Introduction
Plant species growing in heterogeneous environments may develop a wide variety of functional traits in response to local characteristics (Berg et al., Reference Berg, Becker and Matthies2005). Adaptation to local conditions and response plasticity are essential requirements for the effective establishment of new individuals within a population (Galloway, Reference Galloway2005), directly defining plant fitness, population dynamics and community structure (Donohue et al., Reference Donohue, Casas, Burghart, Kovach and Willis2010; Baskin and Baskin, Reference Baskin and Baskin2014). Thus, unravelling factors that control plant recruitment from seeds represent an essential step for predicting vegetation structure and composition in the future (Leishman et al., Reference Leishman, Wright, Moles, Westoby and Fenner2000; Coomes and Grubb, Reference Coomes and Grubb2003). Since it is difficult to evaluate functional responses for all plant species, the identification of traits that best represent plant responses to environmental conditions represents a promising approach (Chevin et al., Reference Chevin, Lande and Mace2010; Barbosa et al., Reference Barbosa, van Langevelde, Tomlinson, Carvalheiro, Kirkman, de Bie and Prins2014).
Seed germination and post-germination adaptions to environmental filters represent crucial phases in the life cycle of plants (Donohue et al., Reference Donohue, Casas, Burghart, Kovach and Willis2010), and new individuals cannot become part of the community if the local climate is suitable for adults but not for seeds and seedlings (Poschlod et al., Reference Poschlod, Abedi, Bartelheimer, Drobnik, Rosbakh, Saatkamp, van der Maarel and Franklin2013). Regenerative traits strongly influence the distribution and structure of plant communities (Rees and Westoby, Reference Rees and Westoby1997; Moles et al., Reference Moles, Ackerly, Tweddle, Dickie, Smith, Leishman, Mayfield, Pitman, Wood and Westoby2007; Cochrane et al., Reference Cochrane, Yates, Hoyle and Nicotra2015; Jiménez-Alfaro et al., Reference Jiménez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016). For example, morphological traits such as seed surface, coat properties and seed mass are functionally related to regeneration steps as dispersal, soil persistence and responses to biotic and abiotic disturbances (Westoby et al., Reference Westoby, Falster, Moles, Vesk and Wright2002; Fenner and Thompson, Reference Fenner and Thompson2005). Seed mass, in particular, was related to seed tolerance to heat shock (Ribeiro et al., Reference Ribeiro, Barbosa, van Langevelde and Borghetti2015). Biophysical traits, such as seed water content (SWC) and desiccation tolerance, are correlated with each other (Hamilton et al., Reference Hamilton, Offord, Cuneo and Deseo2013) and with seed tolerance to water deficit (Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003). Physiological traits represent parameters that estimate the percent (or proportion) of seeds that germinate in a seed batch and the rate and distribution of germination over time (Jiménez-Alfaro et al., Reference Jiménez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016). Such parameters are strongly affected by environmental filters such as light, moisture and temperature (Fenner and Thompson, Reference Fenner and Thompson2005; Donohue et al., Reference Donohue, Casas, Burghart, Kovach and Willis2010), and they have been used for evaluating germination responses to regional constraints, such as water deficit (Flores and Briones, Reference Flores and Briones2001; Kos and Poschlod, Reference Kos and Poschlod2008, Reference Kos and Poschlod2010; Fay and Schultz, Reference Fay and Schultz2009), high temperatures (Flores and Briones, Reference Flores and Briones2001; Ribeiro and Borghetti, Reference Ribeiro and Borghetti2014) and fire (Dayamba et al., Reference Dayamba, Tigabu, Sawadogo and Oden2008; Ribeiro et al., Reference Ribeiro, Barbosa, van Langevelde and Borghetti2015). As these environmental factors represent major filters in neotropical savannas, physiological traits such as seed dormancy, germination percentage and germination time are expected to play an important role in the vegetation dynamics of these ecosystems. However, the relative importance of morphological, biophysical and physiological traits related to seed tolerance to environmental filters is still an open gap in the literature (Jiménez-Alfaro et al., Reference Jiménez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016). Therefore, the comparison of seed responses of species subjected to different environmental conditions would contribute to unravelling which trait(s) best explain seed tolerance to stress.
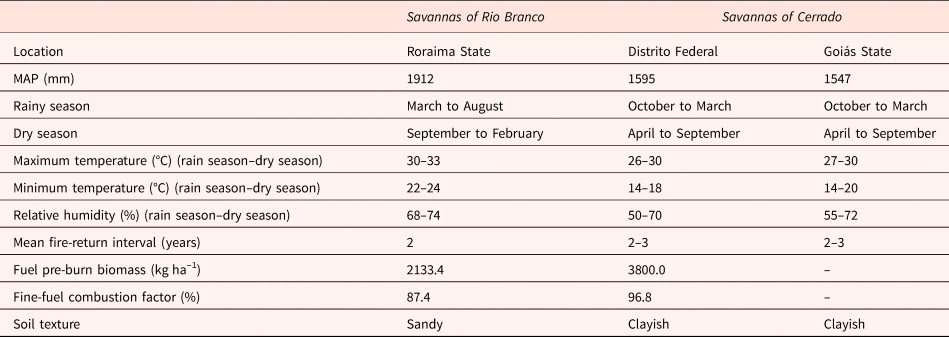
In Brazil, there are two large savanna areas separated by the Amazon basin: (i) Rio Branco savannas, occurring in the state of Roraima, northern Brazil and (ii) Cerrado savannas, occurring within the Cerrado, a biome largely distributed over Central Brazil (Borghetti et al., Reference Borghetti, Barbosa, Ribeiro, Ribeiro, Walter, Scogings and Sankaran2019). They share several plant species and are subjected to a similar fire frequency and mean annual precipitation (see Table 1 for more details). However, annual temperature variation is wider in Cerrado savannas, and the soil is usually clayish, while Roraima savannas are subjected to a narrower temperature range and occur over predominantly sandy soils (Table 1).
Table 1. Environmental characteristics reported for Rio Branco and Cerrado savannas (Castro and Kauffman, Reference Castro and Kauffman1998; Gomes et al., Reference Gomes, Curi, Motta, Ker, Marques and Schulze2004; Barbosa and Fearnside, Reference Barbosa and Fearnside2005b; Benedetti et al., Reference Benedetti, Júnior, Schaefer, Melo and Uchôa2011; INMET, 2013, 2014).
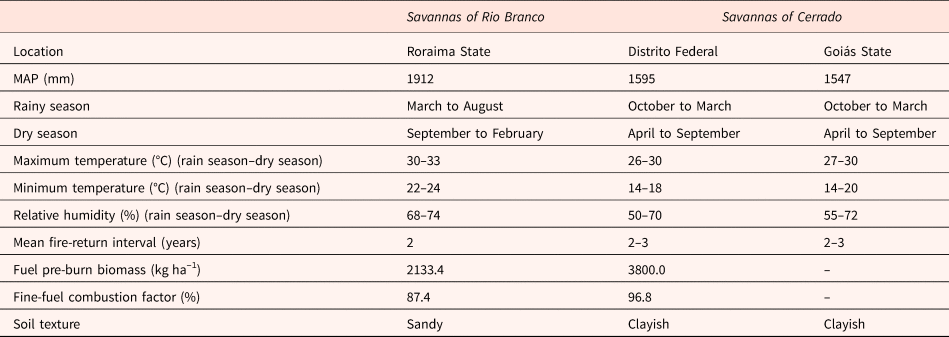
Notes: Mean annual precipitation (MAP), mean maximum and minimum temperature and relative humidity averaged for the period from 2004 to 2013. These data represent regional parameters for the regions selected for this study.
Soils with low vegetation covering, such as those found in Rio Branco savannas (Barbosa and Fearnside, Reference Barbosa and Fearnside2005a), are more affected by solar radiation than soils with high vegetation covering (Oliveira and Silva, Reference Oliveira and Silva1994), such as the Cerrado soils (Castro and Kauffman, Reference Castro and Kauffman1998). Maximum air temperatures recorded for Rio Branco savannas are higher than those recorded for Cerrado savannas (Table 1). Considering that soil temperatures can be positively correlated with air temperatures (Ooi et al., Reference Ooi, Auld and Denham2012), we expect that soils of Rio Branco savannas will experience higher temperatures than those of Cerrado savannas. Due to the higher water holding capacity of clayish soils (Gomes et al., Reference Gomes, Curi, Motta, Ker, Marques and Schulze2004; Benedetti et al., Reference Benedetti, Júnior, Schaefer, Melo and Uchôa2011), water availability in the sandy soils of Rio Branco savannas is expected to be lower than that in the clayish soils of Cerrado savannas. Moreover, due to higher amounts of biomass available for burning and a higher fine-fuel combustion factor (Table 1), soils of Cerrado savannas are expected to experience fires of higher severity (Kauffman et al., Reference Kauffman, Cummings and Ward1994; Castro and Kauffman, Reference Castro and Kauffman1998; Barbosa and Fearnside, Reference Barbosa and Fearnside2005a; Miranda et al., Reference Miranda, Nascimento-Neto, Castro-Neves and Miranda2010) and with increased residence time (Bristow, Reference Bristow1998; DeBano et al., Reference DeBano, Neary and Ffolliott1998; Miranda et al., Reference Miranda, Nascimento-Neto, Castro-Neves and Miranda2010; Nwadibia et al., Reference Nwadibia, Ugwu and Aduloju2010) than soils of Rio Branco savannas.
Variations in seed attributes and germination patterns among populations subjected to different environmental conditions are expected (Baskin and Baskin, Reference Baskin and Baskin2014), but they can only be accessed if seeds from populations occurring in different regions and/or subjected to distinct environmental conditions are studied under the same experimental settings. In this respect, the use of conspecific and congeneric species may contribute to unravelling trait–environment interactions, variation in micro-environmental conditions, ecological breadth and geographic ranges (Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002; Donohue et al., Reference Donohue, Casas, Burghart, Kovach and Willis2010). Due to their regional particularities with respect to climate and soil properties, savannas of Rio Branco and the Cerrado biome offer an excellent opportunity to conduct studies focusing on intraspecific variation in seed traits. For that, we used congeneric and conspecific tree species occurring in both Rio Branco and Cerrado savannas to compare the variation in physiological (germination time), morphological (seed mass) and biophysical (water content and desiccation tolerance) traits, as well as the extent to which environmental filters and seed origin may influence this variation. Also, considering that physiological traits may be more responsive to, and better reflect seed tolerance to stress than morphological and biophysical traits (Saatkamp et al., Reference Saatkamp, Affre, Dutoit and Poschlod2011; Jiménez-Alfaro et al., Reference Jiménez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016), we made the following predictions:
1. The physiological trait explains better seed stress–tolerance than morphological and biophysical traits.
2. Seed origin and environmental filters are better predictors of seed tolerance to stress than seed traits.
3. Seeds from Rio Branco savannas have a higher tolerance to water deficit, high temperatures and heat shock than seeds from Cerrado savannas.
Materials and methods
Study sites: a general view
Rio Branco savannas are located north of the Amazon basin, covering about 43,000 km2 in the northern and northeastern regions of Roraima State, but they also extend to the country of Guyana (Barbosa and Fearnside, Reference Barbosa and Fearnside2005b; Barbosa et al., Reference Barbosa, Campos, Pinto and Fearnside2007; Meneses et al., Reference Meneses, Costa and Behling2013).
The Cerrado biome covers around 2 million km2 in Central Brazil, representing the largest continuous savanna area in South America (Borghetti et al., Reference Borghetti, Barbosa, Ribeiro, Ribeiro, Walter, Scogings and Sankaran2019). Much of the Cerrado is distributed over the Brazilian Central Plateau (Sano et al., Reference Sano, Rosa, Brito and Ferreira2010). The Cerrado vegetation has a gradient of physiognomies; from pure grasslands to forests, however, savanna predominates (Bueno et al., Reference Bueno, Pennington, Dexter, Kamino, Pontara, Neves, Ratter and Oliveira-Filho2017; Borghetti et al., Reference Borghetti, Barbosa, Ribeiro, Ribeiro, Walter, Scogings and Sankaran2019). Although separated by the Amazon basin, these two regions share several species, suggesting that they were in some way connected in the past (Silva and Bates, Reference Silva and Bates2002; Prance, Reference Prance2006; Furley, Reference Furley2007).
Selected species and seed collection
Seed samples were collected in 2011 from natural populations occurring in the two main Brazilian savanna regions: Rio Branco savannas – one site at the campus of the Federal University of Roraima (UFRR) (02°38′N, 60°49′W), located 15 km north of Boa Vista, in Roraima State, at an elevation of 77 m above sea level (a.s.l); Cerrado savannas – two sites: (i) IBGE Ecological Reserve (15°55′S, 47°52′W), Federal District, at approximately 1100 m a.s.l. and (ii) Experimental Farm of the Goiás State University, campus Ipameri (IPM) (17°41′S and 48°11′W), Goiás State, at approximately 780 m a.s.l. For all these regions, the climate is classified as tropical with a dry winter (Aw) under the Köppen–Geiger classification system (Peel et al., Reference Peel, Finlayson and McMahon2007).
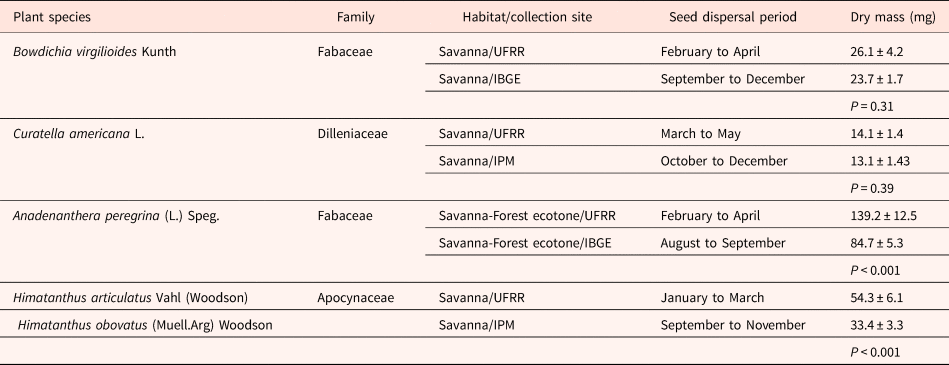
We selected conspecific and congeneric species of common occurrence over both Rio Branco and Cerrado savannas (see Table 2 for more details). For species selection, we also considered their frequency of occurrence in the areas of collection. We collected seeds from at least ten adult individuals of each species, from both savanna sites, except for the two species of Himatanthus (Table 2). After collection, samples of seeds were immediately used for seed morphological and biophysical traits assays, as described in the next section. Another sample of seeds was stored in paper bags under laboratory conditions (22–24°C, 65–85% relative humidity) for a maximum of 4–6 weeks before the germination experiments. Storage under these conditions maintains seed moisture and viability for an extended period of time (Brasil, 2009).
Table 2. Habitat, collection site, seed dispersal period and seed dry mass of the tree species selected for this study

(For dry mass, data are expressed as mean ± standard deviation; P refers to the statistical significance (ANOVA one-way).
Notes: Since neither H. articulatus nor H. obovatus are common to Rio Branco and Cerrado savannas, we worked with different species within this genus. However, phylogenetic studies have shown that Himatanthus has a recent origin, suggesting that morphological and genetic differences within this genus are minimal (Spina, Reference Spina2004; Spina et al., Reference Spina, Bittrich and Kinoshita2013).
Experimental design
Seed morphological and biophysical traits
Samples of 100 seeds of each species were selected to estimate fresh and dry seed mass. Each single seed was weighed before and after being dried in an electric oven, set at 105°C for 24 h (Brasil, 2009). SWC was estimated on a dry weight basis.
For the treatment of desiccation, seed samples of each species had their initial water content reduced to 5% in a hermetically sealed container containing silica gel, which was renewed daily. Seeds were weighed every 2 h during the first 12 h and then every 24 h (Ribeiro and Borghetti, Reference Ribeiro and Borghetti2014) until the mass corresponding to the desired moisture content was achieved. The seed mass corresponding to a water content of 5% was estimated according to Cromarty et al. (Reference Cromarty, Ellis and Roberts1985). The controls consisted of samples of seeds not subjected to desiccation.
Water deficit
For treatments of water deficit, seed samples of each species were placed to germinate in osmotic solutions prepared with polyethylene glycol 6000 P.A. (PEG 6000). This method gives a good estimate of germination behaviour in relation to soil water availability under field conditions (Hardegree and Emmerich, Reference Hardegree and Emmerich1994). We used PEG 6000 solutions at different concentrations to obtain osmotic potentials (OPs) ranging from −0.2 to −1.2 MPa (Villela et al., Reference Villela, Doni-Filho and Siqueira1991). The water potentials chosen for this study represent values registered in the upper soil layers of neotropical savannas, even during the rainy season (Nardoto et al., Reference Nardoto, Souza and Franco1998; Franco, Reference Franco, Oliveira and Marquis2002). The controls consisted of samples of seeds placed to germinate in distilled water (0.0 MPa).
Heat shock
For heat shock treatments, seed samples of each species were placed in a temperature-controlled oven with air circulation set to provide each of the following temperatures: 80, 110, 140, 170 and 200°C. Each heat shock was separately applied for 2.5 or 5.0 min. These combinations of temperatures and times of exposure were chosen based on field recordings taken in the soil of Brazilian savanna ecosystems (surface and/or shallow depths) during a prescribed fire (see Miranda et al., Reference Miranda, Miranda, Dias and Dias1993). The controls consisted of samples of seeds not subjected to heat shock.
Temperature
Seed samples were placed to germinate in chambers set at 20, 25, 30, 35, 40 and 45°C and 12-h photoperiod (white light, 30 μmol m−2 s−1). These temperatures were chosen because they cover a large range of temperature for the germination of savanna tree species of Brazilian ecosystems (Brancalion et al., Reference Brancalion, Novembre and Rodrigues2010; Borghetti et al., Reference Borghetti, Caetano, Colli, Françoso and Sinervo2021).
General procedures
Four replicates of 25 seeds for each species, from each savanna (Rio Branco and Cerrado), were used for each treatment. For the treatments of desiccation, heat shock and temperature, seeds were placed to germinate in 15-cm Petri dishes lined with two sheets of filter paper moistened with distilled water, which was replenished every 2 days. For treatments of OP, PEG solutions were used instead of water, except in the control. To minimize water evaporation from the solutions and, consequently, changes in the PEG concentration, the Petri dishes were sealed with parafilm (Kos and Poschlod, Reference Kos and Poschlod2008). After the treatments of desiccation and heat shock, as well as for the experiments of OP, seeds were placed to germinate in temperature-controlled chambers, set at 30°C and 12-h photoperiod (white light, 30 μmol m−2 s−1), a temperature within the range considered appropriate for the germination of savanna seeds (Zaidan and Carreira, Reference Zaidan and Carreira2008; Brancalion et al., Reference Brancalion, Novembre and Rodrigues2010; Borghetti et al., Reference Borghetti, Caetano, Colli, Françoso and Sinervo2021).
Germination was scored daily by counting and removing the germinated seeds until no signal of germination could be detected. The criterion used for germination was the emergence of the radicle, followed by its geotropic curvature to avoid false germination (Labouriau, Reference Labouriau1983). As the species have different germination kinetics, the experiments ranged from 10 to 40 days, considering species with fastest (e.g. Anadenathera peregrina) and slowest (e.g. Curatella americana) germinations, respectively. After the experiments finished, the viability of non-germinated seeds was tested using 1% w/v tetrazolium chloride solution. Seeds were cut into two halves and the exposed embryos were placed in contact with tetrazolium solution for 24 h in the dark at 30°C. Seeds that had their embryos stained dark pink or red after soaking were considered viable (Moore, Reference Moore and Heydecker1973).
Physiological parameters
The germination percentage was calculated according to Labouriau (Reference Labouriau1983), and the germination time (in hours) according to Farooq et al. (Reference Farooq, Basra, Hafeez and Ahmad2005).
The germination time was the (physiological) trait selected to test for the effects of different environmental filters (desiccation, water deficit, temperature and heat shocks) on seed performance. In those treatments in which the germination percentage was below 10%, the germination time was not calculated.
Statistical analysis
To verify the variation in seed morphological and biophysical traits as well as the effects of different treatments on the germination time, we grouped the species according to their collection site (Cerrado and Rio Branco).
To test variation in seed morphological and biophysical traits between savannas, we used a generalized linear mixed model (GLMM) with log-link function and Gamma distribution of errors. In those analyses, species was included as a random factor and the collection site was included as a fixed factor.
To determine how desiccation, OP, heat shock and temperature treatments affect both germination percentage and germination time (physiological traits), for each species from each collection site, we also performed the GLMM. For germination percentage analyses, the GLMM was performed using germination data (absence or presence) with logit-link function and binomial distribution of errors. For germination time analyses, the GLMM was performed using the germination time with log-link function and Gamma distribution of errors. Fresh mass, dry mass and water content were included as covariates in the models for the analyses of both germination percentage and germination time. Regardless of the treatment, the collection site was included as a fixed factor and species was included as a random factor. Every treatment was included as a fixed variable for analyses of their effects on each germination trait. All statistical analysis was carried out using R v.4.0.2 (R Core Team, 2020).
Results
Morphological and biophysical traits
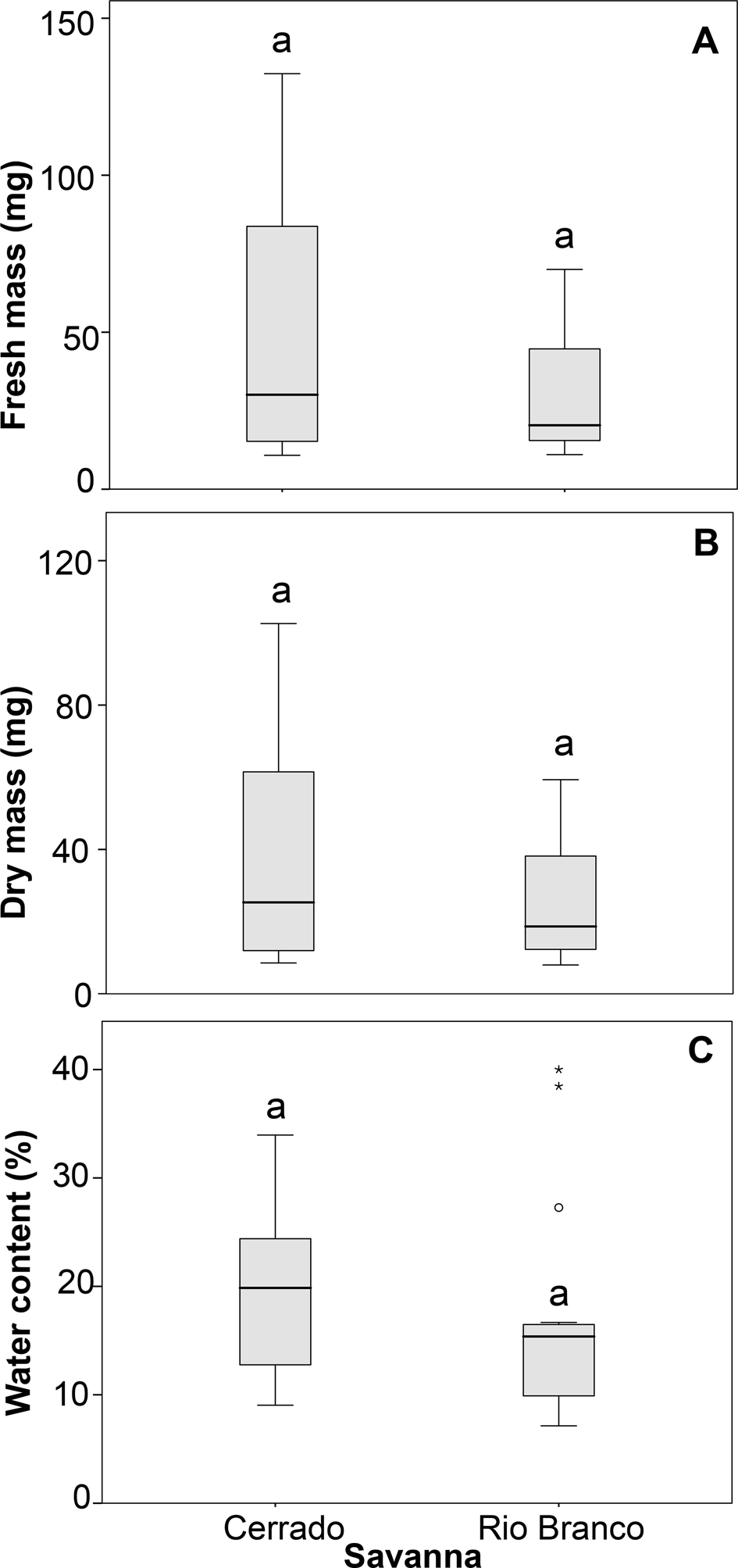
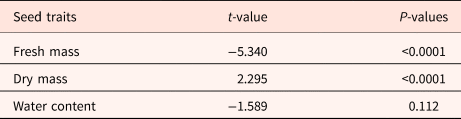
There were no statistical differences between the parameters fresh and dry seed mass and SWC when comparing species from Rio Branco and Cerrado savannas (Table 3 and Fig. 1). Also, there was no statistical difference in seed tolerance to desiccation when comparing both savannas (Fig. 2A). In this case, the best-fitting model selected took into account only SWC, being the significant factor within the model (Z = −3.256; P < 0.01). Desiccation did not significantly affect the germination times of the seeds from both savannas (Fig. 3A). In this case, the best-fitting model selected took into account the factors collection site (S) and SWC. However, only SWC was significant within the model (t = 3.606; P < 0.001).
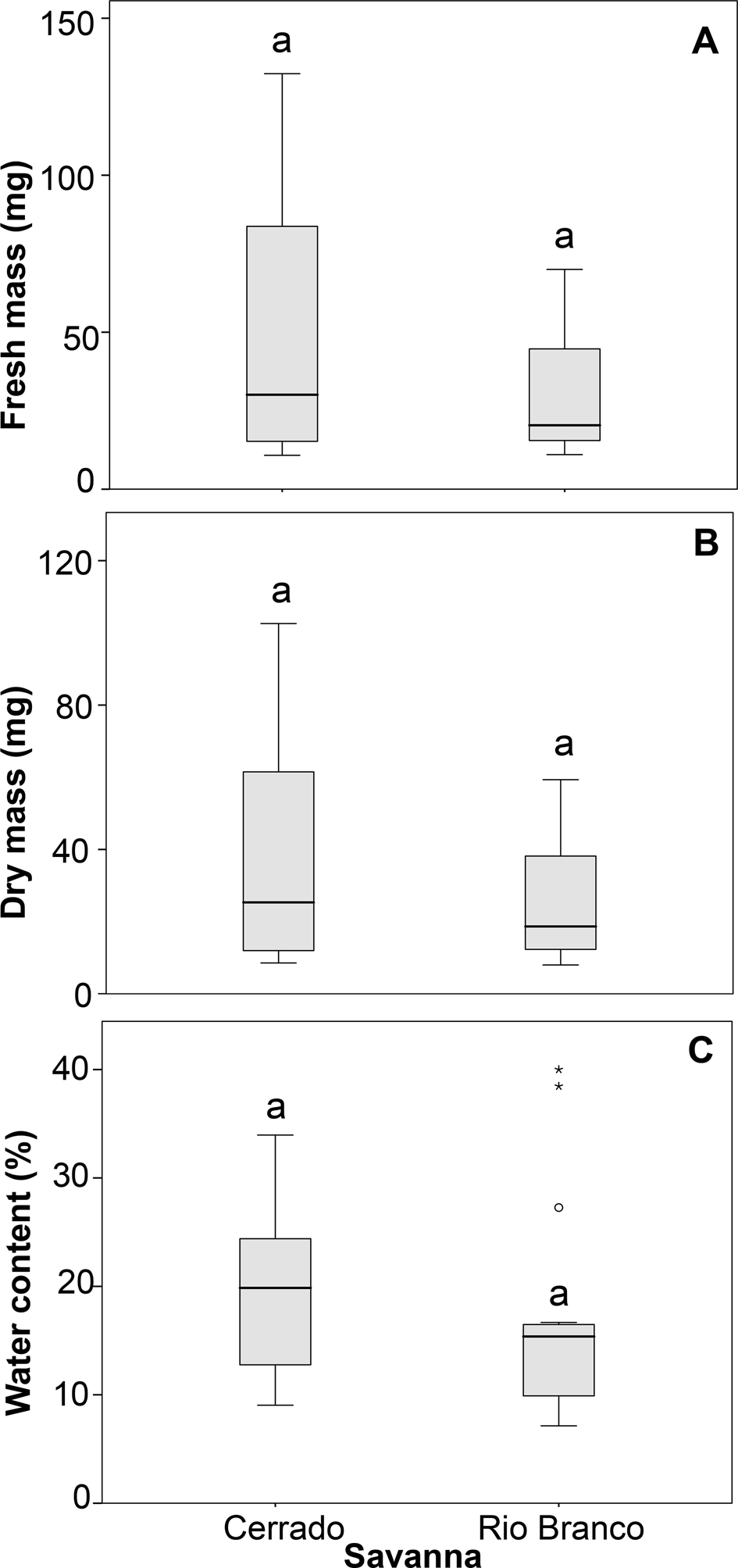
Fig. 1. Fresh mass (A), dry mass (B) and water content (C) of seeds of populations of trees from Cerrado and Rio Branco savannas, Brazil. The same letters above the boxes indicate there is no significant difference.
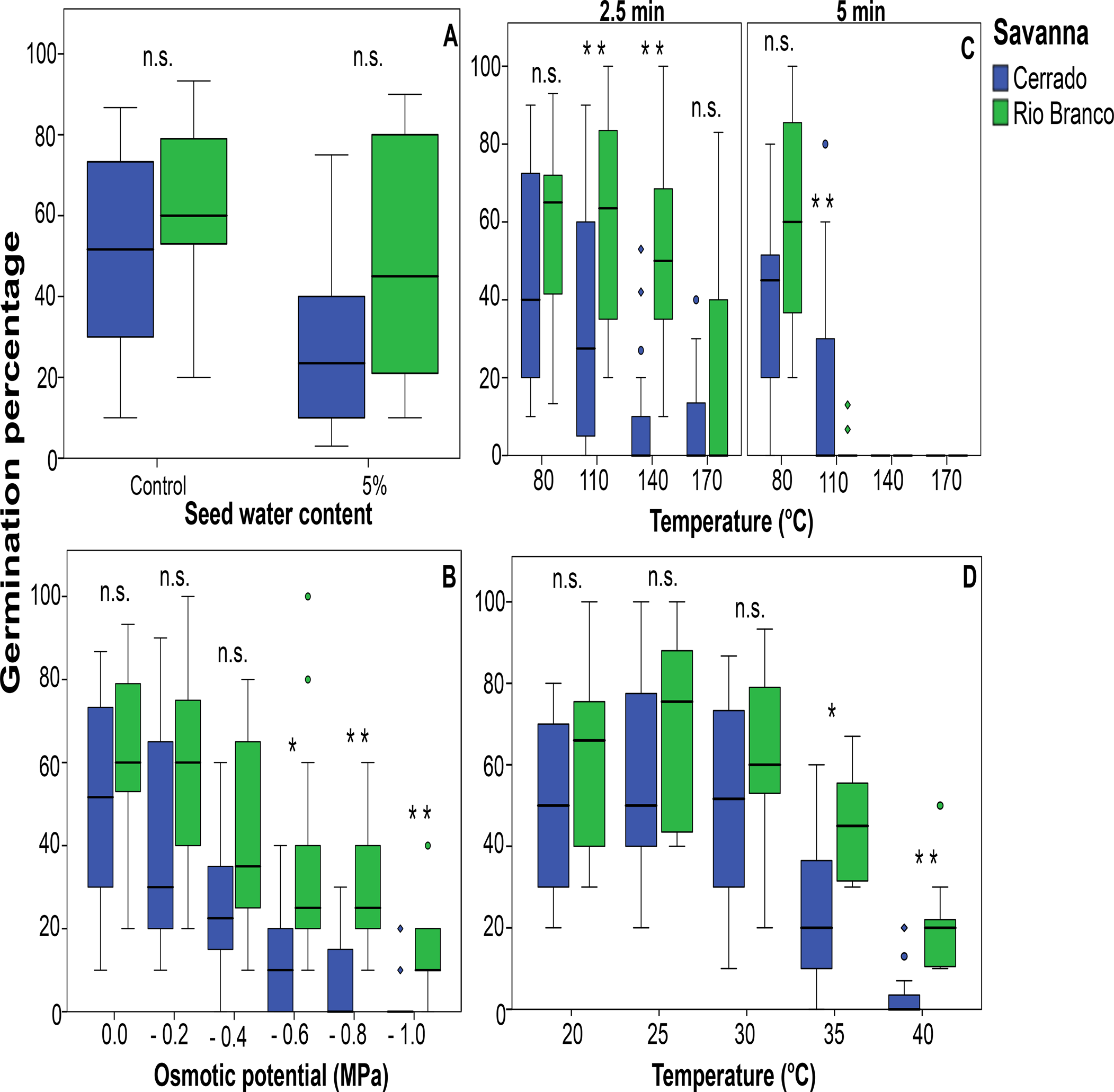
Fig. 2. Effects of desiccation (A), water deficit (B), heat shock (C) and temperature (D) on the germination percentage of populations of trees from Cerrado and Rio Branco savannas, Brazil (*P < 0.05; **P < 0.01; n.s., not significant).
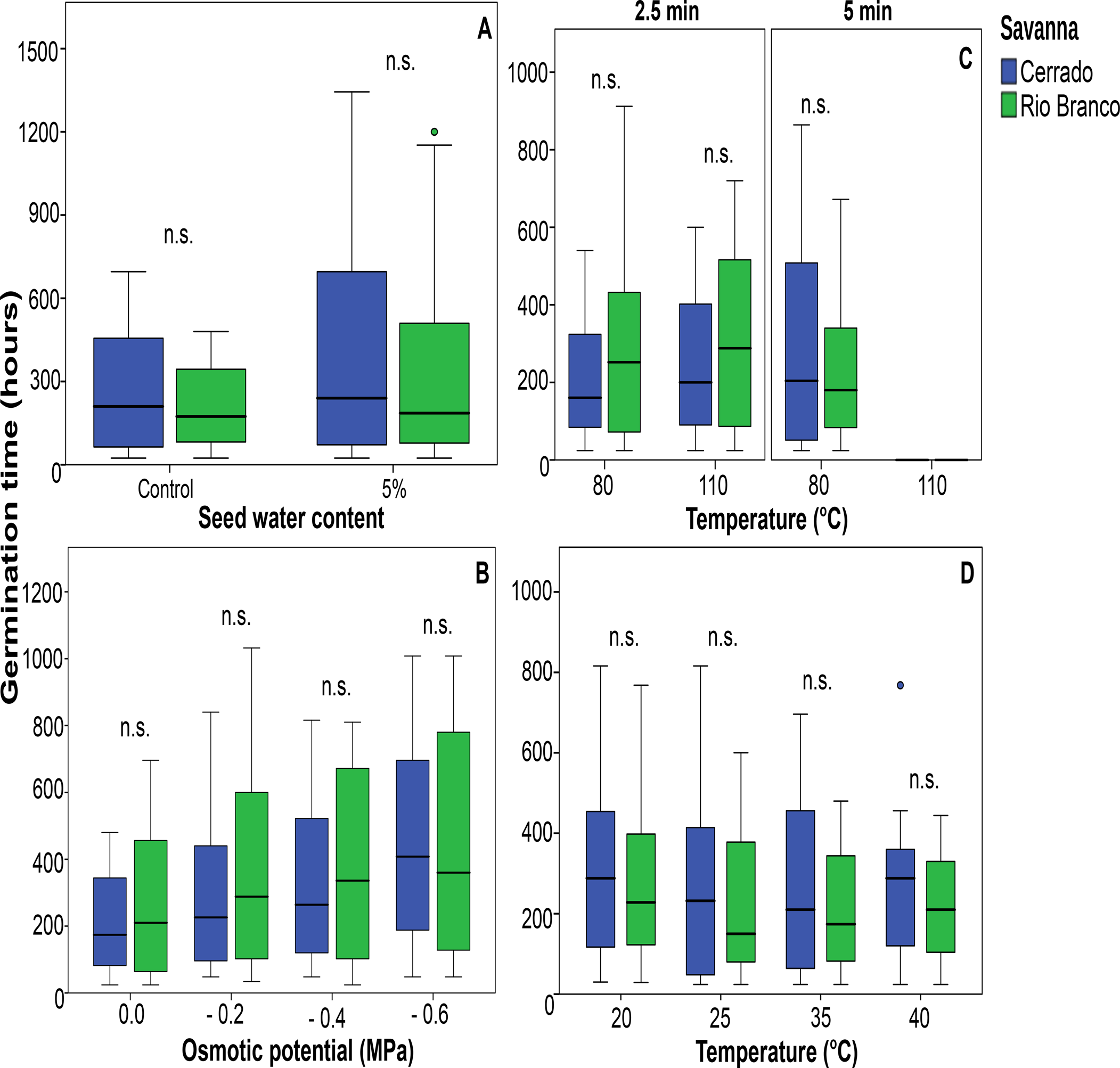
Fig. 3. Effects of desiccation (A), water deficit (B), heat shock (C) and temperature (D) on the germination rate of populations of trees from Cerrado and Rio Branco savannas, Brazil (*P < 0.05; **P < 0.01; n.s., not significant).
Table 3. GLMMs of morphological and biophysical seed traits for conspecific and congeneric species from Cerrado and Rio Branco savannas, Brazil
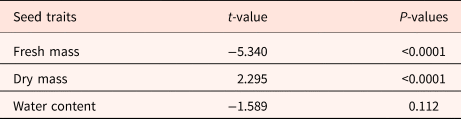
Physiological traits and treatments
Water deficit
Seeds collected from species occurring in both Cerrado and Rio Branco savannas showed similar germination percentages at OPs ranging from zero (control) to −0.4 MPa (Fig. 2B). However, seeds from Rio Branco showed statistically higher germination percentages at lower water potentials than seeds from Cerrado (Fig. 2B). No germination was observed at −1.2 MPa, suggesting that for both savannas this treatment would represent the limiting water potential for seed germination. For the germination percentage, the best-fitting model selected took into account the factors collection site (S) and OP, as well as their interaction (S × OP). However, only OP (Z = −9.212; P < 0.0001) and the interaction S × OP (Z = 4.226; P < 0.0001) were significant within the model.
More negative OP did not lead to significant increases in germination times of seeds from Cerrado and Rio Branco savannas (Fig. 3B). The germination times were very similar within OPs ranging from zero (control) to −0.6 MPa (Fig. 3B). Germination times at OPs more negative than −0.8 MPa were not calculated because the germination percentages in these treatments were below 10% for Cerrado species (see Fig. 2B). For the trait germination time, the best-fitting model selected took into account the factors S, OP as well as their interactions (S × OP). However, only S (t = 2.390; P < 0.05) and OP (t = 5.111; P < 0.0001) were significant within the model.
Heat shock
Seeds from both savannas tolerated heat shocks of 80°C, regardless of the exposure time (Fig. 2C). In other words, no significant differences were observed comparing seeds subjected to 80°C and control, within and between savannas. However, higher temperatures for longer times led to a significant reduction in germination percentages, these results being savanna-dependent. Heat shocks of 110 and 140°C for 2.5 min of exposure were more harmful to seeds from Cerrado than those from Rio Branco (Fig. 2C). When the exposure time was increased to 5.0 min, seeds from Cerrado showed a slightly higher tolerance to heat shock than those from Rio Branco (Fig. 2C). Heat shock of 170°C significantly reduced the germination percentages and was lethal for several seeds from Cerrado and Rio Branco (Fig. 2C). No seeds germinated after heat shock of 200°C (data not shown). For the germination percentages, the factors site of seed collection (S), temperature (Tp) and time (Ti), as well as their interactions were all significant within the best-fitting model selected, being S (Statistic = −3.002; P < 0.01), Tp (Z = −4.932; P < 0.0001), Ti (Z = −2.454; P < 0.05), S × Tp (Z = 6.110; P < 0.0001), S × Ti (Z = 4.428; P < 0.0001), Tp × Ti (Z = 3.473; P < 0.001) and S × Tp × Ti (Z = −5.900; P < 0.001).
Heat shock treatments did not significantly affect the germination times for seeds of Cerrado and Rio Branco savannas (Fig. 3C). Germination times after heat shocks of 140 and 170°C for 2.5 min were not calculated because the germination percentages were below 10% for Cerrado seeds (see Fig. 2C). Also, the germination times were not calculated for heat shocks of 110, 140 and 170°C for 5 min for both Cerrado and Rio Branco seeds for the same reason (see Fig. 2C). For the trait germination time, the best-fitting model selected took into account only the factor time (t = 11.027; P < 0.0001).
Temperature
There was no significant difference in the germination percentages at 20, 25 and 30°C when comparing species from Rio Branco and Cerrado (Fig. 2D). However, at 35 and 40°C, seeds from Rio Branco had statistically higher germination percentages than those from Cerrado (Fig. 2D). No seed germinated at 45°C, irrespective of seed origin. For the germination percentages, the best-fitting model selected took into account the factors collection site (S) and temperature (Tp), as well as their interaction (S × Tp). However, only Tp (Z = −7.225; P < 0.0001) and the interaction of S × Tp (Z = 2.409; P < 0.05) were significant within the model.
The increasing temperature did not affect the germination times when comparing seeds from Cerrado and Rio Branco (Fig. 3D). The germination time was not calculated for 40°C because the germination percentage in that treatment was below 10% for Cerrado species (see Fig. 2D). For this trait, the best-fitting model selected took into account the factors S (t = −8.558; P < 0.0001) and Tp (t = −4.052; P < 0.0001).
Seed viability
The tetrazolium test revealed zero viability among the non-germinated seeds subjected to any of the treatments and less than 10% of viability among those non-germinated seeds in their respective controls. These results indicate that viability and germination were substantially equivalent, regardless of the treatment applied. In other words, if a seed failed to germinate during or after any of the treatments, it was because they had lost viability, not because they were dormant.
Discussion
In this paper, we tested the role of environmental filters and seed traits on seed stress–tolerance of congeneric and conspecific tree species from Cerrado and Rio Branco savannas.
We found that (i) the germination time was a better parameter to explain seed stress–tolerance than seed mass, water content and desiccation tolerance (Prediction 1); (ii) seed origin and environmental filters were better predictors of seed tolerance to stress than seed mass and water content (Prediction 2); (iii) seeds from Rio Branco showed higher tolerance to water deficit, heat shock, low-severity heat shock and high temperatures than seeds from Cerrado (Prediction 3).
Morphological and biophysical traits
Since the factors seed mass and water content were not significant in the models for desiccation, water deficit, heat shock and temperature treatments, they do not predict stress–tolerance when comparing congeneric and conspecific pairs subjected to different environmental conditions.
Seed mass greatly differed among species (Table 2), but the differences vanished when the masses were averaged for comparison between the Cerrado and Rio Branco savannas (Fig. 1). Since the seeds from Rio Branco species were shown to be more tolerant to the stress treatments than those from Cerrado species, our results suggest that regional environmental characteristics are a better determinant of seed tolerance (and, possibly, seed persistence in soil) than seed mass (Leishman and Westoby, Reference Leishman and Westoby1994; Leishman et al., Reference Leishman, Wright, Moles, Westoby and Fenner2000; Baraloto et al., Reference Baraloto, Forget and Goldberg2005; Moles et al., Reference Moles, Ackerly, Webb, Tweddle, Dickie and Westoby2005; Rees and Venable, Reference Rees and Venable2007; Muller-Landau, Reference Muller-Landau2010).
All the species selected for this study disperse seeds at the end of the dry season (see Table 2), and our study shows that their SWC does not differ when comparing congeneric and conspecific pairs occurring in the different regions of seed sampling (Fig. 1). The SWC of savanna species seems to be more related to the period of seed dispersal than to the region of occurrence, and previous studies with savanna seeds show that it is usually lower among seeds dispersed during the dry season than during the wet season (Gottsberger and Silberbauer-Gottsberger, Reference Gottsberger and Silberbauer-Gottsberger2006).
At the conclusion of their development, seeds of most species have a water content ranging from 5 to 20% of their total mass (Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003). Our study showed that seeds from both savanna regions have water content within this range, as already reported for seeds of other savanna species (Sales et al., Reference Sales, Pérez-García and Silveira2013; Ribeiro and Borghetti, Reference Ribeiro and Borghetti2014). Seeds with a lower water content are prone to exhibit higher desiccation tolerance than those with a higher water content (Pammenter and Berjak, Reference Pammenter and Berjak2000; Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003; Pritchard et al., Reference Pritchard, Daws, Fletcher, Gaméné, Msanga and Omondi2004; Ribeiro and Borghetti, Reference Ribeiro and Borghetti2014), and this pattern was also observed among the species selected for this study (Fig. 2A).
Physiological trait and treatments
Water deficit
Edaphic factors are major determinants of recruitment when discussing the effects of water deficit on seed survival and germination under field conditions (Schütz et al., Reference Schütz, Milberg and Lamont2002; Kos and Poschlod, Reference Kos and Poschlod2008). For example, in a condition of water shortage, sandy soils will retain less water and dry faster than clayish soils (Fidalski et al., Reference Fidalski, Tormena, Alves and Auler2013). Considering that seed banks in savannas tend to be formed close to the soil surface (Scott et al., Reference Scott, Setterfield, Douglas and Andersen2010; Salazar et al., Reference Salazar, Goldstein, Franco and Miralles-Wilhelm2011; Andrade and Miranda, Reference Andrade and Miranda2014), it is reasonable to expect that seeds occurring in sandy soils will face more negative water potentials and, therefore, will be selected for higher tolerance to water deficit than those occurring in clayish soils. Our results show that seeds of Rio Branco species (sandy soil) had higher germination percentages at lower OPs (Fig. 2B) than those from Cerrado species (clayish soil).
Heat shock
Our study revealed that the germination of seeds exposed to 80°C was barely affected, corroborating that heat shocks at temperatures up to 80°C have a minimal impact on seed viability, irrespective of the species’ habitat (Escudero et al., Reference Escudero, Nuñez and Pérez-García2000; Hanley and Lamont, Reference Hanley and Lamont2000; Thomas et al., Reference Thomas, Morris and Auld2007; Ribeiro and Borghetti, Reference Ribeiro and Borghetti2014; Ribeiro et al., Reference Ribeiro, Pedrosa and Borghetti2013, Reference Ribeiro, Barbosa, van Langevelde and Borghetti2015). However, high temperatures combined with long times of exposure can lead to a reduction of the germination percentages, depending on the environmental characteristics of species’ habitat. The effects of high temperatures generated during a fire on the soil seed banks can vary greatly among different ecosystems depending on the severity (a combination of fireline intensity and duration), fire-return interval (Keeley, Reference Keeley2009; Pivello et al., Reference Pivello, Oliveras, Miranda, Haridasan, Sato and Meirelles2010), fine-fuel amount (Kauffman et al., Reference Kauffman, Cummings and Ward1994; Barbosa and Fearnside, Reference Barbosa and Fearnside2005a) and soil properties (DeBano et al., Reference DeBano, Neary and Ffolliott1998). Fire temperatures in Brazilian savannas can reach 350°C at the soil level, and heat pulses can vary from 1 to 3 min (Miranda et al., Reference Miranda, Sato, Neto, Aires and Cochrane2009). Although it rarely occurs, the 5-min exposure time was included as a treatment representing an extreme condition that could be reached in the case of an intense fire in Brazilian savannas.
In our study, we observed that combining high temperatures with short times of exposure (a low-severity fire) was more damaging to seeds of Cerrado than to seeds of Rio Branco species, while combining low temperatures with long times of exposure (a medium-severity fire) was more damaging to seeds of Rio Branco than to seeds of Cerrado species. However, combining high temperatures with long times of exposure was harmful to seeds from Cerrado and Rio Branco species. According to our findings, environmental factors such as soil properties (e.g. texture and thermal conductivity) and amount of fine-fuel biomass (available for burning) emerge as major factors explaining seed tolerance to heat shock among species from Cerrado and Rio Branco.
Temperature
The germination percentages of tropical savanna species are usually reduced by temperatures of 35°C and above (Tambelini and Perez, Reference Tambelini and Perez1999; Sy et al., Reference Sy, Grouzis and Danthu2001; Ribeiro and Borghetti, Reference Ribeiro and Borghetti2014; Borghetti et al., Reference Borghetti, Caetano, Colli, Françoso and Sinervo2021). Our findings corroborated this pattern and also revealed that seeds from Cerrado were more sensitive to higher temperatures than seeds from Rio Branco. Once again, these differences between the population response and temperature seem to be related to their respective regional climates. Interestingly, differences in germination traits explained by environmental gradients (moisture and temperature) were already reported for congeneric and conspecific pairs occurring in other neotropical savannas (Ranieri et al., Reference Ranieri, Lana, Negreiros, Araújo and Fernandes2003; Garcia et al., Reference Garcia, Jacobi and Ribeiro2007; Sales et al., Reference Sales, Pérez-García and Silveira2013). Taken together, our study shows that soil properties are better determinants of seed tolerance to water stress, high temperatures and heat shocks than the vegetation cover itself, although it is likely that the latter also determines microclimate properties of the former.
Germination time
We found that desiccation, water deficit and heat shock did not affect the germination times of Cerrado and Rio Branco seeds (Fig. 3). Even treatments of increasing temperatures (where an increase in the speed of germination would be expected due to the kinetic effect of temperature on physiological processes) were unable to affect the times of germination of the studied species (Fig. 3D).
Previous studies have reported that for tropical species, the germination times represent a phylogenetically conserved trait (Norden et al., Reference Norden, Daws and Antoine2009), and our study shows that the treatments had a minimal impact on this parameter (Fig. 3). The range of values recorded for the germination times (always above 300 h and frequently above 400 h – Fig. 3) reveals a slow germination pattern, which may represent a strategy of species growing in habitats subject to an unpredictable climate such as the tropical savannas. Also recognized as a bet-hedging strategy, this germination pattern has been reported as an important mechanism for seed persistence under variable environments (Clauss and Venable, Reference Clauss and Venable2000; Simons and Johnston, Reference Simons and Johnston2006; Ooi et al., Reference Ooi, Auld and Denham2009; Ooi, Reference Ooi2012; Tielbörger et al., Reference Tielbörger, Petru and Lampei2012; Gremer and Venable, Reference Gremer and Venable2014).
Conclusions
Our results showed that for conspecific and congeneric populations of widespread neotropical savanna trees: (a) environmental characteristics are better determinants of stress–tolerance (and seed persistence in soil) than innate seed traits; (b) the germination time is a better parameter to explain seed stress–tolerance than seed mass, water content and desiccation tolerance, and represents a conservative trait at the population level and (c) seeds from Rio Branco savannas display a higher tolerance to stress than those from Cerrado savannas. Finally, our results suggest that the intensification of stress conditions as predicted by climate change models will very likely act more strongly on seed recruitment in Cerrado than in Rio Branco savannas.
Acknowledgements
The authors thank Stuart Klorfline for reviewing the language.
Financial support
This work was supported by the Coordination for the Improvement of Higher Education – CAPES (CAPES/NUFFIC grant no. 019/2010, CAPES/PNADB grant no. 451/2010); the National Council for Scientific and Technological Development – CNPq (grant no. 476297/2004-4 and grant no. 312152/2018-3); the University of Brasília, through their Deanship of Research and Post-Graduation, for financial support.
Conflicts of interest
The author(s) declare none.