Introduction
A number of studies have shown how variation in the germination requirements between species may represent adaptation to the local habitat conditions. Baskin et al. (Reference Baskin, Baskin and Chester1999) suggested that the wide range of habitat preferences amongst Leptochloa species might be explained by species differences in dormancy and germination behaviour. Schutz (Reference Schutz1997) also reported marked differences in germination requirements between forest and open-habitat sedges. In a subsequent study of 32 temperate Carex species, Schutz and Rave (Reference Schutz and Rave1999) found that although broadly similar germination patterns were evident, species differences in germination requirements were related to habitat preference. Evidence for a similar pattern of behaviour between related species of Papaver but with differences in germination preference and depth of dormancy was reported by Karlsson and Milberg (Reference Karlsson and Milberg2007). The same authors have also recently reported a similar pattern of behaviour in four annual Lamium species and suggested that local adaptations arise through differences in dormancy strength (Karlsson and Milberg, Reference Karlsson and Milberg2008). Habitat-related differences in germination behaviour have also been reported amongst Rumex species (Van Assche et al., Reference Van Assche, Van Nerum and Darius2002) and Vandelook et al. (Reference Vandelook, Van de Moer and Van Assche2008) have shown that subtle differences in germination requirements amongst four species of Caryophyllaceae correlate with habitat preferences.
Anemone ranunculoides L. ssp. ranunculoides is a native European woodland geophyte (Tutin et al., Reference Tutin, Heywood, Burges, Valentine, Walters and Webb1964; Hulthén and Fries, Reference Hulthén and Fries1986). While often present in the same woodland habitats as the closely related A. nemorosa L., A. ranunculoides has a more restricted geographical distribution and habitat preference. In northern Italy these two species often grow together in shady and damp deciduous woodlands in the lowland (Po plain) and in the mountains (northern Apennines and Alps), from 0 to 1500 m above sea level (asl) (Abrami, Reference Abrami1971; Pignatti, Reference Pignatti1982). Although quite common in the Alps and Apennines (Pignatti, Reference Pignatti1982; Aeschimann et al., Reference Aeschimann, Lauber, Moser and Theurillat2004), A. ranunculoides is rarer in the Po plain (Macchi, Reference Macchi2005; Bonali et al., Reference Bonali, D'Auria, Ferrari and Giordana2006) compared with A. nemorosa. On a European scale A. ranunculoides is again less common than A. nemorosa, and it is rarely found in the Mediterranean and Atlantic regions (Tutin et al., Reference Tutin, Heywood, Burges, Valentine, Walters and Webb1964; Jalas and Suominen, Reference Jalas and Suominen1989). Both species grow in deciduous woodlands (Querco-Fagetea) (Oberdorfer, Reference Oberdorfer1994); however, A. nemorosa also occurs in a range of other communities such as hedgerows, heathlands, grasslands and meadows (Bothmer et al., Reference Bothmer, Engstrand, Gustafsson, Persson, Snogerup and Bentzer1971; Pigott, Reference Pigott1982; Shirreffs, Reference Shirreffs1985; Grime et al., Reference Grime, Hodgson and Hunt1988). A. ranunculoides is a shade-demanding species (Ellenberg, Reference Ellenberg1974, Reference Ellenberg1988) whereas A. nemorosa is shade tolerant but also grows successfully in open areas (350–50,000 lux) (Canullo, Reference Canullo1985).
A recent comparative study of the germination and emergence phenology of lowland and mountain populations of A. nemorosa from northern Italy revealed that radicles emerge during the autumn following a period of embryo growth in the summer (Mondoni et al., Reference Mondoni, Probert, Rossi, Hay and Bonomi2008). Under natural conditions, shoot emergence was delayed until temperatures had decreased further; laboratory tests confirmed the presence of epicotyl dormancy and that a period of cold stratification was required to trigger shoot growth.
As far as we are aware, there have been no previous studies of seed germination behaviour in A. ranunculoides. Because this species can often be found growing alongside A. nemorosa, we examined the hypothesis that A. ranunculoides would display a similar pattern of dormancy loss and germination preference to A. nemorosa but that subtle differences in behaviour might explain its narrower habitat tolerance.
Materials and methods
Seed collection
Collections of achenes (hereafter referred to as seeds) were made at the time of natural dispersal (Baskin and Baskin, Reference Baskin and Baskin1998; Hay and Smith, Reference Hay, Smith, Smith, Dickie, Linington, Pritchard and Probert2003), 5 May 2006, from a lowland population of A. ranunculoides growing in the Ticino Natural Park (Po plain; c. 79 m asl), northern Italy.
Phenology of embryo growth and of radicle and shoot emergence in the wild
At the time of collection, 25 fine-mesh polyester bags with 50 seeds each were buried approximately 5 cm under leaf litter at the collection site. Sachets were retrieved at intervals of 30 d from May to September and then at weekly intervals until mid-January. Embryo growth, radicle emergence and shoot emergence were monitored throughout. Soil temperature at the level of the sachets was recorded at hourly intervals using Tiny Tag data loggers (Gemini Data Logger Ltd, Chichester, Sussex, UK).
Phenology of embryo growth and of radicle and shoot emergence in the laboratory
All laboratory experiments involved sowing three replicates of 50 seeds each on 1% distilled water agar held in 9-cm diameter Petri dishes. Treatments were incubated in temperature- and light-controlled incubators using a 12-h daily photoperiod (photosynthetically active radiation 40–50 μmol m− 2 s− 1).
On the day of harvest, seeds were placed into a temperature regime simulating seasonal changes occurring in the west Po plain (Mariani et al., Reference Mariani, Paolillo and Rasio2001). Summer conditions (May to September) were simulated by 150 d at 20°C; early autumn (October) by 30 d at 15°C; late autumn (November) by 30 d at 10°C; winter (December to February) by 90 d at 4°C; early spring (March) by 30 d at 10°C; and late spring (April) by 30 d at 15°C.
On the same day, 20 seeds were dissected (Kondo et al., Reference Kondo, Miura, Okubo, Shimada, Baskin and Baskin2004; Toshikazu et al., Reference Toshikazu, Yoshiji and Eriko2004) and the embryo length measured under a binocular microscope equipped with a micrometer. Observations on embryo growth and on root and shoot emergence were made every 30 d during summer and then at 5-d intervals until the end of the simulated cycle.
Constant temperatures were used because the forest cover and surface leaf litter are effective insulators of daily temperature variations (Ellenberg, Reference Ellenberg1988; Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002; Mondoni et al., Reference Mondoni, Probert, Rossi, Hay and Bonomi2008) and because it was assumed that seeds would be more responsive to seasonal rather than diurnal temperature variations. However, additional germination tests were set up to investigate the effect of diurnal alternating temperatures. The following regimes were used: 150 d at 25/15°C (summer) followed by 30 d at 20/10°C (early autumn), 30 d at 15/5°C (late autumn), 90 d at 4°C (winter), 30 d at 15/5°C (early spring) and 30 d at 20/10°C (late spring). In each case a 12 /12 h thermoperiod was used and illumination was provided during the warm phase.
Controls were also set up to investigate the effects of prolonged incubation at each of the mean seasonal constant temperatures (4, 10, 15 and 20°C). Seeds were checked for root and shoot emergence at regular intervals over 360 d.
Effects of summer and autumn conditions on root emergence
To examine the importance of summer conditions, samples of seeds were held at 20°C for 0, 30, 60, 90, 120 and 150 d. After each period, seeds were moved to autumn and then winter conditions. The importance of autumn conditions was examined by placing seeds into simulated summer conditions (150 d at 20°C) and then moving them to the other seasonal conditions, skipping the early autumn (15°C) treatment, the late autumn (10°C) treatment, or both. For each test the number of seeds and replications, method of sowing, light conditions and observations were as described above.
The importance of winter for shoot emergence
The aim of this experiment was to investigate whether cold stratification may affect shoot emergence. After the summer and autumn phases of the seasonal sequence, seeds with emerged radicles were given 0, 30, 60 or 90 d of cold stratification (4°C) before transfer to early spring (30 d at 10°C) followed by late spring conditions (30 d at 15°C). The number of seeds and replications for each test and other details were the same as before.
Data analysis
Embryo growth data were analysed using linear regression analysis in Minitab 14 (Minitab Inc., State College, Pennsylvania, USA) on subsets of the data. To describe the progress of germination in the laboratory, the Gompertz function (Brown and Mayer, Reference Brown and Mayer1988) was fitted to cumulative germination data using Sigma Plot 7, according to the following formula:
where y = germination percentage at time x (d), a = final germination, x0 = germination delay and b = germination rate. In addition, χ2 tests (maximum likelihood) were carried out in Genstat 11 (VSN International Ltd., Hemel Hempstead, Herts, UK) to compare the final proportions of germinated seeds under different conditions.
Results
Phenology of root and shoot emergence under natural conditions
Embryos of seeds buried in leaf litter at the collection site grew continuously during the summer (May–September; data not shown) but radicle emergence did not occur until mid-October when soil surface temperature had dropped to c. 15°C (Fig. 1). The radicle emerged from almost 60% of the seeds within 2 weeks at this temperature, while it emerged from the remaining 40% of the seeds during the next 3 weeks when the temperature had dropped to c. 10°C. Shoot emergence did not commence until mid-November but then proceeded at a similar rate, with c. 95% shoot emergence in 4 weeks, despite the temperature having dropped further to c. 5°C (Fig. 1).

Figure 1 Radicle (Rr) and shoot (Rs) emergence from seeds of A. ranunculoides in the wild. Also shown is the mean daily soil temperature (°C) calculated from measurements made at hourly intervals at the study site.
Phenology of embryo growth and of root and shoot emergence in the laboratory
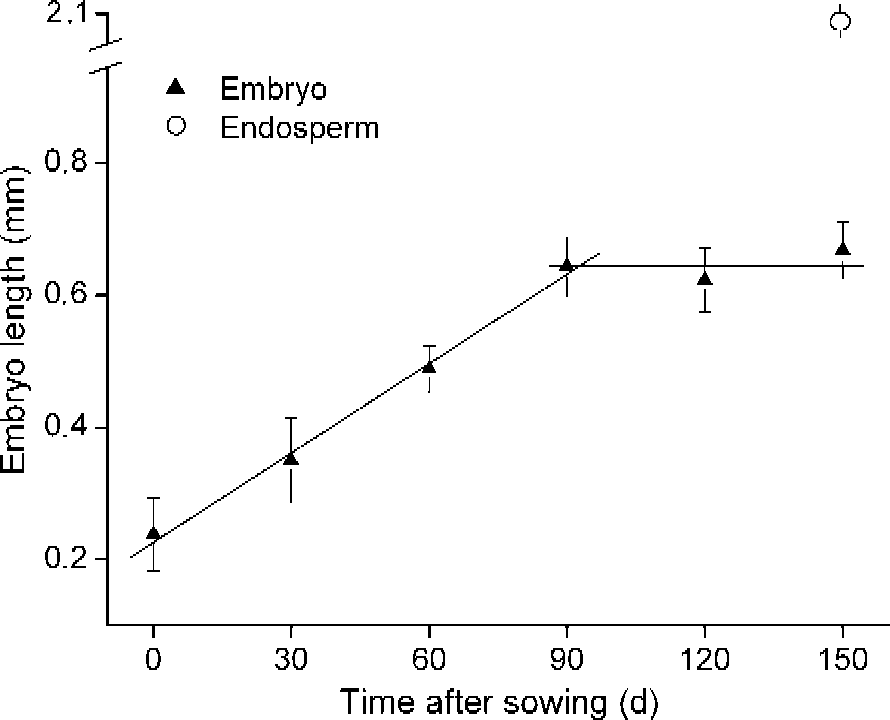
Embryos of A. ranunculoides, undifferentiated at the time of dispersal, grew at a constant rate under continuous, simulated summer conditions in the laboratory (20°C) from about 0.2 to 0.67 mm after 90 d on agar, when they occupied approximately 32% of the whole endosperm length (2.1 mm; Fig. 2). There was no further significant embryo growth after this point prior to radicle emergence.
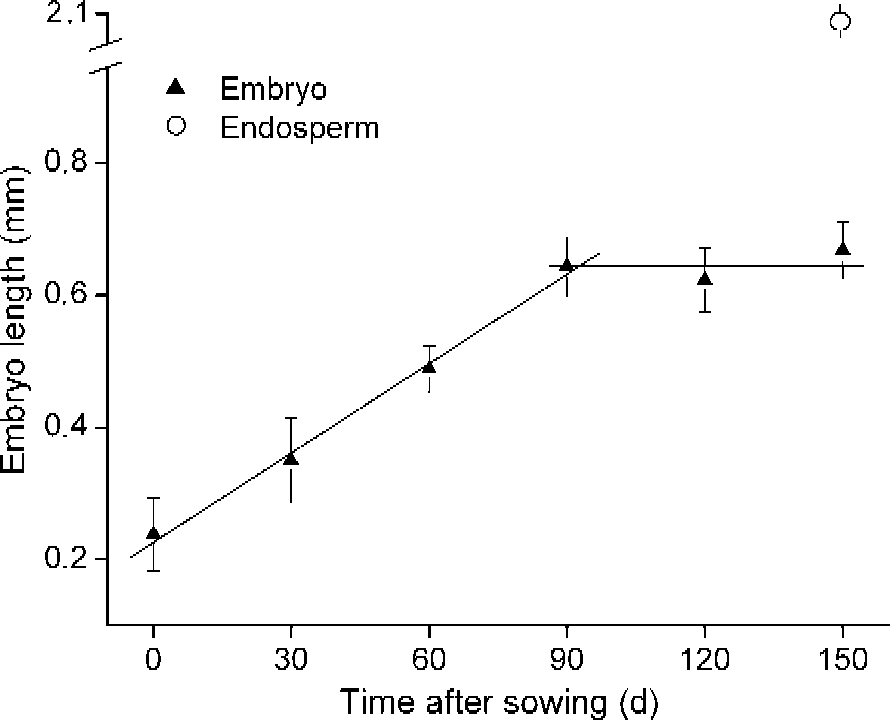
Figure 2 Linear regressions of embryo growth of A. ranunculoides in the laboratory at simulated summer conditions (20°C). Bars are ± SE. Also shown is the length of the mucilaginous endosperm (open circle), within which the embryo develops.
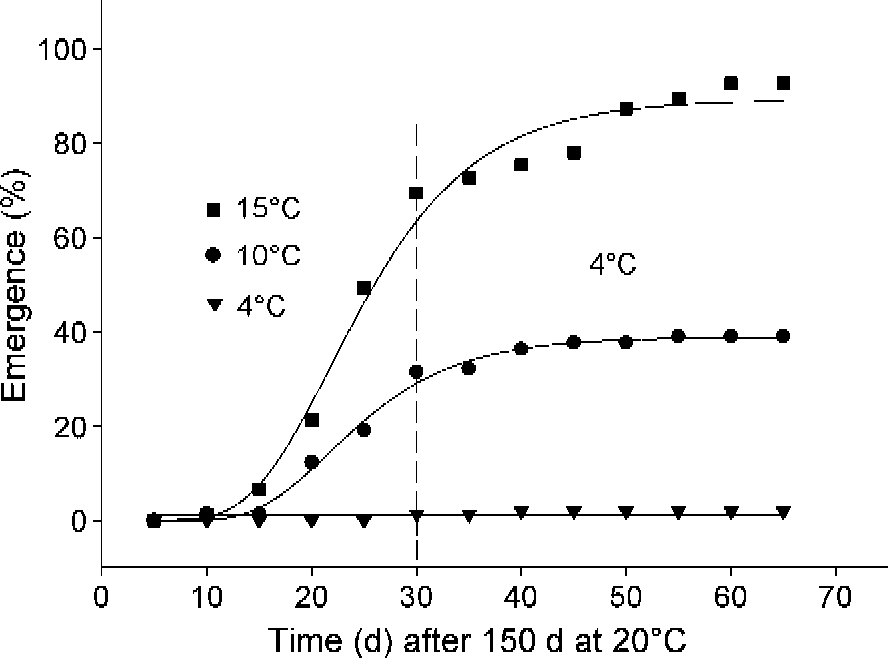
Under simulated seasonal temperatures in the laboratory about c. 94% of radicles of A. ranunculoides had emerged after seeds were transferred from summer conditions (150 d at 20°C) to the early autumn condition (15°C). However, shoot emergence was delayed until seeds were moved to winter conditions (4°C; Fig. 3).

Figure 3 Germination progress curves for seeds of A. ranunculoides at simulated seasonal temperatures. Radicle (closed symbols) and shoot (open symbols) emergence after summer conditions (150 d at 20°C). Curves were fitted using the Gompertz function.
Effects of summer condition on root emergence
In the absence of summer conditions, there was no germination of A. ranunculoides seeds. For seeds given 30 d of summer conditions, only c. 57% germinated after they had been moved through autumn conditions (30 d at 15°C followed by 30 d at 10°C) and under winter conditions (4°C) for 90 d (Fig. 4). Although the difference in the final germination after 30 or 60 d of summer conditions was not significant (χ2(1df) = 0.01, P = 0.907), germination was faster after 60 d of summer conditions. There was 53% radicle emergence after 45 d of autumn conditions for seeds given 60 d of summer conditions, compared with 37% for seeds given 30 d of summer. Increasing the length of simulated summer conditions to 90, 120 or 150 d resulted in further increases in both the total percentage of seeds germinating and the rate of germination, while the time before germination was first observed decreased (Fig. 4).

Figure 4 Germination progress curves for seeds of A. ranunculoides at simulated seasonal autumn (15 and 10°C) and winter (4°C) temperatures, after increasing durations of summer conditions (0, 30, 60, 90, 120 and 150 d at 20°C, as indicated). Curves were fitted using the Gompertz function.
Effects of autumn condition on root emergence
After 150 d of summer conditions, 63% of the seeds had an emerged radicle after 30 d at 15°C and 92% by the time seeds were transferred to winter conditions (4°C), with no more radicle emergence after this time. In contrast, when summer-treated seeds were placed under late autumn conditions (10°C), only low levels of radicle emergence occurred (c. 30%; Fig. 5). When seeds were placed directly into winter (4°C), there was no radicle emergence.
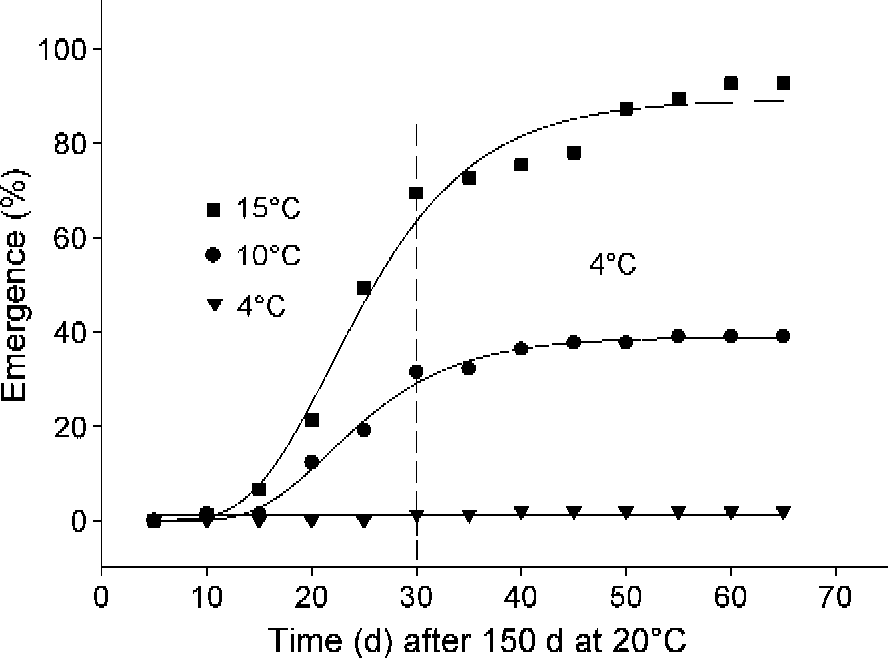
Figure 5 Germination progress curves for seeds of A. ranunculoides at simulated seasonal temperatures given 150 d of summer pre-treatment at 20°C and then incubated at temperatures of early autumn (15°C) and winter (4°C) (squares), late autumn (10°C) and winter (4°C) (circles) and winter (4°C) (triangles). Curves were fitting using the Gompertz function.
The importance of cold stratification for shoot emergence
Differences in final shoot emergence of seeds given 0, 30, 60 or 90 d of winter conditions were not significant (χ2(2df) = 1.93, P = 0.380). Almost complete shoot emergence was observed in A. ranunculoides even in the absence of winter conditions (Fig. 6), with 95% of shoots emerging by the time seeds were transferred to the simulated late spring condition (15°C). However, shoot emergence did not occur in the absence of cooler autumn conditions (10°C).

Figure 6 Radicle and shoot emergence progress curves for seeds of A. ranunculoides at simulated seasonal temperatures in the absence of winter conditions. Curves were fitted using the Gompertz function.
The effect of diurnal alternating temperatures
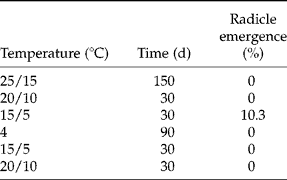
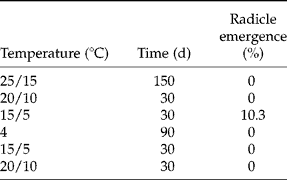
In the simulated regime of diurnal alternating temperatures, no seeds had emerged radicles at 25/15 or 20/10°C and only c. 10% had emerged radicles when seeds were transferred to late autumn conditions (15/5°C) (Table 1).
Table 1 Radicle emergence (%) of seeds of A. ranunculoides under simulated seasons using diurnal alternating temperature cycles (except for winter). Seeds were moved progressively through the seasons with germination scored at each step
Continuous constant temperature controls
No seeds germinated at continuous 5, 10 and 20°C. After 80 d at 15°C, however, seeds began to germinate slowly, with 97% radicle emergence recorded after a further 80 d (data not shown). Shoot emergence did not occur at continuous 15°C.
Discussion
Under natural conditions, the pattern of radicle and shoot emergence phenology in seeds of A. ranunculoides was remarkably similar to that recently reported for seeds from the closely related species A. nemorosa from identical low-altitude woodland habitats (Mondoni et al., Reference Mondoni, Probert, Rossi, Hay and Bonomi2008). Like A. nemorosa, differentiation and growth of A. ranunculoides embryos began immediately after seed dispersal and continued during the summer, culminating in radicle emergence and then shoot emergence when temperatures fell in the autumn.
Although there is clear evidence that shoot emergence under natural conditions is delayed until temperatures fall to late autumn values, c. 80% of shoots had emerged before temperatures had dropped below 10°C (Fig. 1). By contrast, in all three populations of A. nemorosa studied by Mondoni et al. (Reference Mondoni, Probert, Rossi, Hay and Bonomi2008), the same level of shoot emergence was not observed until temperatures had dropped well below 10°C. This subtle, but significant difference in temperature response between these two related species was further revealed in laboratory experiments.
Ali et al. (Reference Ali, Probert, Hay, Davies and Stuppy2007) and Mondoni et al. (Reference Mondoni, Probert, Rossi, Hay and Bonomi2008) concluded that radicles of A. nemorosa should be regarded as non-dormant because they grew continuously with no evidence of developmental arrest prior to emergence as temperatures fell in the autumn. Here we have clear evidence of arrest in embryo growth in A. ranunculoides after about 90 d under simulated summer conditions (Fig. 2). Compared with A. nemorosa, seeds of A. ranunculoides also appear to require more prolonged exposure to summer conditions prior to germination. Under simulated seasonal temperatures, radicle emergence occurred in the autumn, but high levels of germination were only recorded after 150 d of summer conditions (Fig. 4). In seeds of A. nemorosa, just 30 d of summer conditions were enough to elicit high levels of radicle emergence when they were transferred to simulated autumn temperatures (Mondoni et al., Reference Mondoni, Probert, Rossi, Hay and Bonomi2008). Moreover, unlike seeds from three populations of A. nemorosa from identical woodland habitats that were capable of radicle emergence at simulated early autumn, late autumn and winter temperatures, seeds of A. ranunculoides appeared to have a much narrower temperature range for germination, giving high levels of radicle emergence only when seeds were incubated at early autumn temperatures (Fig. 5).
Ellenberg (Reference Ellenberg1974) ascribed so-called indicator values for six ecological factors, including, for example, light and temperature, to the native flora of central Europe. A species given a low value occurs mainly where the factor is less pronounced; species could also be defined as indifferent. In our study the narrower temperature tolerance of A. ranunculoides seeds compared with that of A. nemorosa was reflected in the lower Ellenberg index for temperature, based on the mean annual temperatures occurring in the growing areas of the two species in central Europe.
Kos and Poschlod (Reference Kos and Poschlod2007) have recently shown that the germination of canopy-associated species was inhibited by high-amplitude, diurnal-alternating temperatures typical of matrix (open) sites (Ellenberg, Reference Ellenberg1988). Although this study was based on species from xeric Kalahari savannah, Kos and Poschlod (Reference Kos and Poschlod2007) argue that mechanisms that serve to detect canopy shade, based on sensitivity to the amplitude of diurnal temperatures, might be widespread, especially in bird-dispersed, fleshy-fruited species.
Although seeds of A. ranunculoides are neither bird-dispersed nor fleshy-fruited, very few seeds were able to germinate under a regime of diurnal temperature alternation (Table 1). By contrast, unpublished data from our laboratory showed that seeds of A. nemorosa were capable of c. 90% germination under the same conditions. Although further work is needed, this might help to explain why A. ranunculoides is restricted to closed-canopy sites and why A. nemorosa can be found in both open- and closed-canopy habitats.
Our laboratory experiments reveal that radicles of A. ranunculoides clearly have more stringent germination requirements than those of A. nemorosa. Despite the fact that germination of A. ranunculoides is possible at a constant temperature of 15°C, the arrest of embryo growth after 90 days at summer temperatures (Fig. 1), combined with the requirement for additional time at these temperatures in order to elicit high levels of germination (Fig. 4), leads us to conclude that the radicle should be regarded as dormant.
Mondoni et al. (Reference Mondoni, Probert, Rossi, Hay and Bonomi2008) reported clear evidence that shoots of A. nemorosa possess non-deep epicotyl morphophysiological dormancy (sensu Baskin and Baskin, Reference Baskin and Baskin1998). In that study, laboratory experiments confirmed that shoots required a period of cold stratification at winter temperatures or application of GA3 (to substitute for cold stratification). Here we have shown that shoot emergence in seeds of A. ranunculoides is not dependent on exposure to winter temperatures. Supporting the field data, laboratory tests showed that although shoot emergence is possible at winter temperatures (Fig. 3), it can also occur in the absence of winter (Fig. 6), but not in the absence of the cooler autumn temperature (10°C) and thus the level of epicotyl dormancy in seeds A. ranunculoides is clearly much weaker than that expressed in seeds of A. nemorosa from the same habitat. The general pattern of germination and emergence phenology in A. ranunculoides under natural conditions is more or less identical to that of populations of A. nemorosa occupying the same woodland habitats in northern Italy. However, seeds of A. ranunculoides complete germination and shoot emergence in the autumn, whereas those of A. nemorosa do so during the winter.
Other papers have described similar patterns of behaviour between species in a number of genera across a range of plant families and attributed subtle differences in depth of dormancy or response to specific factors to habitat preference (Schutz, Reference Schutz1997; Baskin et al., Reference Baskin, Baskin and Chester1999; Schutz and Rave, Reference Schutz and Rave1999; Daws et al., Reference Daws, Burslem, Crabtree, Kirkman, Mullins and Dalling2002; Van Assche et al., Reference Van Assche, Van Nerum and Darius2002; Karlsson and Milberg, Reference Karlsson and Milberg2007, Reference Karlsson and Milberg2008; Vandelook et al., Reference Vandelook, Van de Moer and Van Assche2008). Moreover, in a comparative study of germination and emergence phenology in three lowland and one mountain population of A. nemorosa, Mondoni et al. (Reference Mondoni, Probert, Rossi, Hay and Bonomi2008) reported significant differences in germination behaviour and clear evidence that the timing of germination and emergence was well adapted to the climatic differences between the two sites. It is therefore tempting to speculate that the subtle differences in dormancy and germination behaviour between A. ranunculoides and A. nemorosa growing in the same woodlands might explain the more restricted ecological distribution of A. ranunculoides.
High levels of germination and seedling recruitment in the wild have been reported for A. nemorosa (Eriksson, Reference Eriksson1995; Holderegger, Reference Holderegger1996) and it is reasonable to assume that this would be true for A. ranunculoides. Therefore, it is likely that the differences in behaviour we have highlighted are ecologically meaningful and may help to predict future changes in the relative abundance of these two species in the woodlands of northern Italy. Seed germination in both species is clearly highly sensitive to seasonal temperatures and the subtle differences between them in the optimum temperatures for radicle and shoot emergence suggest that climate change might affect their relative reproductive success. For example, the absence of a cold stratification requirement for shoot emergence could favour A. ranunculoides over A. nemorosa in a trend of warmer winters. However, the absence of A. ranunculoides in Mediterranean regions suggests that it has a low tolerance to warm, dry summers, which could become a more important limiting factor. Thus, since the climate is expected to become warmer and drier in several mid-latitude European areas, including northern Italy (IPCC, 2007), A. ranunculoides may become more scarce.
Laboratory and field studies of seed germination and emergence phenology are clearly an important tool for detecting ecologically meaningful differences between closely related plant species, and thus they may help to predict future trends in plant distribution. However, such studies will need to be backed up by long-term monitoring of plant populations (Pauli et al., Reference Pauli, Gottfried, Reiter, Klettner and Grabherr2007) if we are to properly understand the relationship between seed germination events and plant population dynamics in a changing climate.
Acknowledgements
Financial support was provided by the University of Pavia, the Centro Flora Autoctona of the Lombardy Region and the MIUR (Italian Ministry for Education, University and Research) through the project no. 2007JNJ7MX.