salsula, the highest germination resulted after 30 min of acid scarification, but there were no significant differences between the effects of 30 min and 60 min treatments for E. songoricum and 10 min and 20 min treatments for A. lehmannianus (Fig. 2). Germination of G. glabra, S. alopecuroides and C. tora reached 84.88% at 60 min of acid treatment, 73% of seeds of Oxytropis sp. and M. suaveolens were non-dormant after 20 min treatment, and 77.78% of A. bifolium seeds germinated at 6 h of acid treatment. There was no significant difference between the effects of treatments for 4 h and 6 h.

Figure 2 Germination percentages (mean ± SE) of intact seeds at 25/10°C (12 h light/12 h dark) of ten species after different durations of sulphuric acid scarification. Different lower-case letters indicate significant differences in germination percentages of seeds between treatment times.
Table 3 Effects of different times of acid scarification on germination percentage of ten species of Fabaceae (one-way ANOVA)
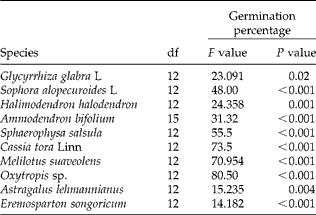
Effects of simulated summer high temperatures under wet and dry conditions on dormancy breaking
Simulated summer high temperatures, wet and dry treatments, treatment time and their interactions had statistically significant effects on the breaking of physical dormancy and increasing the germination percentage. The germination response to simulated summer high temperatures under dry and wet conditions varied among species (Fig. 3, Table 4). The physically dormant seeds could be classified into four types based on their response to the wet-heat and dry-heat treatments, as follows.

Figure 3 Germination percentages (mean ± SE) of intact seeds at 25/10°C (12 h light/12 h dark) of ten species after different periods and various temperatures of dry-heat and wet-heat treatments. Different lower-case letters indicate significant differences in germination percentages of seeds between dry and wet treatments at different temperatures.
Table 4 Three-way ANOVA of effects of temperature, dry–wet heat treatment, treatment time and their interactions on seed germination of ten species
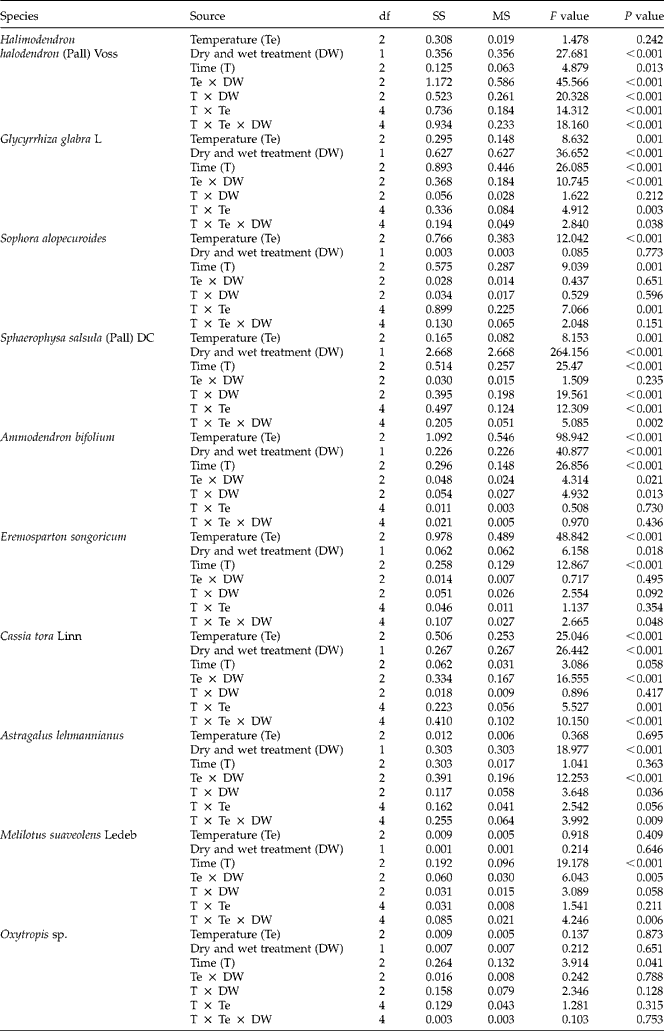
Temperature insensitive ( M. suaveolens and Oxytropis sp.)
Although the seeds of M. suaveolens and Oxytropis sp. showed lower levels of dormancy breaking after temperature treatments, they did not respond significantly to the different simulated temperatures and dry–wet treatments (P > 0.05); germination was less than 30% in each treatment.
Wet-heat sensitive (S. alopecuroides, S. salsula, E. songoricum, A. bifolium and C. tora)
For these seeds, the percentage of seeds breaking dormancy significantly increased with increasing temperature (Fig. 3). The percentage of A. bifolium and C. tora seeds that broke dormancy did not differ significantly between wet and dry conditions at 40°C (P > 0.05); at 65°C and 80°C, the percentage of dormancy breaking was significantly higher in the wet-heat treatment compared to dry heat (P < 0.05; Fig. 3). For the S. alopecuroides and E. songoricum seeds, the percentage of breaking dormancy was higher in the wet-heat treatment compared to dry heat, but the differences were not significant (P > 0.05). Significant effects were found, however, for the 3 d treatment at 80°C for S. alopecuroides and the 1 d treatment at 65°C and 7 d treatment at 80°C for E. songoricum seeds. The presence or absence of statistically significant interaction effects among temperature, dry-wet treatments and treatment duration differed among species (Table 4).
Dry-heat sensitive (G. glabra)
The percentage of G. glabra seeds breaking dormancy was significantly higher with dry than wet heat (P < 0.001; Table 4); however, the difference between dry and wet treatments was not significant at 40°C (P >0.05; Fig. 3). The highest percentage of dormancy break occurred in the 7 d treatment at 65°C. The percentage of dormancy break increased with treatment time. However, the 7 d treatment at 80°C caused a decrease in dormancy breaking under dry conditions and killed the seeds under wet conditions (Fig. 3). A three-way ANOVA showed that dormancy break was significantly affected by temperature (P= 0.001), dry–wet treatment (P < 0.001), treatment duration (P < 0.001) and the interactions of temperature and dry–wet treatment (P< 0.001), temperature and treatment duration (P < 0.05) and temperature, dry-wet treatment and treatment duration (P < 0.05; Table 4).
Both dry-heat and wet-heat sensitive (H. halodendron and A. lehmannianus)
The percentage of H. halodendron seeds breaking dormancy gradually increased at 40°C and was higher at different durations of wet heat at 40°C and 1 d wet heat at 65°C. After that, however, the percentage of dormancy break was higher under dry-heat than wet-heat conditions (Fig. 3). Dormancy break was significantly affected by dry–wet treatment (P < 0.001), treatment duration (P= 0.013) and their interaction (P < 0.001) and by the interactions of temperature and dry-wet treatment (P< 0.001), temperature and treatment duration (P< 0.001) and temperature, dry-wet treatment and treatment duration (P< 0.001; Table 4). For A. lehmannianus, the percentage of dormancy break was higher when treated with wet heat at 40°C; however, it was higher under the dry–wet treatment at 65°C and 80°C (Fig. 3). A three-way ANOVA showed that the dry–wet treatment (P < 0.001) and the interactions of temperature and dry–wet treatment (P< 0.001); dry–wet treatment and treatment duration (P< 0.05); and temperature, dry–wet treatment and treatment duration (P= 0.009) significantly affected dormancy release (Table 4). However, the 7 d treatment at 65°C and 80°C killed the seeds of both species (Fig. 3).
Discussion
An earlier study has shown that >98% of Fabaceae species have orthodox seeds (Jayasuriya et al., Reference Jayasuriya, Wijetunga, Baskin and Baskin2012). Seeds of the 19 studied species had low ( < 11.24%) moisture content and high viability (>90%); 15 of the species possess a water-impermeable seed coat. The data suggest that these species generate orthodox seeds with high tolerance to desiccation (Dickie and Pritchard, Reference Dickie, Pritchard, Black and Pritchard2002). Desiccation tolerance may delay dormancy break and germination until adequate moisture occurs, thus extending the period of viability for the seeds so the species can regenerate successfully over broader spatial and temporal scales (Baskin and Baskin, Reference Baskin and Baskin1998; Taylor, Reference Taylor2005). Seeds from each species, however, have imbibition characteristics that are determined by species-specific traits or degrees of seed hardness. Previous studies have shown that seed hardness degree of impermeability was strictly related to the moisture content of the seeds (Hyde, Reference Hyde1954; Hu et al., Reference Hu, Wu and Wang2009). Hu et al. (Reference Hu, Wu and Wang2009) found that S. alopecuroides seeds growing in arid conditions entered dormancy when the seed moisture content was 18%, whereas seeds from moister habitats became dormant when the moisture content was 20%; the seed coat thickness differed between the two environments. This variation shows that a hard seed coat has great ecological importance in enabling legumes to persist under a wide range of environmental conditions and that seed coat thickness is related to the environmental setting in which an individual plant is found.
The breaking of physical dormancy requires rupture of the seed coat and subsequent absorption of water by the embryo (Baskin and Baskin, Reference Baskin and Baskin1998). The fresh mechanically scarified seeds of G. uralensis, A. bifolium, V. costata and E. songoricum germinated to < 90%; they may have physiologically dormant embryos. The fresh mechanically scarified seeds of the 11 other species germinated to >90%; < 22% of seeds imbibed water and germinated during the incubation period. This result confirms that G. uralensis, A. bifolium, V. costata and E. songoricum seeds have combinational dormancy (PY+PD); they may need cold stratification or sand burial to break physiological dormancy after physical dormancy is broken, thus completing regeneration in the subsequent year. Combinational dormancy may prevent germination after sporadic summer rains that would be insufficient to support the growth of seedlings (Van Assche and Vandelook, Reference Van Assche and Vandelook2010). Mechanical and acid scarifications were most effective in breaking physical dormancy. They facilitated water imbibition or increased the permeability of the seed coat to water and oxygen; therefore, they could be used to artificially re-establish populations.
Dry heat and wet heat also effectively released physical dormancy. Sensitivity to the heat treatments was likely species-specific (Van-Klinken and Flack, Reference Van-Klinken2005). The results of the wet-heat and dry-heat treatments showed that S. alopecuroides, S. salsula, E. songoricum, A. bifolium and C. tora seeds were wet-heat sensitive. However, G. glabra seeds were dry-heat sensitive. This difference may be attributed to species-specific requirements for releasing dormancy (Loi et al., Reference Loi, Coclts, Howieson and Carr1999) and to different seed coat thicknesses (Bewley and Black, Reference Bewley and Black1982). The wet-heat and dry-heat treatments and dormancy release patterns coincide with temperatures typically encountered in field conditions. G. glabra, S. alopecuroides, C. tora and S. salsula are widely distributed, occurring mostly in grasslands (Lee et al., Reference Lee, Jang, Lee, Kim and Kim2006; Shen et al., Reference Shen, Li and Yan2011), which have higher soil moisture content than other areas. The soil moisture content during the hottest summer weather may create a beneficial microenvironment for releasing dormancy in these species. E. songoricum and A. bifolium, in contrast, are narrowly distributed in the dry desert (Yin et al., Reference Yin, Tan and Wang2006; Liu et al., Reference Liu, Shi, Wang, Yin, Huang and Zhang2011). Although the main period for the breaking of seed dormancy is likely to be during the dry summers, when sand surface temperatures are highest (Commander et al., Reference Commander, Merritt, Rokich and Dixon2009), insufficient precipitation causes low germination rates and poor seedling establishment in the field (Liu et al., Reference Liu, Shi, Wang, Yin, Huang and Zhang2011). Therefore, wet heat provides a strong environmental signal for breaking dormancy in seeds of these species. Many seeds may break dormancy after ‘drought-breaking’ rains in hot summer conditions. This phenomenon may be the key to maximizing the success of species recruitment in these ecosystems.
The H. halodendron and A. lehmannianus seeds were sensitive to both dry heat and wet heat. Thus, they can germinate in their habitat any time between spring and autumn when dormancy is broken (Wang et al., Reference Wang, Jiang, Wang, Luo, Song and Chen2006). This attribute may be a result of their adaptation to a wide range of sandy conditions. The response of the seeds to the temperature treatment is advantageous for effective regeneration in the desert. The optimal duration of high temperatures differed among species and had a greater effect on physical dormancy release (Oakes, Reference Oakes1984). However, the seed embryos of our studied species were damaged after treatment for 7 d at 65°C and 80°C, and germination decreased or did not occur. For this reason, temperature and treatment durations may need adjustment to minimize damage that could kill seeds. The seeds of M. suaveolens and Oxytropis sp. were temperature insensitive; the different wet-heat and dry-heat treatments released dormancy in less than 30% of the seeds. A possible explanation for the insensitivity is that the two species are found in forests and along roadsides (Shen et al., Reference Shen, Li and Yan2011), where soil moisture conditions are better than in the desert and high temperatures are unlikely. Dormancy release may therefore be achieved with sand burial, without a need for high temperatures.
This study shows that seeds of 4 of the 19 species do not have physical dormancy. The simulated wet-heat and dry-heat conditions effectively broke physical dormancy in seeds of 13 of the 15 tested species; the exceptions were M. suaveolens and Oxytropis sp. For most of the species, the wet-heat treatment was more effective than the dry-heat treatment. Although a 65°C temperature could be effective for breaking dormancy in summer, the highest levels of germination were achieved at a temperature of 80°C. However, a maximum air temperature of 40°C was sufficient to release dormancy in a small portion of the seeds. Although the strong relationship between wet-heat and dry-heat treatments and dormancy release revealed a mechanism for releasing dormancy that was common across most species, the proportion of seeds breaking dormancy under favourable conditions varied by species. This result suggests that favourable conditions for regeneration differ among species.
Acknowledgements
We thank Wang, X.Y. (Turpan Botanic Garden, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences), for providing seeds, and Zhuo, L. and Li, W.J. (Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, University of the Chinese Academy of Sciences) for their help during this experiment.
Financial support
Funds for this study were provided by Grant Project of Xinjiang Committee of Science and Technology (201330122-3).
Conflicts of interest
None.









