Introduction
Physically dormant seeds fail to imbibe water when placed on a wet substrate. Physical dormancy (PY) has been documented in 18 plant families of angiosperms (no gymnosperms) and is caused by an impermeable palisade cell layer in the seed or fruit coat (endocarp) (Baskin and Baskin, Reference Baskin and Baskin2014). Water impermeability in seeds with PY has also been reported to be due to the waxy cuticle on the outer macrosclereid cells. This was recently reinforced by a mutant screen in the model species Medicago truncatula that identified a Knox gene associated with cuticle development as critical to maintaining physical dormancy (Chai et al., Reference Chai, Zhou, Molina, Fu, Nakashima, Li, Zhang, Park, Tang, Jiang and Wang2016). Dormancy release in seeds with PY principally occurs by disruption or dislodgement of water-gap morpho-anatomical structures associated with the palisade layer (Baskin, Reference Baskin2003). In addition to specific water-gap structures, cracks that occur in the outer coat layers may play an additional role in water entry in some physically dormant seeds (Morrison et al., Reference Morrison, Auld, Rish, Porter and Mcclay1992; Ma et al., Reference Ma, Cholewa, Mohamed, Peterson and Gijzen2004; Zeng et al. Reference Zeng, Cocks, Lailis and Kuo2005).
Water-gaps are located in the hilar, micropylar or chalazal regions of the seed, as well as the carpellary hilar or micropylar regions of the pericarp, and their opening allows water to enter the seed (Baskin et al., Reference Baskin, Baskin and Li2000). The water-gap is often associated with more than one morpho-anatomical structure that forms specialized areas referred to as water-gap complexes (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2013b). Therefore, a water-gap complex consists of one or more morpho-anatomical structures directly associated with the gap opening (water-gap) as well as with ancillary tissues associated with the water-gap that may be mechanistically important in opening the water-gap.
Water-gap complexes act as environmental signal detectors that sense conditions favourable for seed germination and plant establishment. Temperature and moisture are the major environmental signals sensed by water-gap complexes, but the environmental sensing systems for PY break differ between species even within the same genus.
This mini-review describes the features of water-gap complexes and is organized into four basic parts: (1) water-gap complex types; (2) spatial aspects of the water-gap complex; (3) temporal aspects of physical dormancy break in water-gap complexes; and (4) form and function for water-gap complexes.
Water-gap complex types
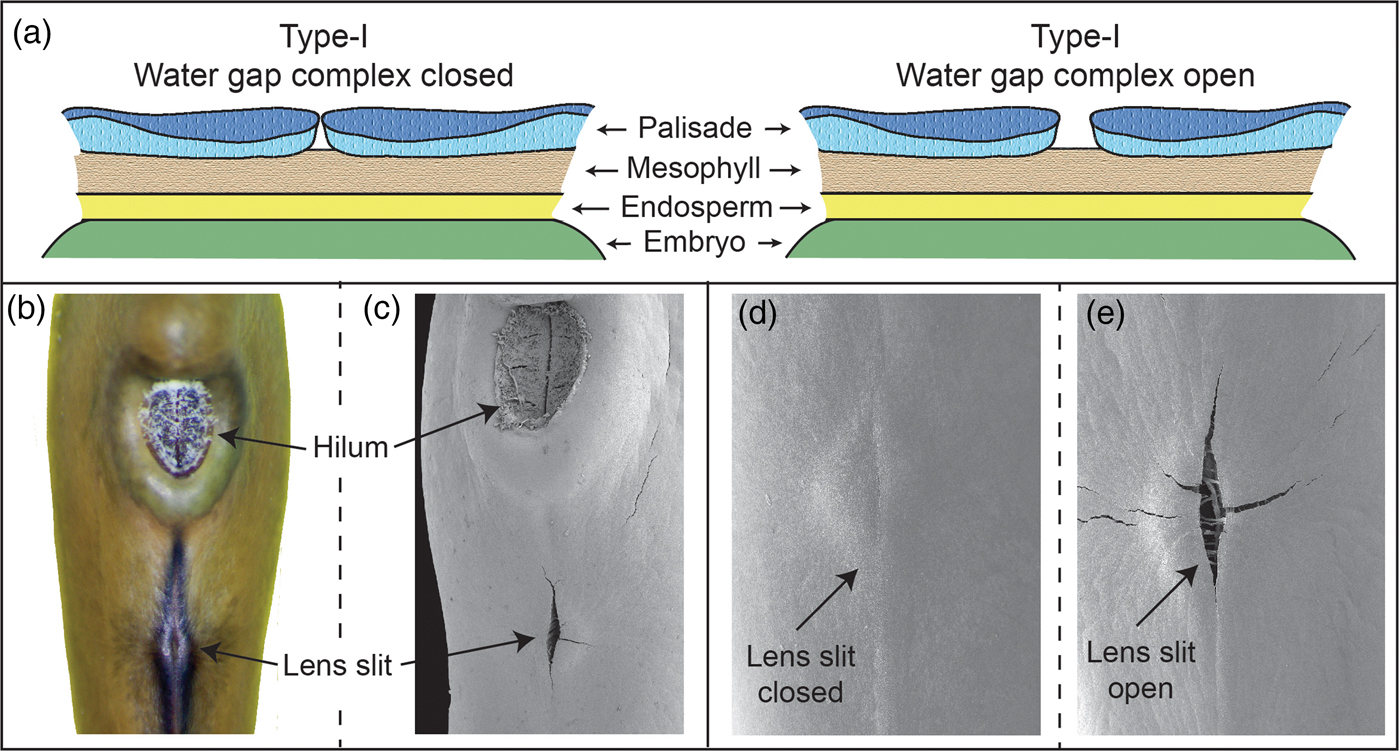
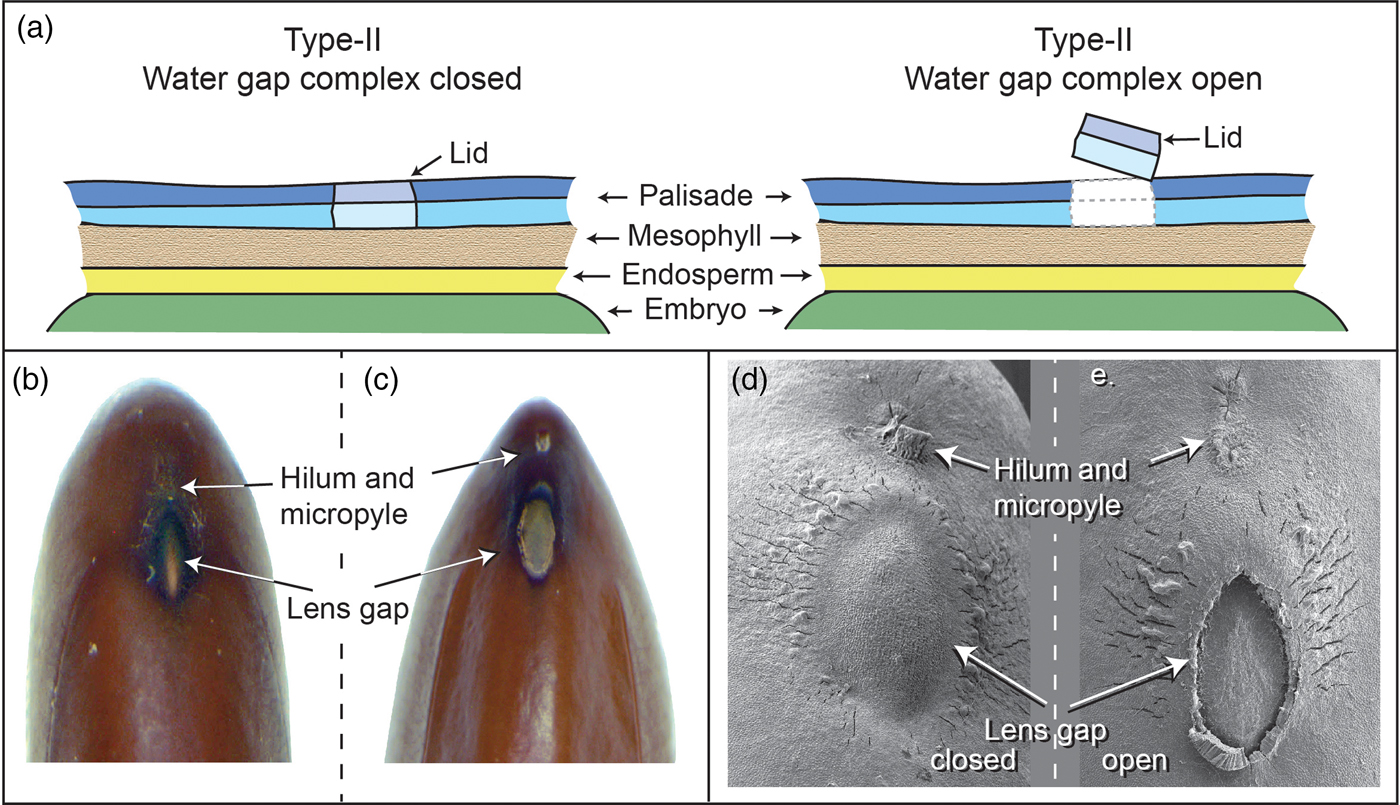
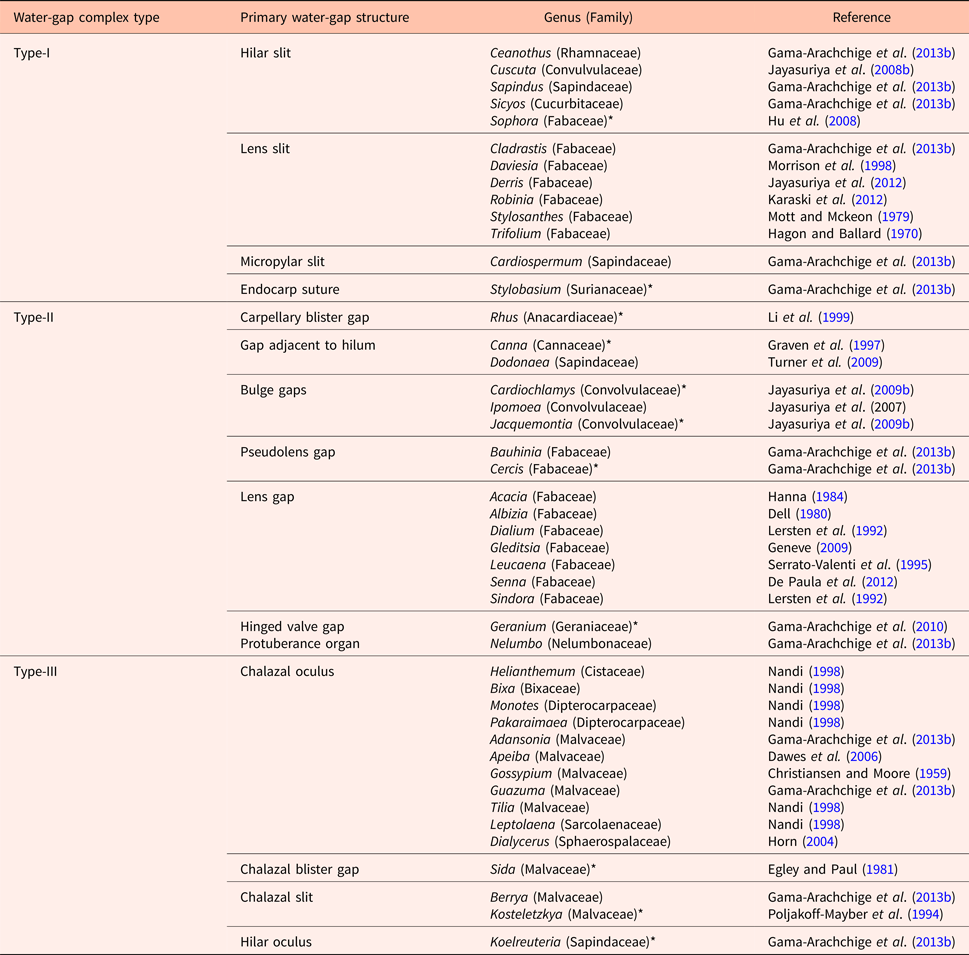
Numerous water-gap structures have been described for physically dormant seeds that are associated with the gap opening such as the hilar slit, carpellary blister or chalazal oculus (Baskin and Baskin, Reference Baskin and Baskin2014). However, Gama-Arachchige et al. (Reference Gama-Arachchige, Baskin, Geneve and Baskin2013b) suggested that regardless of location on the seed or fruit, these diverse structures have common morpho-anatomical release mechanisms and could be organized into three basic types: Type-I, Type-II and Type-III (Table 1). In Type-I, the water-gap forms as a narrow, linear opening of occluded palisade cells that separate or are pulled apart and thus allow water entry through the seed or fruit wall (Fig. 1). In Type-II water-gap complexes, a group of specialized cells forms a circular or linear lid-like structure that is dislodged and separates from the seed surface, whereupon water enters the seed (Fig. 2). In Type-III, a circular plug-like structure (oculus) formed by water-impermeable sclerenchyma cells separates from the adjacent palisade cells, thereby causing the seed (or fruit) coat to become permeable (Fig. 3).
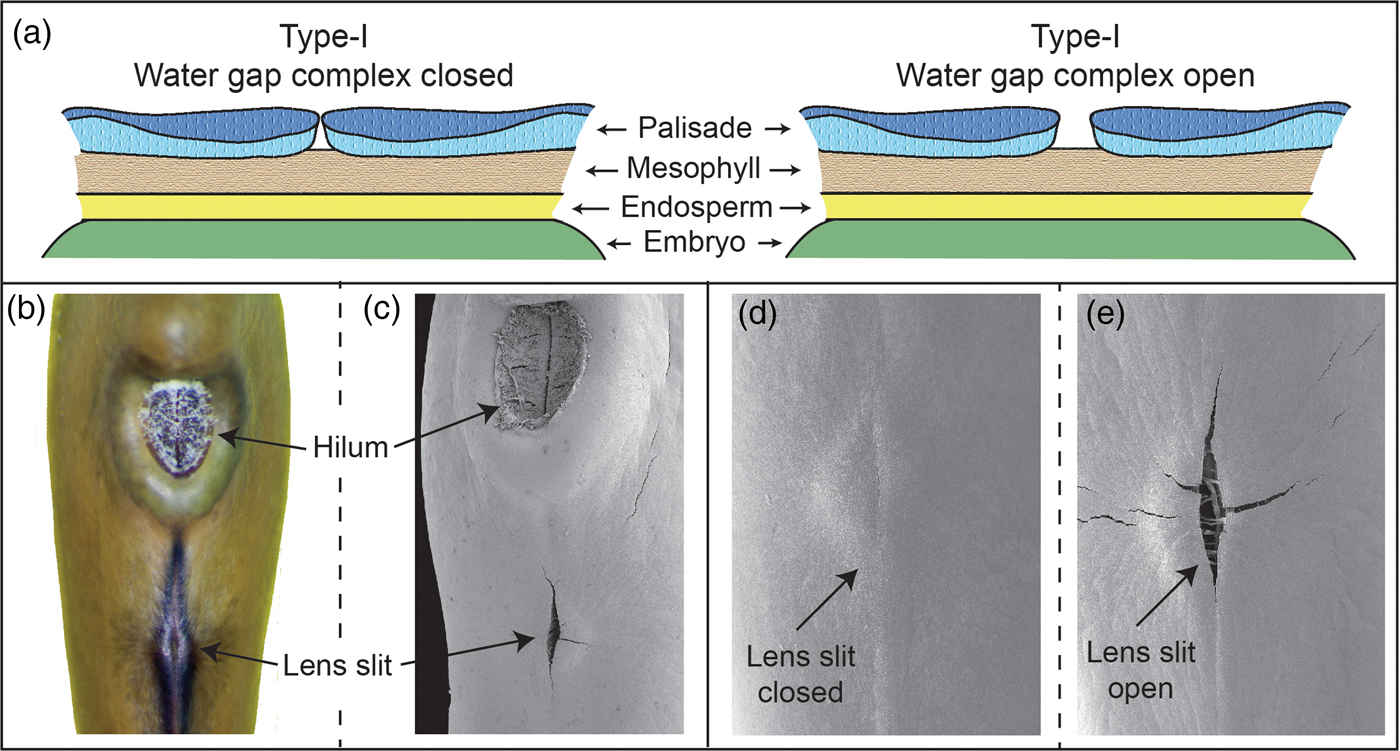
Figure 1. Type-I water-gap complex. (a) Cartoon illustrating tissues involved in a closed and open Type-I water-gap. (b and d) Photograph and electron micrograph of a closed lens slit; (c and e) electron micrographs of an open lens slit in a yellowwood (Cladrastis kentukea) seed.
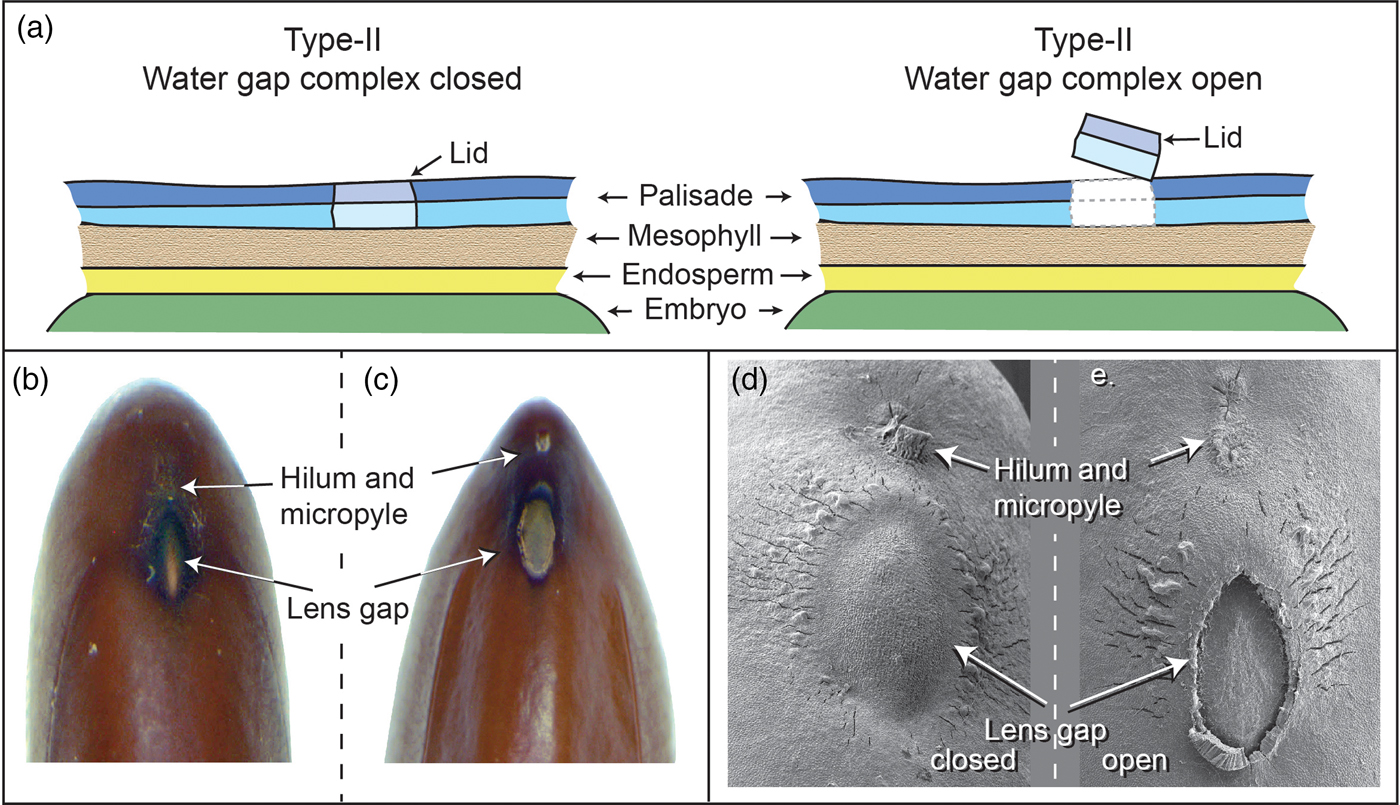
Figure 2. Type-II water-gap complex. (a) Cartoon illustrating tissues involved in a closed and open Type-II water-gap. (b and d) Photographs and electron micrograph of a closed lens gap; (c and e) an open lens slit in mimosa (Albizia julibrissin) seed.
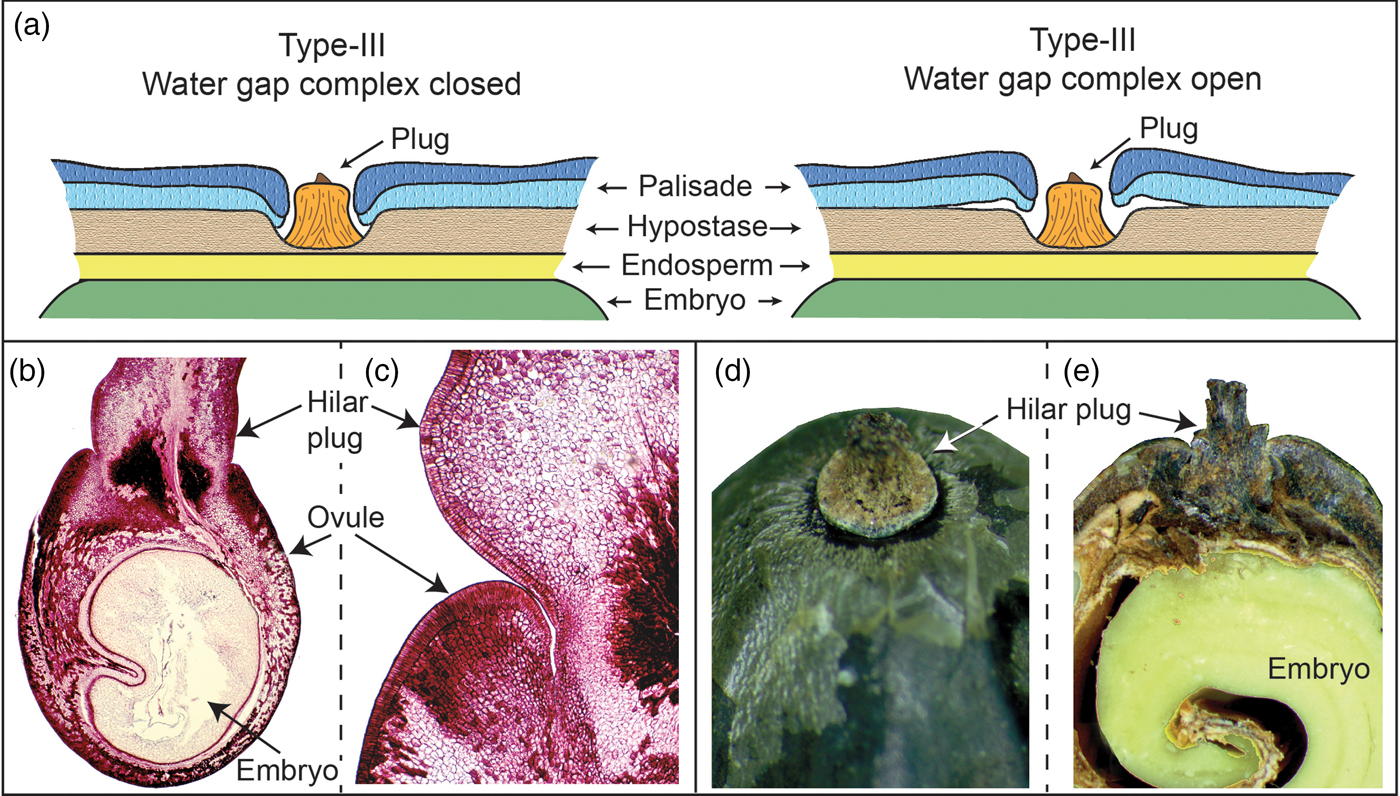
Figure 3. Type III water-gap complex. (a) Cartoon illustrating tissues involved in a closed and open Type-III water-gap. (b and c) Development of the hilar plug relative to the palisade layer in the ovule in golden raintree (Koelruteria paniculata). (d and e) Hilar plug.
Table 1. Types of water-gap complexes
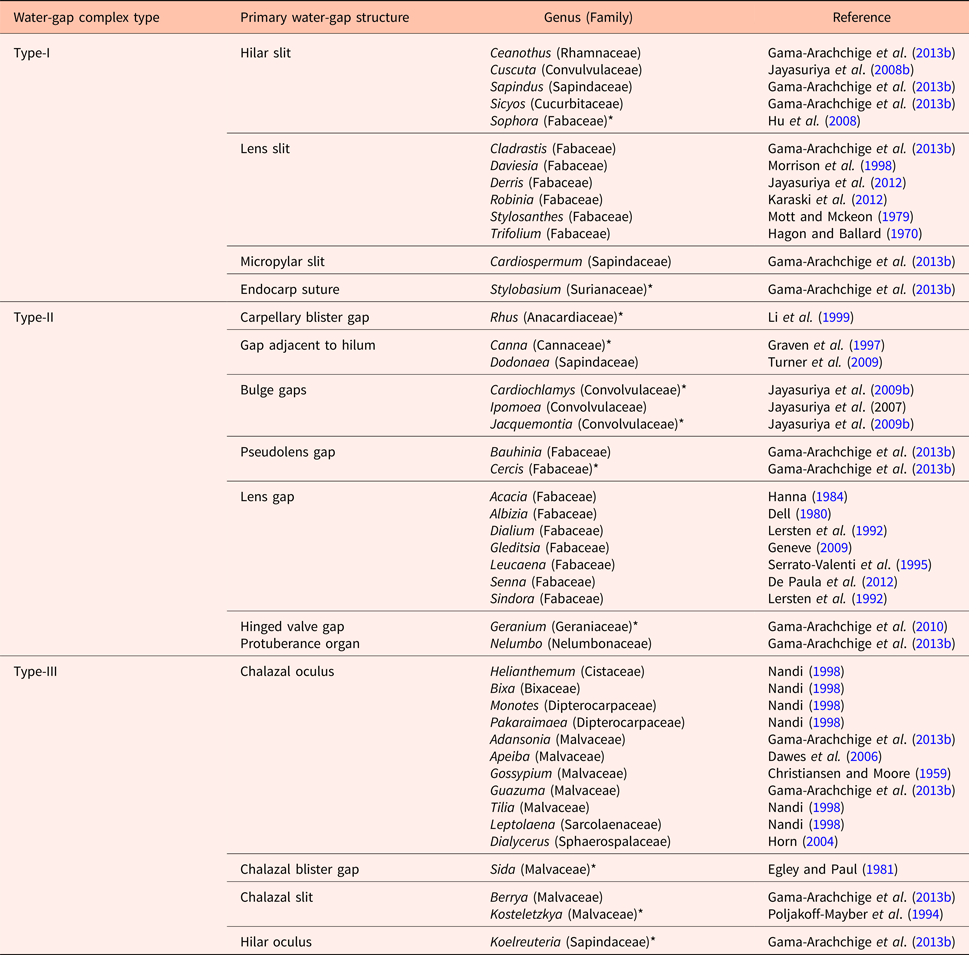
* Indicates a complex water-gap.
Spatial aspects of the water-gap complex
Two spatially related aspects associated with the water-gap complex can be recognized: (a) more than one morphological surface feature on the seed or fruit is involved in forming multiple gap openings, and (b) coordinated changes in surface and subsurface tissue layers that lead to water-gap opening. In seeds of most species with PY, a single seed- or fruit-structure forms the water-gap, while in other species more than one structure on the seed or fruit surface act in concert to form multiple water-gap openings. Using the terminology of Gama-Arachchige et al. (Reference Gama-Arachchige, Baskin, Geneve and Baskin2013b), seeds with a single water-gap are simple water-gap complexes and those with multiple openings are compound water-gap complexes (Fig. 4a,b). For seeds with compound water-gap openings, the associated tissues may be distinctly different (e.g. lens and micropyle) or less frequently the multiple openings utilize identical tissues (i.e. two bulge gaps in Ipomoea). In addition, the multiple tissue types in a compound water-gap complex have a primary (i.e. lens) and a secondary (i.e. micropyle) site for water entry. For example, in the woody legume Cercis, the primary water-gap is the pseudolens, and the secondary openings are the micropyle and hilar slit (Fig. 4d).
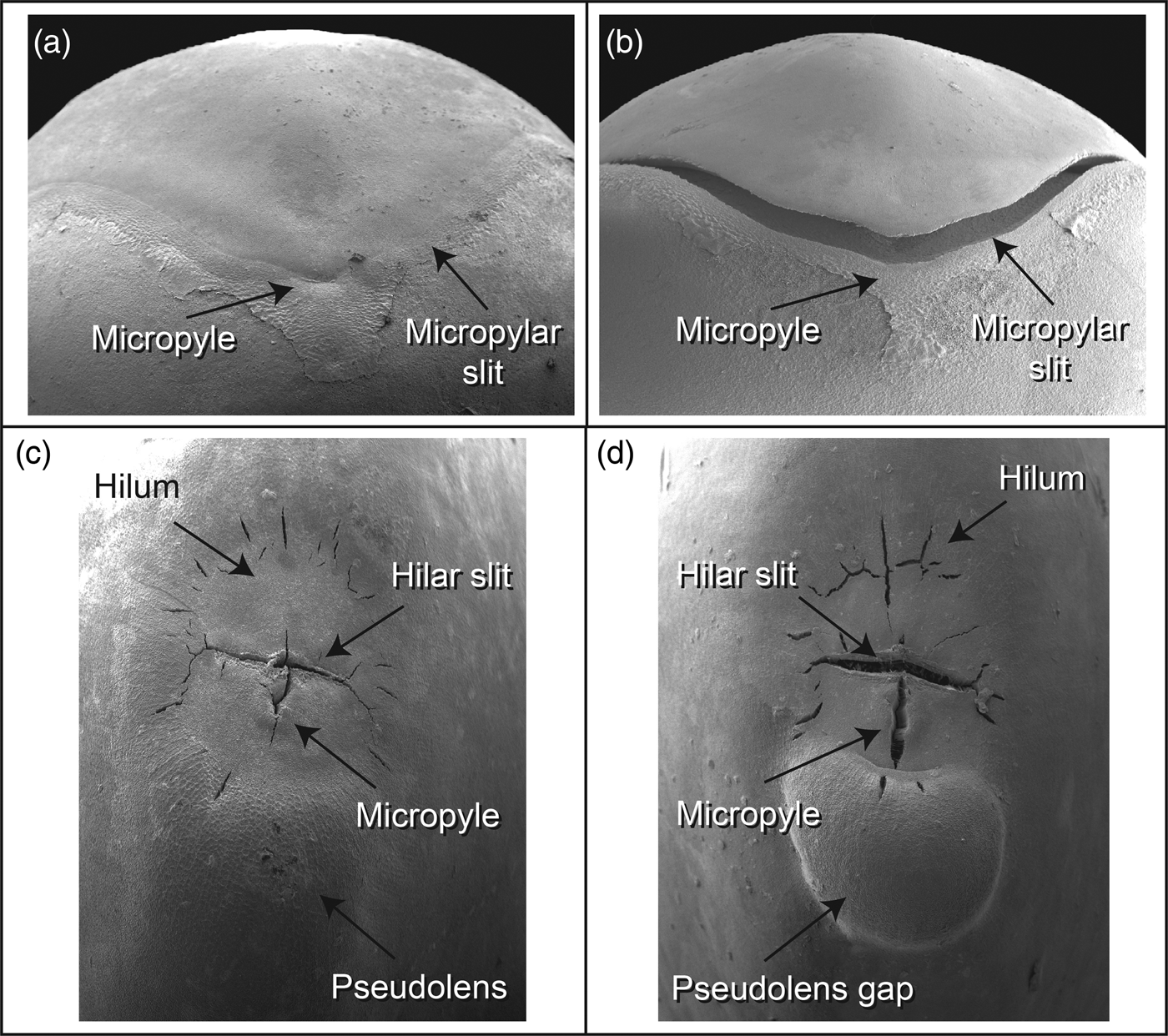
Figure 4. Simple and compound water-gap complexes. Simple Type-I (a) closed and (b) open water-gap complex in Cardiospermum halicacabum. Compound Type-II (c) closed and (d) open water-gap complex in Cercis canadensis.
Most studies involving PY have focused on the environmental signals responsible for triggering dormancy break (Baskin, Reference Baskin2003), but few have attempted to describe changes at the tissue or cell level involved in water-gap opening. The tissues involved in the opening of a water-gap are non-living seed coat or pericarp. Therefore, the proposed mechanisms involved in creating a water-gap opening rely on physical changes in the cells associated with the water-gap complex. These changes may involve simple separation of tissue layers in response to internal or external forces or possible expansion or contraction of individual cells in response to temperature changes.
Seeds of some species with PY require moist heat to initiate dormancy break (Baskin and Baskin, Reference Baskin and Baskin2014; van-Klinken et al., Reference van-Klinken, Flack and Pettit2006). It is paradoxical that these seeds are dormant due to an impermeable seed coat or pericarp and yet require a wet environment for heat-induced dormancy release. Hanna (Reference Hanna1984) first showed that portions of the seed coat associated with the hilum and lens in a physically dormant seed (Acacia) were partially open and could take up water or water vapour. He speculated that heating of the internal water vapour may increase internal pressure on cells in the lens, causing the water-gap to open. Using blocking experiments and liquids with different vapour pressure expansion coefficients and relative humidity storage environments, Jayasuriya et al. (Reference Jayasuriya, Baskin, Geneve and Baskin2009c) provided experimental evidence that water vapour enters through the hilar pad in dormant seeds of Ipomoea lacunosa. Water vapour trapped internal to the hilar pad expanded following a heat treatment, and this expansion was the driving force to open the bulge gap tissue responsible for water entry. The hilar area of other species has been shown to act as a valve that regulates moisture movement into physically dormant seeds (Rangaswamy and Nandakumar, Reference Rangaswamy and Nandakumar1985; Serrato-Velenti et al., Reference Serrato-Valenti, Devries and Cornara1995), suggesting this could be a common feature for seeds requiring moist heat for dormancy break.
For seeds that do not require moisture to induce the initial stages of physical dormancy break, expansion or contraction of cells associated with the water-gap in response to temperature appears to be responsible for cell weakening at the gap opening. In general, cells within the outer palisade and inner mesophyll tissue are uniform except for particular areas of the seed or fruit in the hilar, micropylar and chalazal regions. Cells within the water-gap complex can be physically heterogeneous, e.g. longer palisade cells in the lens of some legumes than in those away from the lens (Manning and van Staeden, Reference Manning and van Staden1987; Martens et al., Reference Martens, Jakobsen and Lyshede1995, Rodrigues-Junior et al., Reference Rodrigues-Junior, Faria, Vaz, Nakamura and José2014). In Geranium carolinianum, several different cell shapes and orientations were observed in palisade and subpalisade cells associated with the water-gap (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2011). The initial step in seed dormancy break of this species requires seeds to be sensitized at warm (≥20°C) temperature followed by a decrease in temperature (base temperature of 17.2°C) to initiate a physical change in water-gap complex cells. When a temperature difference builds up across the seed coat, the palisade and subpalisade layers shrink differentially causing them to slip. The Q 10 temperature coefficient calculated for dormancy break (Q 10 between 0.02 and 0.01) indicated a physical rather than chemical process (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2013a). Mechanical force applied to the same tissue area mimicked the changes observed by the temperature-treated Geranium seeds supporting physical weakening between cells in the palisade and subpalisade layers as the cause for water-gap opening. A similar response to physical pressure was observed in Sida spinosa seeds leading to chalazal blister formation (Egley and Paul, Reference Egley and Paul1981). Once the seeds become permeable, cell hydration becomes the driving force that lifts or separates the water-gap structure, allowing water entry into the seed.
Temporal aspects of dormancy break in water-gap complexes
In some cases, the water-gap complex may sense an abrupt environmental change (i.e. direct heat shock produced by fire) that could lead to immediate gap opening. For example, in Sicyos angulatus seeds exposure to a flame (30 s) causes the hilar slit gap to open (Gama-Arachchige et al. Reference Gama-Arachchige, Baskin, Geneve and Baskin2013a). However, direct heat shock caused by fire or laboratory boiling water treatments may not be representative of what happens in nature (Santana et al., Reference Santana, Baeza and Blanes2013). In most cases, where environmental change is gradual, it seems probable that there is a temporal separation between the events leading to tissues in the water-gap attaining the ability to open and the actual opening process. Following signal perception, there may be no obvious morpho-anatomical indication of change occurring in water-gap tissues (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003). However, in some cases there are readily recognized morphological changes that make it easy to follow a step-wise progression leading to gap opening such as the temperature-induced colour change in G. carolinianum that occurs in the micropylar area prior to opening of the hinged valve gap (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2011).
Tissues in the water-gap complex can proceed in a step-wise pattern where one set of signals induce tissues to become sensitive, while a second set of signals allows sensitive tissue to perceive environmental signals after which they will irreversibly open. Temperature and moisture cues are the usual signals inducing sensitivity as well as water-gap opening. Several spring-germinating members of the Fabaceae require a chilling (5°C) temperature period to become sensitive to perceive the alternating temperatures (15/6°C or 20/10°C) required for dormancy break (Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003). The opposite temperature sequence was observed in autumn-germinating G. carolinianum, in which seeds become sensitive only at temperatures ≥20°C and consequently responded to open the water-gap when the temperature dropped below 20°C (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2011).
After water-gap tissues have responded to the dormancy-breaking signal, the seeds are non-dormant and canalized to open in a wet environment. At this point, water-gap tissues cannot re-enter a dormant condition, and the gap will open when exposed to water (Hamly, Reference Hamly1932). However, prior to responding to the dormancy-breaking signal, water-gap complex tissues in the sensitive stages have shown the capacity to cycle back to an insensitive state. This process has been termed sensitivity cycling (Jayasuriya et al., Reference Jayasuriya, Baskin and Baskin2009a), and it plays the same role in seeds with PY as dormancy cycling in seeds with physiological dormancy (PD) (Baskin and Baskin, Reference Baskin and Baskin2014). That is, both kinds of pre-germination cycling ensure that the seed germinates only when the environment is favourable for plant establishment and that they are insensitive (PY) or dormant (PD) when the environment is not suitable for plant establishment. For species with PY, the prevalence of sensitivity cycling across plant families is not known. However, it has been documented in seeds of Melilotus, Ornithopus and Trifolium in the Fabaceae (Hagon and Ballard, Reference Hagon and Ballard1970; Taylor, Reference Taylor1981; Taylor and Revell, Reference Taylor and Revell1999; Van Assche et al., Reference Van Assche, Debucquoy and Rommens2003) and in I. lacunosa and Cuscuta australis in the Convovlulaceae (Jayasuriya et al., Reference Jayasuriya, Baskin and Baskin2008a; Jayasuriya et al., Reference Jayasuriya, Baskin, Geneve, Baskin and Chien2008b).
Form and function of water-gap complexes
Placing physically dormant seeds into morpho-anatomical ‘Types’ (Table 1) is a useful way to categorize water-gap complexes and may prove to be mechanistically important as studies begin to elucidate the physical steps leading to water-gap opening (Gama-Arachchige et al., Reference Gama-Arachchige, Baskin, Geneve and Baskin2011; Jayasuriya et al., Reference Jayasuriya, Baskin, Geneve and Baskin2009c). However, it is clear that there is no obligate relationship between the water-gap complex type and habitat or optimal environmental signals for dormancy break. For example, in a study comparing legume species in the subfamily Caesalpinioideae adapted to either a wetland or a dry terrestrial habitat, a relationship was found between dormancy break and the degree of high temperature or moisture level associated with the preferred habitat for each species (van-Klinken and Goulier, Reference van-Klinken and Goulier2013). However, each of these species would fall within the same Type-II water-gap complex that includes the lens gap as the primary water-gap opening. Similarly, Ipomoea hederacea and I. lacunosa both have a Type-II compound water-gap complex with bulge gaps adjacent to the hilum that act as the primary path for water entry. Although these water-gap complexes appear almost identical, I. hederacea requires dry, warm temperatures for dormancy break, while I. lacunosa requires wet, warm temperatures (Jayasuriya et al., Reference Jayasuriya, Baskin, Geneve and Baskin2009c; Jayasuriya et al., Reference Jayasuriya, Baskin, Geneve and Baskin2009d).
Acknowledgements
This is publication No. 17-11-0 of the Kentucky Agricultural Experiment Station and is published with the approval of the Director.
Funding
This project was supported by the USDA National Institute of Food and Agriculture, hatch project number KY011042.
Conflicts of interest
None.