Introduction
Seed germination and seedling establishment are critical stages in the life cycle of a plant, and their success depends on seeds being non-dormant and in a suitable location when conditions permit germination. It is well established that seed dormancy and germination of higher plants are complex adaptive traits influenced by a large number of environmental factors (e.g. Mayer and Poljakoff-Mayber, Reference Mayer and Poljakoff–Mayber1975). Furthermore, the environmental conditions required to break dormancy may, or may not, be the same as those required for germination (Baskin and Baskin, Reference Baskin and Baskin1998). Vleeshouwers et al. (Reference Vleeshouwers, Bouwmeester and Karssen1995) considered seed dormancy to be important in the life cycle of plants because it prevents germination at a time when environmental conditions are not (or will not remain) favourable for seedling establishment. On the other hand, non-dormant seeds may not germinate because environmental conditions do not correspond to those required for germination (Vleeshouwers et al., Reference Vleeshouwers, Bouwmeester and Karssen1995). That is, correlations between germination requirements of non-dormant seeds and the ecological state of a plant's habitat offer a mechanism to avoid unfavourable conditions (e.g. cold winter) for subsequent plant establishment and reproductive growth (Finch-Savage and Leubner-Metzger, Reference Finch–Savage and Leubner–Metzger2006).
The long-term persistence of any species at a site requires that, at least occasionally, seeds germinate and plants grow to maturity and produce seeds. Thus, timing of seed germination in relation to environmental conditions in the habitat can be an important part of the adaptation of a species to its habitat. Annual species, in particular, have received considerable research attention with regard to timing of germination in nature (Baskin and Baskin, Reference Baskin and Baskin1998); however, little is known about timing of germination in annual holoparasites, such as Cuscuta. In addition to favourable environmental conditions in the habitat, host plants must be available when seeds of holoparasites germinate, or seedling establishment cannot occur (Kuijt, Reference Kuijt and Kuijt1969). Seedlings of Cuscuta, in particular C. epithymum, only have 14–21 d to locate a host and establish a haustorial connection, or they will die (Benvenuti et al., Reference Benvenuti, Dinelli, Bonetti and Catizone2005; unpublished personal observation).
The subject of our study was the annual holoparasite, Cuscuta epithymum L. (Convolvulaceae). In this species, another intriguing aspect of the germination ecology is the presence of water-impermeable seed coats, i.e. physical dormancy (Gaertner, Reference Gaertner1950). Seeds of many species of Cuscuta have a water impermeable seed coat surrounding fully developed coiled embryos (Baskin and Baskin, Reference Baskin and Baskin1998; Benvenuti et al., Reference Benvenuti, Dinelli, Bonetti and Catizone2005); consequently, scarification promotes germination of seeds of many Cuscuta species, including C. epithymum (Gaertner, Reference Gaertner1950; Hutchison and Ashton, Reference Hutchison and Ashton1979; Flynn et al., Reference Flynn, Turner and Stuppy2006). Furthermore, because C. epithymum seeds do not germinate to high percentages following acid scarification (maximal 5% germination: Gaertner, Reference Gaertner1950), they may also have physiological dormancy. As far as we know, this is the first report of combined dormancy in seeds belonging to the Convolvulaceae and the genus Cuscuta.
The holoparasitic C. epithymum grows in the temperate zone of the Northern Hemisphere, with its main distribution areas in western and central Europe and the Atlas Mountains in north-western Africa (Verdcourt, Reference Verdcourt1948; Schaminée et al., Reference Schaminée, Stortelder and Weeda1996). In northwestern Europe, C. epithymum is mainly associated with dry heathland vegetation (Doyle, Reference Doyle1993), and is mostly restricted to the pioneer phase of the heather succession cycle, as described by Gimingham (Reference Gimingham1972). Changes in land use and cessation of traditional management have caused the parasite to become very threatened in Flanders (Belgium), and to be categorized as ‘endangered’ on the Red List of higher plants (Van Landuyt et al., Reference Van Landuyt, Hoste, Vanhecke, Van den Bremt, Vercruysse and De Beer2006). In other countries, such as the United Kingdom (Cheffings et al., Reference Cheffings, Farrell, Dines, Jones, Leack, McKean, Pearman, Preston, Rumsey and Taylor2005) and The Netherlands (Weeda et al., Reference Weeda, Westra, Westra and Westra1988), the rapid decline of its habitat has equally caused a strong decline of this species. Unlike some other holoparasites, for example, in Orobanche and Striga, the germination of C. epithymum does not depend on host exudates to stimulate germination (Baskin and Baskin, Reference Baskin and Baskin1998; Matusova et al., Reference Matusova, van Mourik and Bouwmeester2004). However, relatively little information is available about other factors responsible for seed dormancy break, germination requirements and on the relevance of these characteristics for C. epithymum in dynamic heath systems. For example, it is generally known that seeds of many species with physical dormancy can become non-dormant during dry storage at room temperature (Baskin and Baskin, Reference Baskin and Baskin1998). Little is known about this phenomenon in C. epithymum seeds, although Ewart (Reference Ewart1908) reported only 6% germination after 4 years of dry storage.
In spite of this knowledge, several aspects of the germination of C. epithymum are still unclear. The patchy occurrence of the parasite in managed heath communities and the ongoing decline of this species raise several fundamental questions that were objectives of our research. (1) What kind of dormancy (physical and/or physiological) do freshly collected and dry-stored seeds have? (2) What are the temperature requirements for the loss of dormancy and for optimal germination? (3) Do non-dormant seeds require light for germination? (4) If physiological dormancy is present, how much cold stratification is required to break it?
Materials and methods
Species
Cuscuta epithymum (L.) L. is generally described as a summer annual, although it has been reported to be perennial under certain conditions (Dean, Reference Dean1954; Costea and Tardif, Reference Costea and Tardif2006; unpublished personal observation). In this study, the plant is referred to as an annual. Each individual plant may produce hundreds to thousands of spherical to somewhat 3-angled seeds, with an average diameter and mass of 0.9 mm and 0.3 mg, respectively (Gaertner, Reference Gaertner1950; Gómez, Reference Gómez1994; Costea and Tardif, Reference Costea and Tardif2006; unpublished personal observation). Mature Cuscuta seeds have a four-layered seed coat consisting of an epidermis, outer and inner palisade layers and compressed parenchyma cells (Lyshede, Reference Lyshede1992; Costea and Tardif, Reference Costea and Tardif2006). The testa epidermis is peculiar in that the outer walls of dry seeds are invaginated, while they bulge outwards in wetted seeds. Together with the epidermis, the specialized two-layered cell palisade tissue is likely responsible for impermeability (Lyshede, Reference Lyshede1992), and the loss of dormancy is correlated with anatomical changes in the seed coat (Hutchison and Ashton, Reference Hutchison and Ashton1979). Seeds show no morphological adaptations for dispersal (Kuijt, Reference Kuijt and Kuijt1969).
Seed collection
Seeds were collected from ripe fruits during the last 2 weeks of September 2004, 2005 and 2006 from different C. epithymum populations located in heath vegetation of three nature reserves. These reserves [‘Heiderbos’ (2004, 2005, 2006), ‘Kikbeekbron’ (2005, 2006) and ‘Ziepbeekvallei’ (2005)] are situated in the province of Limburg (Flanders, Belgium: 50°56′–51°00′N and 5°27′–5°38′E), and separated by less than 9 km. The climate is temperate, with an annual mean temperature of 10–11°C and an annual total rainfall of 750–800 mm. In 2004 and 2005, seeds were stored dry at room temperature after collection until the start of imbibition treatment, germination experiment or the stratification treatment. In contrast, seeds collected in 2006 were used within 1 week for different experiments. While collecting seeds in autumn 2005, we marked some locations where plants occurred, and C. epithymum reappeared (as seedlings) in these exact places the next spring, confirming the summer annual character of this species. Seeds undergo three distinct developmental stages on the mother plant. First, immature seeds are large and green, and then change from green to light brown, finally becoming darker brown and shrinking in size as they dry. For the germination tests, only the last category was used. The diameter of C. epithymum seeds was 0.9 ± 0.03 mm (mean ± SE; n = 20). Freshly collected seeds weighed 0.31 ± 0.01 mg (n = 100), while seeds stored dry for 3 months weighed only 0.26 ± 0.01 mg (n = 160).
Seed viability
To determine the maximum germination potential of fresh seeds, seed viability of seeds collected in 2006 was assessed with tetrazolium tests (Moore, Reference Moore and Heydecker1973). Five replicates of 40 seeds each were placed on moist filter paper (Schleicher and Schuell No. 2282) at 23°C for 16 h, and then cut in half with a scalpel. Seed sections were incubated in a 0.1% aqueous solution of tetrazolium chloride for 24 h at 30°C in darkness. Only seeds showing a strong red-stained embryo were considered viable.
Imbibition experiment
To test if C. epithymum seeds were water impermeable, i.e. possess physical dormancy, the rate of water uptake was monitored for scarified and for non-scarified dry-stored (December 2005) and fresh (2006) seeds. Four replicates of 40 seeds individually scarified with a razor blade and of 40 non-scarified seeds were placed on filter paper moistened with distilled water in 9 cm diameter Petri dishes and kept in the laboratory at room temperature (c. 21°C). Following this procedure, seed mass was determined at 1 h intervals for the first 8 h, then after 13 h, and later at intervals of 24 h for the next 120 h. Following Hidayati et al. (Reference Hidayati, Baskin and Baskin2001), percentage water uptake was calculated as the increase in seed mass based on seed mass at t 0:
where W s = increase in mass of seeds, W i = mass of seeds after a given interval of imbibition, and W d = seed mass at t 0. Initial (at t 0) seed mass was determined for air dried seeds that had been wetted for c. 2 min, blotted dry (standard treatment), and weighed to the nearest 0.1 mg (mean of four seeds).
Increase in mass of the seed may not necessarily indicate that germination will occur. Thus, to determine if the scarified and non-scarified seeds would germinate, seeds were incubated under a 12 h photoperiod at 23°C for 3 weeks.
Germination experiments
Different germination experiments were carried out to study the effect of temperature and light on the loss of dormancy and on germination. In a first experiment, a preliminary treatment of cold stratification and three temperature regimes (15/6, 23 and 30/20°C) after cold stratification were chosen, based on results from a pilot study in 2005. The 15/6°C regime simulates spring conditions in a temperate climate, 23°C represents a constant temperature and 30/20°C was chosen because a previous study had found optimum germination of C. epithymum seeds at relatively high temperatures, and because we expected a positive influence of fluctuating temperatures. Lados (Reference Lados1999) obtained high germination percentages at 26°C.
To determine if cold stratification is a prerequisite for germination, 18 dishes of 50 non-scarified seeds each were placed on moist filter paper at 5°C for 12 weeks in December 2005. To obtain dark conditions, nine of the 18 dishes were placed in light-tight boxes. Filter paper was replaced every 2 weeks to avoid fungal infection. The controls consisted of 18 dishes of 50 non-scarified seeds, each on dry filter paper stored at room temperature (21°C) for 12 weeks. Dry stored and cold stratified seeds were tested for germination under a 12 h daily photoperiod or in continuous darkness (Petri plates inside wooden light-tight boxes and only opened under green light) at alternating (12/12 h) temperature regimes of 15/6 and 30/20°C, or at a constant temperature of 23°C. Light (PAR = c. 36 μmol m− 2 s− 1, 400–700 nm photon irradiance) was provided by fluorescent tubes (Philips TLD 80) during the 12 h higher temperature portion of the cycle.
In 2006, during an extensive experiment we determined if C. epithymum seeds had both physical and physiological dormancy. Three replicates of 50 scarified and of 50 non-scarified fresh seeds were cold stratified at 5°C for 0, 4, 8 and 12 weeks, and then tested for germination at 15/6, 23 and 30/20°C in a 12 h daily photoperiod for 9 to 15 weeks. Several periods of cold stratification were used to determine how much cold stratification was required to break physiological dormancy. During stratification, a low concentration (3 ppm) of merthiolate (Na–ethylmercury–thiosalicylate) was added to the Petri dishes to prevent microbial infection. No inhibition of germination was observed. Following Baskin and Baskin (Reference Baskin and Baskin1998), nongerminated seeds were checked for viability at the end of the experiment. Seeds that collapsed when pinched gently with forceps were considered non-viable, while those that did not collapse following pinching and had a firm, white embryo, were regarded as viable.
In all experiments, distilled water was added as needed. Germinated seeds were counted three times a week until no germination was observed for 10 d. Protrusion of the radicle was the criterion for germination.
Effect of dry storage
Seeds collected in September 2004 and stored for 16 months at room temperature were used to determine the effect of dry storage on dormancy level and germination. Nine replicates of 50 seeds were placed in 9 cm diameter Petri dishes on moistened filter paper. Seeds in three replicas were individually scarified with a razor blade, while those in six replicates were not scarified. The scarified seeds were incubated in a 12 h daily photoperiod at 23°C, and the non-scarified seeds were incubated under 12 h daily photoperiod at 23°C and at an alternating temperature of 15/6°C. All seeds were incubated until no germination was observed for 10 d (after 4 weeks). In addition, the effect of dry storage was tested by comparing germination of fresh seeds (2006) with that of seeds stored dry for 3 months (2005).
Data analysis
Following Meyer and Monsen (Reference Meyer and Monsen1991) germination rate was expressed as the number of days until 50% of the final germination was reached. The maximal potential germination percentage was calculated as the sum of per cent germinated seeds and per cent non-germinated, but viable seeds. Means and standard errors were calculated for germination percentages and percentage increase in seed mass. To achieve normality and homogeneity of variances, data were arcsine transformed before analysis. Effects of cold stratification, scarification, temperature regimes and/or light regimes on the (maximum) germination of C. epithymum seeds were analysed with a two- or three-way ANOVA, followed by a Tukey pairwise means comparison test (P < 0.05). Potential differences between treated and untreated seeds (scarification or dry storage), differences between the temperature regimes and differences between various stratification cold periods were analysed using an independent sampled t-test and one-way ANOVA test, respectively. All statistical analyses were performed with SPSS for Windows 14.0 (SPSS, 2006).
Results
Seed viability
Tetrazolium tests revealed that 26 ± 1 of 40 freshly collected seeds were viable, indicating a maximum potential germination percentage of 65%. Of the non-viable seeds, 9 ± 1% coloured only light red and 26 ± 1% showed no colouring. Therefore, 35% of freshly collected seeds were non-viable, rather than dormant.
Imbibition experiment
After 2 h (dry-stored seeds) or 1 h (fresh seeds), scarified seeds had imbibed significantly more water than non-scarified ones (Fig. 1a, at time 2 h for dry stored: t = 2.99, P < 0.05; Fig. 1b, at time 1 h for fresh seeds: t = − 14.81, P < 0.001). After 4–5 h, the increase in seed mass of scarified seeds reached a plateau value of 126 ± 9% for dry-stored seeds and 90 ± 9% for fresh seeds. Only 0.6 ± 0.6% dry-stored and 0% fresh, non-scarified seeds germinated within 20 d at 23°C, while 27 ± 4% dry stored and 21 ± 3% fresh scarified seeds germinated in the same germination period (germination % after 21 d: t = 4.46, P < 0.05 and t = − 14.56, P < 0.001, respectively). However, after 21 h of incubation, all scarified seeds (fresh and dry-stored) were totally swollen, and 0% of the dry-stored and 21 ± 2% of the fresh non-scarified seeds were partly swollen.

Figure 1 Mean ( ± 1 SE) water uptake by intact and by scarified C. epithymum seeds (a) stored dry for 3 months or (b) freshly collected. Seeds were incubated at room temperature (23°C) on moist filter papers for c. 6 d. Filled symbols: scarified seeds; open symbols: non-scarified seeds. W s = percentage increase in seed mass.
Germination experiments
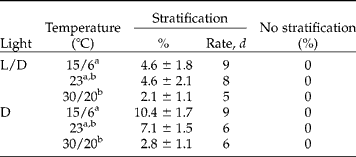
After 4 weeks of incubation in either light or darkness at the three temperature regimes, none of the non-stratified seeds had germinated (Table 1). Stratification significantly increased germination both in light and darkness (t-test after 21 d: t = 8.49, P < 0.001, Table 1), but at all test temperatures germination percentages were relatively low (Table 1). Furthermore, a significant temperature effect was observed, with significantly less germination at 30/20°C compared to the other temperature regimes (Tukey test, Tables 1 and 2). Daily alternation of temperature did not enhance germination (Table 1). Light was also not required for germination of the stratified seeds, and seeds germinated as fast in the dark as in light conditions (Table 1). When only considering the seeds incubated at 15/6 and 23°C, more seeds germinated in dark than in light, although this result was not significant (Table 2).
Table 1 Germination of non-scarified C. epithymum seeds at constant (23°C) and alternating temperatures (15/6 and 30/20°C) at a 12 h daily photoperiod (L/D) or continuous darkness (D) for 30 d. Stratified (non-scarified) seeds were kept at 5°C for 8 weeks. Germination percentages (%) are means±SE after 3 weeks of incubation. Germination rate was scored as the time (d) to 50% of the final germination percentage. Different letters correspond to significant differences (Tukey's pairwise means comparison test, P<0.05)
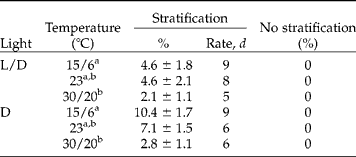
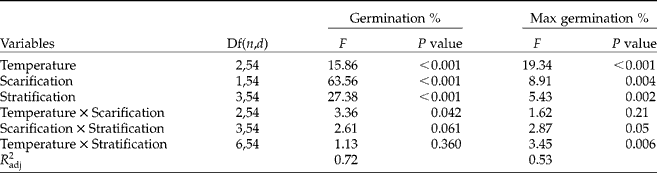
Table 2 F and P values of the two-way ANOVAs testing the effects of germination temperature, light regime and interaction on germination percentages of 3-month–old dry stored C. epithymum seeds after 30 d of incubation. Df(n, d)=degrees of freedom (nominator, denominator)

For scarified fresh seeds at all temperatures, germination percentages increased with increasing length of the cold stratification period (Fig. 2, Tukey pairwise means comparison test, P < 0.05). Increasing germination was most significant for seeds incubated at 15/6°C (Fig. 2, effect of stratification on germination: one-way ANOVA F = 32.49, P < 0.001). Increasing germination with increasing length of cold period was not significant for the temperature regime of 30/20°C (one-way ANOVA: non-scarified seeds: F = 6.94, scarified seeds: F = 1.69, P>0.05). An 8-week period of cold stratification gave optimal results; 12 weeks of this treatment showed no further increase in germination of seeds at 15/6, 23 or 30/20°C. After 8 weeks of cold stratification, the percentage of germinated seeds came close to the maximal potential (Fig. 2). None of the seeds germinated at 5°C. Germination only occurred after temperature elevation. Temperature and scarification, as well as stratification, significantly affected seed germination (Table 3). The effect of scarification was dependent on temperature regime (Fig. 2, Table 3), and scarified seeds incubated at 15/6 or 23°C germinated to significantly higher percentages than those at 30/20°C (t-test: t = − 3.98, P = 0.001; t = − 3.06, P = 0.006; t = − 2.23, P = 0.04, respectively, for 23, 15/6 and 30/20°C). In all treatments, the slowest germination was for seeds incubated at 15/6°C (Fig. 2); it generally took 1 week before these seeds started germinating. Furthermore, after 3 weeks of observation, some seeds, especially those not exposed to a cold period, continued germinating even after more than 50 d of incubation (last observed germination after 110 d of incubation, 15/6°C, scarified seeds). At the end of this experiment, 49 ± 3% of the seeds were dead (i.e. collapsed when pinched gently), 25 ± 3% had germinated and 26 ± 3% were viable, but not germinated (Fig. 2). The maximum potential germination percentage was influenced by temperature and scarification, as well as by stratification (Table 3). At 30/20°C or after scarification, viability was significant lower than at the other temperature regimes or without scarification (Fig. 2, Tukey pairwise means comparison test, P < 0.001).

Figure 2 Cumulative germination percentages of scarified (right) or non-scarified (left) freshly harvested C. epithymum seeds in a 12 h photoperiod, under temperature regimes of 15/6°C (○), 23°C (▿) or 30/20°C (□) for 50 d, after a stratification period of 0, 4, 8 or 12 weeks at 5°C (open symbols). Maximal potential germination percentages for the three temperature regimes, i.e. the sum of the percentages of germinated seeds and the viable ungerminated seeds, are shown by closed symbols.
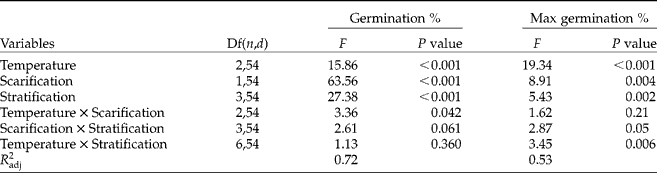
Table 3 F and P values of the three-way ANOVAs testing the effects of germination temperature, scarification, stratification and interactions on germination percentages of fresh C. epithymum seeds after 50 d of incubation and on the maximum potential germination percentage. Df(n, d), degrees of freedom (nominator, denominator); Max germination, sum of percentage germinated seeds and percentage ungerminated, but viable seeds
Effect of dry storage
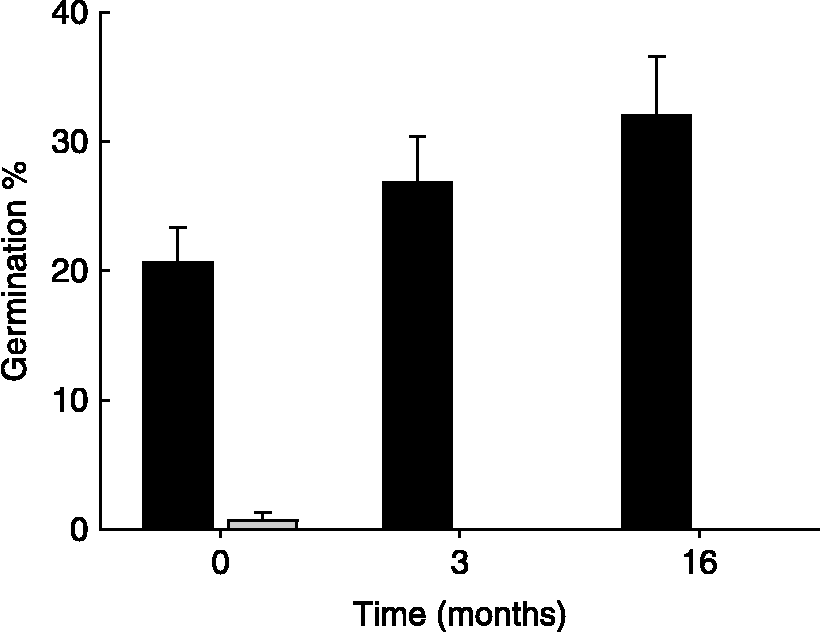
No germination of unscarified seeds was observed after 16 months of dry storage at room temperature, followed by 4 weeks of incubation at 15/6 or 23°C. In contrast, after 4 weeks of incubation at 23°C, 32 ± 5% of the scarified seeds had germinated. Although not significant, the germination percentage of scarified seeds at 23°C increased with the length of dry storage (Fig. 3; one-way ANOVA after 21 d: F = 2.13, P = 0.19).
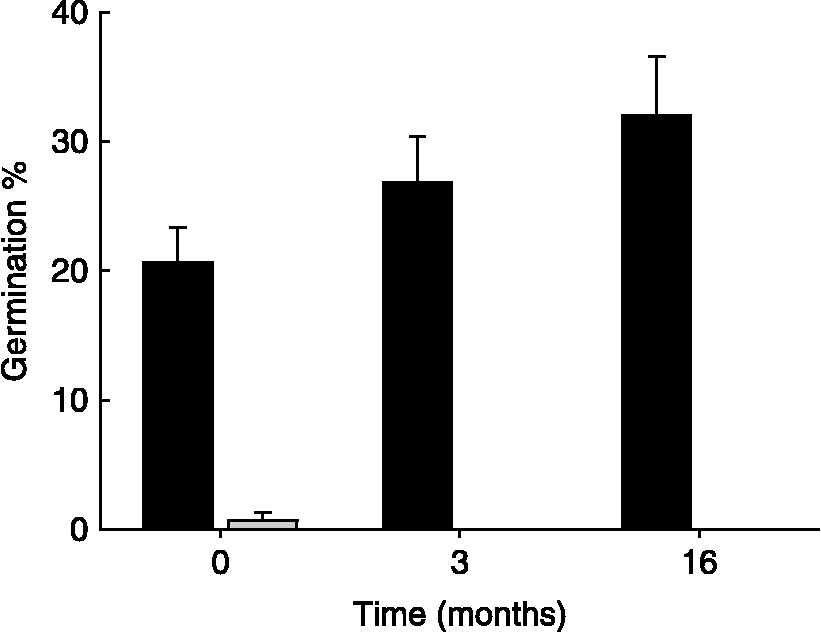
Figure 3 Germination percentages (mean+SE) after 21 d of incubation at 23°C of scarified (black bars) and non-scarified (grey bar) C. epithymum seeds stored dry for 0, 3 and 16 months at room temperature.
Discussion
Environmental control of dormancy breaking
Various mechanisms regulate seed germination of C. epithymum in its natural habitat, some of which are internal, whereas others are external environmental factors. These experiments revealed that both naturally matured and dry-stored seeds were physically dormant, and physical dormancy was not broken during long periods (3 and 16 months) of dry storage (Figs 2 and 3). In accordance with data from other Cuscuta spp. (e.g. C. campestris: Hutchison and Ashton, Reference Hutchison and Ashton1979; Benvenuti et al., Reference Benvenuti, Dinelli, Bonetti and Catizone2005), the exclusion of water by the seed coat was the main factor preventing germination. Indeed, physical dormancy had to be broken before germination was possible. Consistent with Hutchison and Ashton (Reference Hutchison and Ashton1979) for C. campestris, impermeability of C. epithymum seeds probably develops during maturation on the mother plant and remains after shedding under dry conditions [but Gaertner (Reference Gaertner1950) reported seed germination when still in the capsule]. Furthermore, in contrast to physically dormant seeds from other families that become water-permeable (non-dormant) during dry storage [e.g. Sida spinosa (Malvaceae) (Egley, Reference Egley1976) and Stylosanthes macrocephala (Fabaceae) (Silva and Felippe, Reference Silva and Felippe1986)], our imbibition experiments suggest that dry storage strengthened physical dormancy of C. epithymum seeds. While 20% of the fresh, non-scarified seeds imbibed fully, none of the dry-stored non-scarified seeds did so. While water absorbed by the epidermis of dry-stored Cuscuta seeds causes them to bulge outwards, water could pass through the two palisade cell layers lying just underneath the epidermis (Lyshede, Reference Lyshede1992); thus, the seeds do not imbibe. The ecological significance of physical dormancy can be seen as an adaptive seed trait allowing C. epithymum seeds to germinate over time and thus spread germination risks across generations. Physical dormancy may allow species to quickly colonize burned areas, be dispersed over long distances and/or survive for long periods in the seed bank (Fenner and Thompson, Reference Fenner and Thompson2005). Indeed, C. epithymum grows on recently burned heath (Meulebrouck et al., 2006, Reference Meulebrouck, Ameloot, Verheyen, Van Assche and Hermy2007); it appears after management of the vegetation (e.g. mowing and turf cutting; Meulebrouck et al., 2006, Reference Meulebrouck, Ameloot, Verheyen, Van Assche and Hermy2007); and may therefore possess a persistent seed bank, as suggested for Cuscuta spp. by Costea and Tardif (Reference Costea and Tardif2006). Many other heathland species have a persistent soil seed bank (Grime, Reference Grime1981; Bossuyt and Hermy, Reference Bossuyt and Hermy2003), and 15% of C. campestris seeds were viable after 12 years of storage, suggesting that this species may be able to form a persistent seed bank (Benvenuti et al., Reference Benvenuti, Dinelli, Bonetti and Catizone2005). However, survival of seeds in dry storage is not necessarily an indication of viability in the soil.
Although Hutchison and Ashton (Reference Hutchison and Ashton1979) found that mechanical scarification of C. campestris seeds increased germination to over 90% at 30°C, scarified seeds of C. epithymum without cold stratification germinated to a maximum of only 24% (Fig. 2). Thus, physiological dormancy occurs in seeds of C. epithymum; germination only occurred in scarified and/or stratified seeds, with the highest germination observed in scarified seeds that were cold stratified (Fig. 2). Thus, although seed coats are made permeable and can imbibe water, only some of C. epithymum seeds are capable of germinating (Fig. 3 and Fig. 2), while other seeds possess dormant embryos. Germination after dry storage increased slightly for scarified seeds and decreased for non-scarified seeds, suggesting afterripening or a progressive loss of physiological dormancy as a function of time (Fenner, Reference Fenner2000). Another possible explanation is the presence of annual cycles of physiological dormancy, as found for various species, including annuals [e.g. Baskin and Baskin (Reference Baskin and Baskin1985), Bouwmeester and Karssen (Reference Bouwmeester and Karssen1993), Van Assche and Vanlerberghe (Reference Van Assche and Vanlerberghe1989) and Van Assche et al. (Reference Van Assche, Debucquoy and Rommens2003)], but both hypotheses need further research for seeds of Cuscuta spp. (e.g. burial experiments). We conclude that C. epithymum seeds possess both physical and physiological dormancy (combinational dormancy), and seed dormancy is a crucial safety mechanism in its parasitic life cycle. Previously, embryo dormancy combined with coat impermeability has also been observed in both leguminous and non-leguminous species, and is interpreted as a double safety mechanism preventing accidental premature germination of seeds (Kigel, Reference Kigel, Kigel and Galili1995), although, as far as we know, this is the first report of combinational dormancy in the genus Cuscuta and in the Convolvulaceae.
As reported for seeds of many other plant species germinating in spring under field conditions (Baskin and Baskin, Reference Baskin and Baskin1998), our experiments showed that spring germination would be strongly influenced by temperature. A treatment of 8 weeks at 5°C (stratification) was most effective in releasing both physical and physiological dormancy (Fig. 2). This safety mechanism ensures that intact, as well as slightly damaged, seeds germinate in early spring and not before the winter, or when they are exposed to the high temperature fluctuations occurring in summer. The advantage of this low temperature requirement is obvious for C. epithymum, which sheds its seeds in autumn, but its seedlings require young host plants to parasitize, which are mostly available in spring. Furthermore, if the parasite would germinate in autumn, it would not have enough time to complete its life cycle before winter. As mentioned before, after seed shed, some C. epithymum seeds may still be water permeable, but at that time physiologically dormant embryos cannot germinate. Soon, after drying, all seeds become impermeable until a cold winter period leads to changes in the dormancy status of the seeds. A cold stratification likely inactivates the physical barrier in the seed coat by means of structural and biochemical alterations in the cell walls so that water can reach the developing embryo (personal comment, M. Costea). More importantly, while 65% of the fresh C. epithymum seeds were viable, only 22% of the intact stratified seeds germinated after a cold period of 8 weeks. Our research thus emphasizes that a cold winter period of 8 weeks, followed by a temperature elevation, totally inactivates the physiological dormancy, but that this cold period only partially (30%) eliminates physical dormancy since more seeds germinated after scarification (Fig. 2). As a result, only a part of the seed crop will germinate in the following spring, while the other, still physically dormant, fraction will remain in the seed bank until some subsequent growing season. In other words, physiological dormancy ensures germination in spring, while physical dormancy provides a spread of germination in time, illustrating the presence of a double safety mechanism. In managed heathland landscapes, where the vegetation undergoes successional dynamics due to regular human disturbance activities, a stock of viable seeds is an advantageous regenerative strategy. In this way, the parasite may remain in the seed bank when heather is too old and quickly reappears after management, such as mowing, turf-cutting or burning. The chief advantage of such a risk-spreading strategy is the avoidance of total reproductive failure of any given generation. C. campestris also possesses a mechanism that allows germination of a few seeds at a time for several years (Dawson, cited in Kuijt, Reference Kuijt and Kuijt1969). Confirmation of this hypothesis needs further experimentation, including sowing and burial experiments.
Germination requirements
In western European heathlands, C. epithymum occurs mostly in early successional stages, disappears after c. 6 years, but reappears in young vegetation when heathland is managed by mowing, turf-cutting or burning (Meulebrouck et al., Reference Meulebrouck, Ameloot, Verheyen, Van Assche and Hermy2007). Therefore, an absolute light requirement or the need for fluctuating soil temperatures, seen as indicators of the surrounding vegetation and the depth of seeds in the soil, are expected to occur in C. epithymum seeds. These mechanisms frequently occur in small-seeded pioneer species (Putz and Appanah, Reference Putz and Appanah1987; Van Assche and Vanlerberghe, Reference Van Assche and Vanlerberghe1989; Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003) and in buried seeds of many annual weeds (Pons, Reference Pons1991). Nevertheless, our laboratory data showed no evidence of requirements for light or fluctuating temperature for germination (Table 1 and Fig. 2). This is in accordance with Benvenuti et al. (Reference Benvenuti, Dinelli, Bonetti and Catizone2005), who demonstrated the photo-insensitive germination ecology of C. campestris. In general, seeds with physical dormancy germinate over a wide range of temperature and light conditions after they become permeable (Baskin et al., Reference Baskin, Davis, Baskin, Gleason and Cordell2004). How C. epithymum seeds avoid germination in older heath vegetations still remains unclear, knowing that germination does not depend on chemicals produced by hosts (Baskin and Baskin, Reference Baskin and Baskin1998). Maybe the seeds cannot germinate because of other factors, e.g. inhibition through allelopathy by other plants (Costea and Tardif, 2006; Baskin and Baskin, Reference Baskin and Baskin1998).
At least in some species, temperature can have a dual effect on seeds, since it not only affects dormancy, but it may also have a direct effect on germination (Bouwmeester and Karssen, Reference Bouwmeester and Karssen1993). As mentioned above, low autumn and winter temperatures tend to partially break physical dormancy in the summer annual C. epithymum. Our results showed that seeds never germinated at 5°C, and germinated best after being exposed to moderate temperatures (15/6 or 23°C, Fig. 2), suggesting that chilling followed by rising spring temperatures is needed to remove physical dormancy. After physical dormancy is broken, seeds with non-dormant embryos can germinate at prevailing spring temperatures. For some legumes with physical dormancy, germination also occurred as a response to sudden shift to higher, alternating temperatures after a cold winter period (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003), and such a sudden shift to higher temperatures may be a widespread season-sensing mechanism among species with persistent seed banks. When seeds are near the soil surface, seeds undergo a cold period, followed by higher temperatures in spring, resulting in germination. Indeed, fresh C. epithymum seeds germinated best at a constant temperature of 23°C, while 15/6°C yielded almost comparable results. The latter exhibited no germination in the first week, followed by a slow germination during several weeks, but the final germination percentages were as high as at 23°C (Fig. 2). This may be a mechanism for preventing germination on one or a few relatively warm days during the cold winter period. Furthermore, the seeds may be non-dormant, but they may not germinate because it is too cold. Once temperature becomes suitable in spring, a large number of seeds of this parasite may germinate within a few days, followed by a period with intermittent germination. High temperatures (e.g. 30/20°C) would not stimulate cold stratified seeds to germinate, causing C. epithymum seeds to remain ungerminated in the summer period. This suggests the existence of the annual cycling of physiological dormancy, although confirmation is needed.
We do not know if the processes observed under laboratory conditions occur in the field, and more work is certainly needed, but this study provides evidence that double seed dormancy may be an important survival strategy of C. epithymum, allowing for its persistence in dynamic heathland vegetation from north-western Europe. The combined dormancy creates a double safety strategy: because of physiological dormancy, germination can occur in spring, and due to the physical dormancy only a portion of the seeds will germinate, which will potentially lead to the formation of a persistent seed bank. In this way, the parasite may be adapted to the specific conditions that now occur in managed heathlands in a temperate climate.
Acknowledgements
We would like to thank Nick Sibbett, Mihai Costea and Stefano Benvenuti for helpful comments on the manuscript. K.M. holds and E.A. held a grant from the Fund for Scientific Research (F.W.O. − Vlaanderen). We further acknowledge the Agency of Nature and Forest of the Flemish government and Natuurpunt vzw for giving us the opportunity to perform research within their nature reserves. This research was partly funded by F.W.O. − Vlaanderen (G. 0296.07).