Introduction
Plant species in deserts have developed various adaptive mechanisms, including seed dormancy (Gutterman, Reference Gutterman2002; Baskin and Baskin, Reference Baskin and Baskin2014) and delay of seed dispersal (Venable and Lawlor, Reference Venable and Lawlor1980) that prevent germination until soil moisture is sufficient for successful seedling establishment. Retention of seeds on the parent plant is often coupled to seed release being triggered by an environmental factor. Delay of seed release is a common phenomenon in fire-controlled (Lamont, Reference Lamont1991; Enright et al., Reference Enright, Lamont and Marsula1996) and desert (Günster, Reference Günster1994; Gutterman, Reference Gutterman2002) ecosystems. Delayed seed release can be adaptive by (1) spreading seed dispersal in time (Venable and Lawlor, Reference Venable and Lawlor1980), (2) retaining seeds in a favourable microhabitat (Gutterman, Reference Gutterman1994), (3) controlling the time of seed germination (Lamont, Reference Lamont1991), (4) changing the spatiotemporal pattern of soil seed banks (Günster, Reference Günster1994) and (5) decreasing the opportunity for seed predation and death (Günster, Reference Günster1994). The number of mature seeds retained on the mother plant and length of time vary with the species and ecosystem (Cowling and Lamont, Reference Cowling and Lamont1987; Bastida and Talavera, Reference Bastida and Talavera2002; Ma and Liu, Reference Ma and Liu2008; Peters et al., Reference Peters, Martorell and Ezcurra2009). Furthermore, the pattern of seed release and distribution away from mother plants, i.e. seed shadow, is an important part of plant fitness (Wenny, Reference Wenny2000).
The timing of seed dispersal may have an effect on the soil seed bank (Günster, Reference Günster1994; Li et al., Reference Li, Wang and Zhang2005), and the spatiotemporal pattern of soil seed banks plays an important role in regulating the structure and dynamics of communities (Nathan and Muller-Landau, Reference Nathan and Muller-Landau2000). Thus, in the restoration of degraded plant communities it is critical to have a good understanding of the soil seed bank potential of the species that are proposed for use in the initial stages of restoration. In addition to timing of seed dispersal, other biotic and environmental factors, including seed traits (Thompson et al., Reference Thompson, Band and Hodgson1993; Liu, Reference Liu2010), viability and longevity of the seeds (Garcia-Fayos and Verdu, Reference Garcia-Fayos and Verdu1998; Orscheg and Enright, Reference Orscheg and Neal2011), soil structure (Guo et al., Reference Guo, Rundel and Goodall1998; Kurova, Reference Kurova2016) and predation by animals and microbes (Narita and Wada, Reference Narita and Wada1998; Bastida and Talavera, Reference Bastida and Talavera2002; Zhang et al., Reference Zhang, Zhang and Yang2014), may have an effect on the soil seed bank.
The Dabancheng region of Urumqi in the Xinjiang Autonomous region of northwest China is the second largest region in China with chronically strong winds, and it is very dry (Fig. 1A). The natural vegetation in this temperate cold desert is dominated by shrubs, but many areas have been badly degraded because of human impacts. Revegetation practices usually involve the planting of non-indigenous trees and shrubs that require irrigation, and thus there is much cost in terms of manpower and water usage. Native pioneer species that do not require irrigation have great potential for use in revegetation projects in this arid area (Zhao et al., Reference Zhao, Chen and Sun2001), but little is known about the use of native species for restoration projects.

Figure 1. Cold desert habitat (A) and winged fruits (B and C) of Zygophyllum xanthoxylon. The simultaneous presence of brown (previous year) and green immature (current year) fruits on a mother plant is shown in C.
Although the native cold desert shrubs can be long-lived, there must be sexual reproduction if a species that lacks asexual reproduction is going to persist in an area or become established in degraded areas. Thus information on the seed dispersal and germination stages of the life cycle increases our understanding of how shrubs persist in the desert. Zygophyllum xanthoxylon (Bunge) Maxim. (Zygophyllaceae) is a super-xerophilous shrub that is widely distributed in the cold deserts of northwest China. It is the dominant species in Dabancheng and is an important candidate species for use in revegetating similar regions of China. Our preliminary observations indicated that fruit release by this species is slow and that some fruits were retained on the mother plants until fruiting occurred the next year (Fig. 1C). As timing of fruit dispersal could have an effect on timing of germination of seeds in the field and on size of the soil seed bank, we undertook an investigation of fruit dispersal and soil seed bank formation of Z. xanthoxylon. Due to the strong winds in the region and the extended period of fruit dispersal, we hypothesized that the spatial pattern of fruit dispersal would be irregular, fruits would be deposited beside any plants that served as a wind break and the number of fruit/seeds in the seed bank would fluctuate greatly, depending on time of year.
Materials and methods
Study site and species
The study area is located in the Dabancheng region of Urumqi in Xinjiang Autonomous region, China (43° 33′ 5.0′′ N, 87° 55′ 11.3′′ E, altitude approximately 1119 m above sea level). Dabancheng is a Gobi-type desert; the land is flat with stones on the soil surface. The area has a temperate continental climate, with a mean annual precipitation and temperature of 71.8 mm (Cheng, Reference Cheng2010) and 6.9°C (Cao et al., Reference Cao, Wang, Lu, Wei and Jia2015), respectively. Annual potential evaporation is about 2754 mm (Cao et al., Reference Cao, Wang, Lu, Wei and Jia2015). Total precipitation was 49.6, 68.9 and 47.2 and 68.6 mm in 2010, 2011, 2012 and 2013, respectively (Dabancheng Metro Bureau in Xinjiang). Rain may occur throughout the growing season (April–October), but most of it usually falls in summer (Fig. 2). There is little snow in winter. Based on wind data (2010–2013) from the Dabancheng Metro Bureau, mean annual wind speed ranges between 3.6 and 4.2 m s–1. The prevailing wind directions during the growing season and winter are shown in Fig. 3.
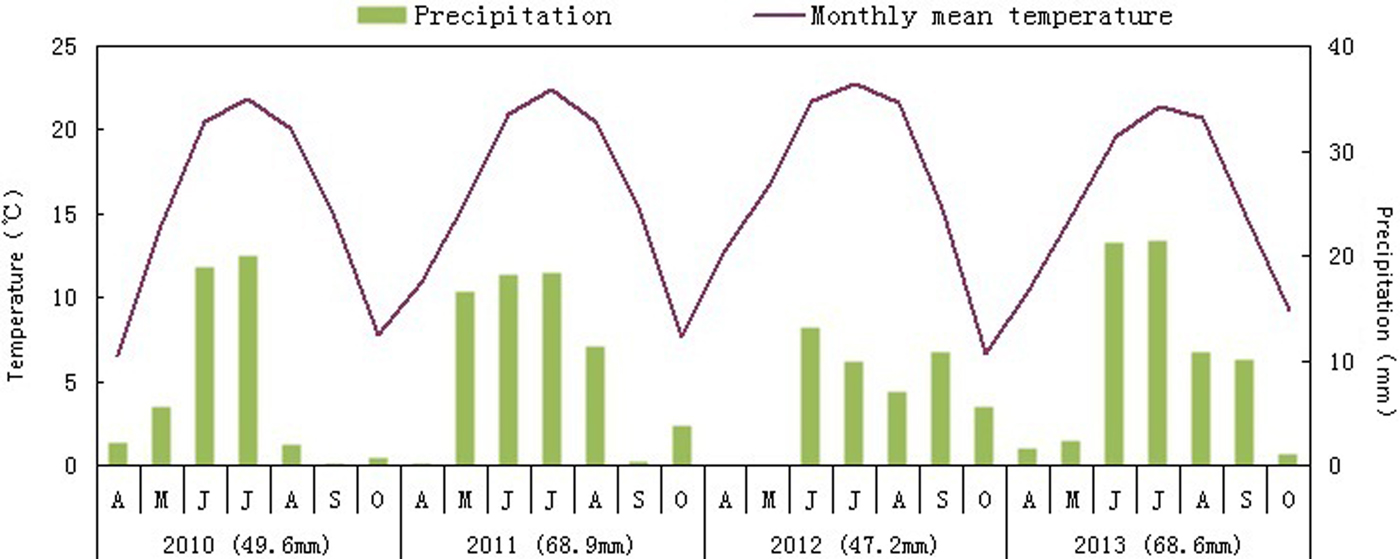
Figure 2. Monthly precipitation and mean monthly air temperatures during the growing seasons of 2010 to 2013 (from Dabancheng Metro Bureau, Xinjiang, China).

Figure 3. Wind speed and frequency for 16 compass directions measured at 1-hour intervals each day over the dispersal period of Z. xanthoxylon: from April to October (A) and November to March (B) in 2010–2013. Bars are ±1SE.
Vegetation cover in the region is less than 10% (Parhat, Reference Parhat2015), and it consists mainly of drought-tolerant shrubs, with Z. xanthoxylon and Ephedra przewalskii (Ephedraceae) being the dominant species (Fig. 1). Zygophyllum xanthoxylon is a super-xerophilous shrub 50–100 cm in height that occurs in Xinjiang, Inner Mongolia and Gansu provinces in China and in Mongolia (Shen, Reference Shen2011)). The dispersal unit is a 3-winged capsule (Fig. 1B,C) that contains 1 to 5 seeds (mean 2.2), and in the field seeds germinate while they are inside the fruit, i.e. the fruit is the natural germination unit. The fruit and seed of Z. xanthoxylon are, respectively: 2.1 and 0.85 cm in length; 0.62 and 0.12 cm in width; and 0.62 and 0.24 cm in height. Fruit and seed mass are 9.4 and 1.2 g, respectively (Wang, Reference Wang2017).
Temporal pattern of fruit release
Our study was conducted from 2010 to 2013 in two stands of Z. xanthoxylon in Dabancheng. The first stand was a natural population subject to strong chronic wind and drought. The second stand was near an artificial Populus forest that was irrigated at regular intervals. Although the second stand was not irrigated, Z. xanthoxylon roots may have received some additional moisture due to water movement through the soil. Zygophyllum xanthoxylon plants in the second stand experienced reduced wind velocity due to presence of the Populus forest.
At the onset of fruit release in June 2010, August 2011 and July 2012, eight plants were randomly selected and tagged in each stand. The stands were visited throughout the fruit release period, generally at 15-day intervals during the growing season and at 30-day intervals during winter. During each visit, we recorded the number of fruits remaining attached to each tagged plant. The percentage of fruits dispersed since the previous visit was calculated.
Spatial patterns of seed deposition
Seeds on the soil surface were sampled only in the first stand. Four transects were established in the stand: two transects received only natural rainfall and were classified as very dry habitat, while the other two received additional water via run-off following rainfall and were classified as dry habitat. Based on our observations in the study area, the fruits were mainly deposited in clusters, leeward of individuals and on bare low ground. In each habitat, 20 sampling sites (8 m × 8 m) were established at about 100-m intervals along each of the two 1000-m transects. In each sampling site, there were four microsites: (1) big cluster of shrubs (about 0.5–5 m2) formed mainly by E. przewalskii but also including other shrubs such as Z. xanthoxylon and/or Calligonum junceum; (2) small cluster of shrubs (<0.5 m2) consisting mainly of E. przewalskii; (3) four sides [northwest (windward), southeast (leeward), northeast and southeast] from single individuals of Z. xanthoxylon; and (4) bare low ground (depressions). In each sampling site, a 1 m2, 0.25 m2, 0.25 m2 and 0.25 m2 sampling plot was established in a big cluster, in a small cluster, around a single plant and on bare ground, respectively. Each transect was 1000 m in length, and 40 soil samples (0.5 m × 0.5 m × 5 cm deep) were collected on 10 September 2012, after most fruits had been released, and on 2 April 2013. Each soil sample was passed through a graded series of sieves, and fruits of Z. xanthoxylon were removed. Fruits were opened, and seeds were removed from them and tested for viability using the TTC (triphenyltetrazolium chloride) staining method. The number of viable seeds per sample was counted and number of viable seeds per 1 m2 calculated.
Statistical analysis
A general linear model (GLM) analysis was performed to test the effects of year, month, habitat and their interactions on fruit dispersal and to evaluate the effects of time of soil/seed sample collection and habitat on the soil seed bank. The least significant difference (LSD) test was used to estimate the least significant range between means. All statistical methods were performed using SPSS, version 19.0.
Results
Temporal pattern of fruit dispersal
In both Z. xanthoxylon stands, fruits dispersed over an extended period of time (Fig. 4). Fruit release began in summer (June 2010, August 2011, July 2012), and in both habitats 60.1–88.6% of the fruits had been released 3–4 months following maturation. Fruits remaining on the plants after 3–4 months were released gradually until the next spring (April or May). This pattern did not vary among the three years (Table 1). Ninety per cent of the fruits had been released from plants in the natural habitat after a mean time of 3.7 months (range 3–7) and from those growing near the Populus forest after a mean time of 4.7 months (range 4–5) (Fig. 4). The dispersal period was about 9–10 months; however, it should be noted that sometimes plants could be found in the population with previous- and current-year fruits attached to them. We have not observed fruit/seed predation.

Figure 4. Cumulative fruit release for each of three years in two stands of Z. xanthoxylon
Table 1. GLM analysis of the effect of year, month, habitat and their interactions on fruit release of Zygophyllum xanthoxylon

Spatial patterns of seed deposition
The cumulative number of fruits deposited on the soil surface varied between habitats. The number of deposited fruits was significantly higher in the dry than in the very dry transect. Each year in the big clusters of shrubs, the maximum number of fruits on the ground was higher in the dry than in the very dry habitat (Fig. 5). In the dry and very dry habitats, 98.4 and 95.2%, respectively, of the dispersed fruits/seeds in September were deposited in shrub clusters, and 98.0 and 93.2%, respectively, were in the big and small shrub clusters in April. Seed densities were very low on the leeward side of single individuals in the dry (3.6% in September, 3.5% in April) and very dry (2.6% in September, 0.4% in April) habitats (Fig. 5). Almost no fruits/seeds were deposited on bare low ground or on the windward side or the other two (non-leeward) sides of single plants. A few isolated seeds were found in soil samples in September and April; most fruits in the soil samples were not open.

Figure 5. Soil seed bank density in different microsites in two habitats of natural populations of Z. xanthoxylon. BC, big shrub cluster; SC, small shrub cluster; LSP, leeward of single plant; BLG, bare low ground. Different uppercase letters indicate significant differences between sampling dates for the same location in the dry or in the very dry habitat, and different lowercase letters indicate significant differences between locations within each sampling date in each habitat.
Discussion
Temporal pattern of fruit dispersal
Our study showed that >50% of the Z. xanthoxylon fruits were released during the first 3–4 months following maturity. Thus <50% of the fruits were retained on the mother plant and were released gradually over a period of 7–8 months in the natural habitat and in the habitat near the Populus forest. This temporal pattern of dispersal was similar in 2010, 2011 and 2012, when amount of precipitation was 49.6, 68.9 and 47.2 mm, respectively (Fig. 2). This implies that fruit release of Z. xanthoxylon was not affected by precipitation.
The dispersal pattern of Z. xanthoxylum in which many fruits are released shortly after maturity, whereas others are released gradually might have two advantages. Firstly, the initial period (first 3–4 months) of fruit dispersal is summer when rain may occur (Fig. 3), thereby providing suitable conditions for the non-dormant seeds to germinate and seedlings to become established (Wang et al., Reference Wang, Zhao and Yuan2016). Seeds of Z. xanthoxylon sown in the field germinated (to ca 60%) during the period when soil was continuously wet for several days (number not given) and were covered by 2 or 3 cm of sand in Neimeng (Zeng et al., Reference Zeng, Wang and Bao2005). Furthermore, fresh seeds removed from the fruits in Dabancheng germinated to 66.1% at 25/15°C (Wang, Reference Wang2017).These results imply that some seeds are not dormant and can potentially germinate in the field in summer. If seeds germinate in summer, seedlings have enough time to grow and thus a chance to survive until next spring. Secondly, seeds retained in the fruits are added to the soil seed bank from summer to the following spring. Viability of seeds removed from fruits buried at a depth of 2 cm in soil in situ was 100% after 16 months and 96% after 5 years (X. Zhao et al., unpublished data). Thus if seeds become buried there is high probability that a persistent seed bank will be formed.
Our data for soil seed density (Fig. 5) support our hypothesis that the number of seeds in the soil seed bank varies with the time of year. The numbers were relatively high in all microsites in the two habitats in September due to seed/fruit dispersal. On the other hand, the numbers of seeds in the soil were relatively low in April because many seeds had apparently germinated in early spring.
Spatial patterns of seed deposition
Almost no fruits were deposited on bare land or on the windward side of isolated individual plants of Z. xanthoxylon. Only a few fruits were deposited on the leeward side (i.e. on the southeast side) of the individual plants (Fig. 5). Most fruits landed in the 0.5–5 m2 clusters of shrubs that consisted mainly of E. przewalskii, Z. xanthoxylon and/or C. junceum (Fig. 5). There are several possible reasons for this fruit deposition pattern. (1) Wind speed is high and duration long in Dabancheng, thus fruits are easily blown away if no shrubs are located around the mother plant. The prevailing wind direction during the growing season (April to October) was from the northwest. (2) The clusters of E. przewalskii and other shrubs act as ‘safe islands’ that stop/hold fruits of Z. xanthoxylon. Zygophyllum xanthoxylon regenerates only by seeds, while E. przewalskii regenerates mainly by vegetative expansion. Thus large clusters of E. przewalskii plants form on the landscape. (3) The land surface is flat in Dabancheng, and soil erosion via strong winds has exposed many stones, resulting in a stone pavement that allows fruits to be easily moved about until some object stops them. (4) The winged fruits (Fig. 1B,C) of Z. xanthoxylon are easily moved by wind over the stony soil surface until they become intercepted by clusters of plants. Many studies have demonstrated that fruit deposition pattern depends on the species, wind speed and direction and topography (Li et al., Reference Li, Wang and Zhang2005; Li and Fang, Reference Li and Fang2008; Liu, Reference Liu2010) and on seed size, mass and shape (Günster, Reference Günster1994; Bestida and Talavera, Reference Bastida and Talavera2002).
Recommendations
Based on our results, we suggest that E. przewalskii acts as a ‘resource island’ (sensu Reynolds et al., Reference Reynolds, Virginia, Kemp, de Soyza and Tremmel1999) and ‘seed reservoir’ (Liu, Reference Liu2010). Thus we recommend that E. przewalskii be planted together with Z. xanthoxylon when pioneer species are used for land restoration in the cold desert. In addition, since grazing can disturb the soil seed bank and impact seed germination (Kinucan and Smeins, Reference Kinucan and Smeins1992; Sternberg et al., Reference Sternberg, Gutman and Perevolotsky2003), we suggest that grazing should be prohibited in cold deserts with strong prevailing winds. If livestock trampling disturbs the soil seed bank of Z. xanthoxylon, the winged fruits will be blown away by the strong winds. Thus the soil seed bank would be depleted, and subsequently population regeneration would be impaired.
Acknowledgements
We thank Rezi Gurimre, Jia Wang, Jiang Nan and Aypari Parhat for assistance in the field, Jiao Wang for help in preparing the figures and Peng Cheng for providing the wind speed data.
Financial support
This research was supported by the National Science Foundation of China (31660167, 31260101) and the Xinjiang Key Laboratory of Special Species Conservation and Regulatory Biology.
Conflicts of interest
None.









