Introduction
Dormancy could be defined as the failure of an intact viable seed to complete germination in a specified period of time under any combination of normal physical environmental factors that are otherwise favourable for its germination (Bewley, Reference Bewley1997; Baskin and Baskin, Reference Baskin and Baskin2007). Dormancy is a common trait in non-domesticated plants which increases the ability of a species to avoid competition between individuals of the same species and prevents germination out of place or season (Finkelstein et al., Reference Finkelstein, Reeves, Ariizumi and Steber2008). Seeds are dormant at the time of dispersal from the mother plant (Hilhorst, Reference Hilhorst1995) and dormancy is progressively lost as a consequence of seed interaction with environmental signals such as soil temperature and moisture (Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000). However, in many species, dormancy is terminated only when seeds are exposed to a second set of signals, such as light, nitrate and fluctuating temperatures (Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011). This second set of signals indicates if existing conditions are suitable to terminate dormancy and induce the completion of germination (Finch-Savage and Footitt, Reference Finch-Savage and Footitt2012).
In species with physiological dormancy, abscisic acid (ABA) and gibberellins (GA) have an antagonistic role (Yamaguchi, Reference Yamaguchi2008; Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010; Linkies and Leubner-Metzger, Reference Linkies and Leubner-Metzger2012). The action of ABA and GA is supported by numerous genetic studies using biosynthesis and signalling mutants. In particular, the ratio between the contents of these two hormones and their respective signalling pathways are important in regulating induction, maintenance and termination of dormancy (Finkelstein et al., Reference Finkelstein, Reeves, Ariizumi and Steber2008). The termination of dormancy requires low ABA/GA ratios. In contrast, the maintenance of dormancy is associated with high ABA/GA ratios (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). In addition to hormone content, dormancy termination is characterized by a decrease in ABA sensitivity and an increase in GA sensitivity (Linkies and Leubner-Metzger, Reference Linkies and Leubner-Metzger2012).
Hormone content is the result of two processes: synthesis and catabolism (Umezawa et al., Reference Umezawa, Okamoto, Kushiro, Nambara, Oono and Seki2006). A reduction in ABA content by the effect of a dormancy-terminating factor is due to a slow, or even turned off, synthesis, an increased inactivation or to the co-ordinated action of both processes. ABA synthesis is mainly modulated by NCED (9-cis-epoxycarotenoide dioxygenase) and the activity of this enzyme has been proposed as a key regulatory step in ABA synthesis (Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008). ABA inactivation comprises two processes: ABA hydroxylation or conjugation (Yamaguchi et al., Reference Yamaguchi, Kamiya, Nambara, Bradford and Nonogaki2007). ABA hydroxylation is accomplished by the activity of ABA 8′-hydroxylase (Millar et al., Reference Millar, Jacobsen, Ross, Helliwell, Poole, Scofield, Reid and Gubler2006) and ABA conjugation is achieved by the ABA-glycosyltransferase (Nambara and Marion-Poll, Reference Nambara and Marion-Poll2005). Likewise, an increment of GA content could be related to a higher GA synthesis, a reduction in GA deactivation or the parallel action of both processes (Yamauchi et al., Reference Yamauchi, Takeda-Kamiya, Hanada, Ogawa, Kuwahara, Seo, Kamiya and Yamaguchi2007).
The way in which light terminates seed dormancy altering ABA/GA ratios has been well studied (e.g. Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009). Light modulates the transcription of many genes involved in ABA and GA synthesis and catabolism (Yamaguchi, Reference Yamaguchi2008; Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010) allowing, as a result, a reduction in the ABA/GA ratio. Toyomasu et al. (Reference Toyomasu, Tsuji, Yamane, Nakayama, Yamaguchi, Murofushi, Takahashi and Inoue1993, Reference Toyomasu, Kawaide, Mitsuhashi, Inoue and Kamiya1998) observed that light promotes GA synthesis through an up-regulation of a gene that encodes a GA 3-β-hydroxylase (i.e. the enzyme that catalyses the last step in bioactive GA synthesis). In addition to this, Yamauchi et al. (Reference Yamauchi, Takeda-Kamiya, Hanada, Ogawa, Kuwahara, Seo, Kamiya and Yamaguchi2007) determined that light down-regulates a GA 2-oxidase gene, which codes for an enzyme that inactivates GA. On the other hand, light reduces seed ABA content; in this regard, Seo et al. (Reference Seo, Hanada, Kuwahara, Endo, Okamoto, Yamauchi, North, Marion-Poll, Sun, Koshiba, Kamiya, Yamaguchi and Nambara2006) observed a negative effect on the expression of AtNCED6, while Toyomasu et al. (Reference Toyomasu, Yamane, Murofushi and Nick1994) determined an up-regulation of the expression of CYP707A2, a gene encoding an ABA 8′-hydroxylase. Light treatments are also known to modify hormone signalling networks (Arana et al., Reference Arana, De Miguel and Sánchez2006; Piskurewicz et al., Reference Piskurewicz, Jikumaru, Kinoshita, Nambara, Kamiya, Lopez-Molina and Gene2008; Graeber et al., Reference Graeber, Nakabayashi, Miatton, Leubner-Metzger and Soppe2012).
In contrast, the way in which fluctuating temperatures alter ABA/GA ratios to terminate dormancy is poorly understood (Hu et al., Reference Hu, Huang and Wang2012). Cynara cardunculus (L.) var. sylvestris seeds display an almost absolute requirement for fluctuating temperatures for dormancy termination. A water relations analysis of seed germination at fluctuating and constant temperatures using Gummerson's hydrotime model (Gummerson, Reference Gummerson1986) was carried out using C. cardunculus seeds and revealed that incubation under fluctuating temperatures displaces the mean base water potential (ψ b (50)) towards more negative values (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2005). This implies that fluctuating temperatures terminate dormancy in this species through an enhancement of embryo potential to overcome a physical restraint for germination. This displacement of ψ b (50) towards more negative values has also been observed in ABA mutants or in seeds incubated in the presence of GA (Ni and Bradford, Reference Ni and Bradford1992, Reference Ni and Bradford1993; Alvarado and Bradford, Reference Alvarado and Bradford2005) suggesting a hormonal control behind the above-mentioned displacement as a result of incubation under fluctuating temperatures. Indeed, studies in C. cardunculus (L.) have shown that fluctuating temperatures terminate dormancy by reducing ABA content and sensitivity (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010). Furthermore, germination behaviour at constant and fluctuating temperatures and changing ABA/GA ratios, using exogenously applied ABA, GA and their synthesis inhibitors (i.e. fluridone and paclobutrazol, respectively), revealed that fluctuating temperatures could terminate dormancy by eliciting a reduction in ABA/GA ratio (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010). Indeed, GA3-treated and fluridone-treated seeds incubated under constant temperatures germinate as if they had been incubated under fluctuating temperatures; in contrast, the presence of paclobutrazol in the incubation medium when the seeds were incubated under fluctuating temperatures, inhibited germination as if they had been incubated under constant temperatures (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010). Taken together, these results suggest that fluctuating temperatures terminate dormancy by means of a change in ABA/GA ratio through a reduction in ABA content and sensitivity, but also through an increase in GA content and/or sensitivity. Alternatively, fluctuating temperatures could modify ABA/GA ratio just by reducing ABA content and sensitivity without modifying GA synthesis and sensitivity. In this context, the reduction of ABA content at fluctuating temperatures might be expected to be triggered at the level of gene expression through a negative regulation in the expression of an ABA synthesis gene (NCED) or a positive regulation of an ABA inactivation gene (CYP707A2) as has been observed for other signals that terminate dormancy, such as light or nitrate (Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). Likewise, the reduction in ABA sensitivity at fluctuating temperatures could be exerted by a reduction in the expression of ABA signalling genes (e.g. ABI5) (Raghavendra et al., Reference Raghavendra, Gonugunta, Christmann and Grill2010). ABI5 is a positive regulator of ABA signalling through the induction of transcription of genes involved in processes promoted by ABA (Chen et al., Reference Chen, Zhang, Neff, Hong, Zhang, Deng and Xiong2008). Similarly, if fluctuating temperatures stimulate GA synthesis or sensitivity and this stimulation takes place at the level of gene expression, this should be reflected in a positive regulation of a GA synthesis gene (e.g. GA 3-oxidase) (Yamaguchi, Reference Yamaguchi2008) or a negative regulation of GA signalling genes such as RGL2 and GAI (Hartweck, Reference Hartweck2008).
The objectives of this paper were to determine if: (1) the reduction of ABA content that results from incubation under fluctuating temperatures is exerted through changes in the transcription of ABA synthesis (NCED) and/or inactivation genes (CYP707A2); (2) dormancy termination by fluctuating temperatures includes the promotion of GA synthesis and/or sensitivity; and (3) if an eventual enhancement of GA content or sensitivity by fluctuating temperatures is exerted at the level of transcription of GA synthesis genes (i.e. GA3ox) and/or GA signalling genes (i.e. RGL2 and GAI).
Materials and methods
Plant material
C. cardunculus (L.) mature achenes (hereafter termed ‘seeds’) were hand collected from a plot at the School of Agricultural Sciences, Argentine Catholic University, Buenos Aires, Argentina (34°34′W, 58°26′S) during January 2010 and 2011, and from an infested roadside in Azul, Buenos Aires Province, Argentina (36°47′W, 59°42′S) at the time of their natural dispersal. After cleaning, seeds were kept in paper bags at − 18°C to maintain their initial level of dormancy.
Role of GA in seed responses to fluctuating temperatures
Gibberellin extraction and quantitation by LC–ESI–MS–MS
Analysis of gibberellins was carried out on seeds collected in 2010. Seeds were incubated in distilled water for 0, 1, 3, 4 and 5 d. After each incubation period, three samples (biological triplicates) of embryos were excised from the achenes and frozen in liquid N2. Samples were freeze-dried, ground to powder with a mortar and pestle, and weighed (100–200 mg per sample). Extraction of gibberellins was performed with 5 ml of methanol:water (80:20, pH 2.8) at 4°C. After centrifugation (1 min, maximum speed), buffer was collected and the pellet was then re-extracted with 2 ml of fresh buffer (pH 2.8) for an additional 4 h. A 50 ng aliquot of each of deuterated GA1, GA3, GA4, GA8 and GA34 (Lew Mander, Australian National University, Canberra, Australia) was added as internal standards. Extracts were transferred to 50-ml tubes and mixed with ethyl acetate and partitioned. Then, the organic phase was extracted and evaporated at 37°C in a Speed-Vac. After methanol evaporation, the volume of the remaining aqueous fraction was adjusted with water to 15 ml, and the pH was lowered to 2.5 with diluted HCl. The 15 ml aqueous extract was then partitioned three times against 5 ml of ethyl acetate (water saturated). After solvent evaporation, samples were taken to the University of Río Cuarto (Córdoba, Argentina) for hormone analysis.
Liquid chromatography (LC)
Analyses were performed using an Alliance 2695 (Separation Module, Waters, Milford, Massachusetts, USA) quaternary pump equipped with auto-sampler. A Restek C18 (Restek, Bellefonte, Pennsylvania, USA) column (2.1 × 100 mm, 5 μm) was used at 28°C, with injected volume 10 μl. The binary solvent system used for elution gradient consisted of 0.2% acetic acid in H2O (solvent B), and methanol (MeOH; solvent A), at a constant flow-rate of 200 μl min− 1. A linear gradient profile with the following proportions (v/v) of solvent A was applied [t (min), % A]: (0, 40), (25, 80), with 7 min for re-equilibration.
MS/MS experiments
MS/MS experiments were performed on a Micromass Quatro UltimaTMPT double quadrupole mass spectrometer (Micromass, Manchester, UK). All analyses were performed using turbo ion spray source (ESI) in negative ion mode with the following settings for gibberellins: capillary voltage − 3250 V, energy cone 35 V, RF Lens1 (20), RF Lens 2 (0.3), source temperature 100°C, desolvation temperature 350°C, gas cone 100 l h− 1, gas desolvation 701 l h− 1, collision cell potential of 15 V and multiplier (650). MS/MS parameters were optimized in infusion experiments using individual standard solutions of each hormone. MS/MS product ions were produced by collision-activated dissociation of selected precursor ions in the collision cell of the double quadrupole mass spectrometer, and mass was analysed using the second analyser of the instrument. In negative mode, the spectrum for each hormone gave deprotonated molecules [M–H]. Quantitation was performed by injection of samples in multiple reaction monitoring (MRM) modes, since many compounds could present the same nominal molecular mass. The combination of parent mass and unique fragment ions was used to selectively monitor hormones. MRM acquisition was performed by monitoring the 348/242 and 350/244 transitions for GA1 and (2H2)-GA1; 345/221 and 347/223 for GA3 and (2H2)-GA3; 332/244 and 334/246 for GA4 and (2H2)-GA4; 364/276 and 366/278 for GA8 and (2H2)-GA8, and 347/ 242 and 349/244 for GA34 and (2H2)-GA34 respectively, with dwell 2000 ms for each transition. Data were acquired and analysed using MassLynxTM 4.1 and QuanLynxTM 4.1 (Micromass) software. For quantitation, values were obtained from a calibration curve previously constructed using known amounts of each hormone and their pure standard (Sigma, St. Louis, Missouri, USA)/deuterated internal standard ratio. Data were subjected to analysis of variance (Statistix 8.0, Analytical Software, Tallahassee, Florida, USA). Tukey's test at 5% level of probability was used for comparison between means.
Sensitivity to GA3
Sensitivity to GA3 as affected by the incubation regime (constant or fluctuating) was determined in the 2011 seed lot by means of two different approaches.
The first approach (test 1) involved incubation in 7 ml of a mixed solution of GA3 (Phytotechnology Laboratories, Shawnee Mission, Kansas, USA) (0, 10, 50, 250, 1000 and 1500 μM) plus 750 μM of Trinexapac-ethyl (TE). The pH of each solution was adjusted to 6.7. To determine the dose of TE that inhibits germination (750 μM), seeds were dark incubated for 14 d at fluctuating (20°C, 12 h/10°C, 12 h) and constant temperatures (15°C) in a range of TE concentrations (0, 125, 250, 500, 750 and 1000 μM). Germination in the presence of 750 and 1000 μM of TE was similarly reduced in both thermal treatments to 15 and 10% (750 and 1000 μM, respectively).
The second approach (test 2) involved incubation in 7 ml of a mixed solution of GA3 (10, 50, 250, 1000 and 1500 μM) plus 250 μM ABA (Phytotechnology Laboratories). To determine the ABA dose that inhibits germination (250 μM) seeds were dark incubated in the presence of ABA (0, 50, 100 and 250 μM) at fluctuating (20°C, 12 h/10°C, 12 h) and constant temperatures (15°C).
In both tests, treatments were factorial combinations of five GA3 doses and two thermal conditions (15°C versus 20/10°C). The germination data were subjected to analysis of variance (Statistix 8.0, Analytical Software). Tukey's test at 5% level of probability was used for comparison between means.
RNA extraction and cDNA synthesis
Three replicates of 20 seeds were incubated for 0, 1, 3 and 5 days at fluctuating (20/10°C) and constant (15°C) temperatures. Between 15 and 17 embryos ( ≥ 350 mg) per sample were isolated from pericarp and frozen in liquid N2 and stored at − 20°C until used for RNA extraction. RNA was extracted from embryos using the mRNA Isolation Kit (Roche Molecular Biochemicals, GmbH, Mannheim, Germany) and cDNA was made with at least 10 ng of RNA sample by means of Revert Aid M-Mul V Reverse transcriptase system (Fermentas International Inc., Burlington, Ontario, Canada). RNA concentration was estimated by absorbance at 260 nm with a fluorometer Qubit® 2.0 (Invitrogen, Carlsbad, California, USA). The integrity and purity of RNA was checked with a 260/280 nm absorbance ratio.
RNA was converted to cDNA as follows: (1) the RNA concentration of each sample was determined; (2) according to the RNA concentration, a solution containing variable volumes of miliQ water and RNA plus 1 μl oligo-p (dT) (Biodynamics, Buenos Aires, Argentina) to reach a final volume of 11 μl was made. Each sample was placed in a thermocycler for 5 min at 70°C and 3 min at 4°C. Afterwards, 8 μl of a solution containing 4 μl of reverse transcriptase buffer (RT), 2 μl of dinucleotides (dNTPs) 10 mM, 0.5 μl of RNAse inhibitor (Fermentas) and 1.5 μl of MiliQ water was added. The reaction was continued for 5 min at 37°C when an additional 1 μl of RT (Fermentas) was added, with the following conditions: for 60 min at 42°C, 10 min at 70°C and finally reducing the temperature to 4°C. The cDNA was stored at − 20°C until used.
Search for Cynara sequences encoding putative orthologues for ABA and GA metabolism and signalling proteins and RT-PCR
Transcript accumulation for NCED, CYP707A2 , ABI5, GA3ox, GAI and RGL2 genes was estimated by semi-quantitative reverse transcriptase–polymerase chain reactions (RT-PCR). The cDNA sequences were obtained from seed RNA through RT-PCR. Degenerate primer pairs were designed to amplify fragments between 192 and 620 bp (Table 1) within highly conserved regions of candidate genes from other species (Table 1). Aligned sequences used for NCED primer design were: DQ173543.1 (GenBank accession number) (Rumex palustris NCED9), XM002862948.1 (Arabidopsis lyrata NCED9), NM106486.2 (Arabidopsis thaliana, NCED9), AB120110.1 (Lactuca sativa, NCED4). For CYP707A2, sequences used were: AB235920.1 (Lactuca sativa LsABA8ox4), NM128466.2 (A. thaliana CYP707A2), GU559990.1 (Prunus aviun CYP707A3), DQ145932.1 (Hordeum vulgare subsp. vulgare ABA 8′-hydroxylase 1). For ABI5, sequences used were: EU964768.1 (Zea mays), NM129185.3 (A. thaliana), AB193553.1 (Triticum aestivum), XM002970700.1 (Selaginella moellendorffii), AY150676.1 (H. vulgare subsp. vulgare). For GA 3-oxidase, the following sequences were used: AB012205.1 (L. sativa), AB303422.1 (Allium fistulosum), AB010991.1 (Solanum lycopersicum), AB613270.1 (Torenia fournieri), AB032198.1 (Nicotiana tabacum), DQ641497.1 (R. palustris), AJ006453.1 (Cucurbita maxima). For GAI, sequences used were: AY781175.1 (Oryza sativa), DQ062091.1 (Sacharum officinarum), NM101361.2 (A. thaliana), EU112606.1 (Helianthus annus). For RGL2, sequences used were: NM111216.2 (A. thaliana), XM002519168.1 (Ricinus communis) and DQ007884.1 (Malus × domestica).
Table 1 Primers used for semi-quantitative RT-PCR

R = A+G; Y = C+T; N = A+C+G+T; M = A+C; W = A+T.
PCR conditions for NCED were as follows: 4 min at 94°C (first cycle); 50 s at 94°C, 1 min at 54°C, 1 min at 72°C (40 cycles) and 7 min at 72°C (last cycle). PCR conditions for ABI5 were as follows: 4 min at 94°C; 50 s at 94°C, 45 s at 55°C, 1 min at 72°C (38 cycles) and 7 min at 72°C. PCR conditions for GA3ox were as follows: 4 min at 94°C, 50 s at 94°C, 50 s at 55°C, 1 min at 72°C (37 cycles) and 7 min at 72°C. PCR conditions for GAI, RGL2 and CYP707A2 were as follows: 4 min at 94°C, 50 s at 94°C, 1 min at 50°C, 1 min at 72°C (38 cycles) and 7 min at 72°C.
Actin was selected as a housekeeping gene and used to normalize the amount of starting template. For Actin a specific primer was designed by means of OligoPerfect™ Designer (Invitrogen) from a sequence of Cynara scolymus (GenBank accession no. AM744951.1). PCR conditions were as follows: 4 min at 94°C (first cycle), 50 s at 94°C, 50 s at 55°C, 1 min at 72°C (34 cycles) and 7 min at 72°C (last cycle).
PCR products were separated on 1.5% agarose gels, stained with SYBR Green (Invitrogen) and visualized by the UVP Doc-It LS Image Acquisition Software (UVP, Upland, California, USA). A 100-bp DNA ladder (Invitrogen) was used as a standard molecular marker. Amplified fragments were cloned into the pGEM®-T Easy vector system (Promega, Madison, Wisconsin, USA). Sequence analysis was performed on an ABI PRISM 377 DNA Sequencer (Applied Biosystems, Foster City, California, USA). The final sequence was analysed and homology searches were carried out using the National Center for Biotechnology Information (NCBI) BLASTx algorithm (www.ncbi.nlm.nih.gov/BLAST). They contained cDNA sequences of genes with very high similarity to NCED, CYP707A2, ABI5, GA3ox, GAI and RGL2 genes of other plant species (GenBank databases). Sequences are shown in Supplementary Figure S1 (available online). They were named and those with a length up to 200 bp were registered in GenBank: CycaNCED, CycaCYP707A (accession no. KF769950), CycaABI5 (KF769951), CycaGA3ox (KF769952), CycaGAI (KF769953), CycaRGL2 (KF769954) and Cycaactin. A ratio of intensities between bands obtained for each gene and those obtained for Cycaactin (in both cases analysed with the National Institutes of Health (NIH) image program) for each thermal treatment and time of incubation was calculated.
Results
Determination of fluctuating temperature requirements to terminate seed dormancy in C. cardunculus seeds
To corroborate the requirement of fluctuating temperatures to terminate C. cardunculus seed dormancy, seeds were incubated at fluctuating (20°C, 12 h/10°C 12 h) or constant (15°C, 24 h) temperatures. The exposure of seeds to fluctuating temperatures increased total germination (P= 0.0019) (Fig. 1). The effect of fluctuating temperatures on germination was observed from day 5 on (mean ± SE: 20 ± 10.4% and 0% for 20/10°C and 15°C, respectively). Maximum germination increased to 81.6 ± 10.1% and 6.6 ± 1.6% for 20/10°C and 15°C, respectively, by day 9 and no further germination was scored until the end of the experiment (day 14).
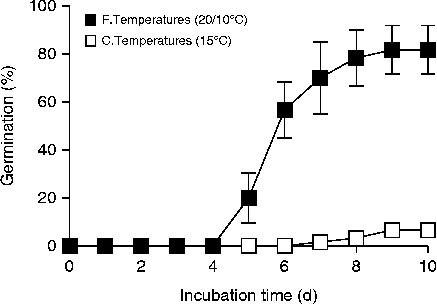
Figure 1 Cumulative germination time courses of Cynara cardunculus seeds incubated at fluctuating (20°C/10°C, 12 h thermoperiod) (closed symbols) or constant temperatures (15°C) (open symbols). Data are means of triplicates ± SEs.
Role of GA in seed responses to fluctuating temperatures
GA content during seed incubation
The embryonic content of the active GAs (GA1 and GA4) [ng (g DW)− 1] was similar in both thermal treatments during a 5 d incubation period (just prior to the onset of germination in seeds incubated at 10/20°C) (Fig. 2). Similarly, GA8 content (GA1 catabolite) did not differ between thermal regimes (Fig. 2C). GA34 (GA4 catabolite) was not detected. To confirm these results, on dry seeds and seeds incubated for 5 d, a second GA quantitation was carried out. In this assay, GA3 and GA34 contents were also studied. The GA content was similar among dry seeds and seeds incubated for 5 d at constant or fluctuating temperatures (P= 0.3, P= 0.65, P= 0.15, P= 0.44 and P= 0.15 for GA1, GA3, GA4, GA8 and GA34, respectively) (Fig. 3). These results do not support the possibility of a stimulation of GA synthesis by fluctuating temperatures.
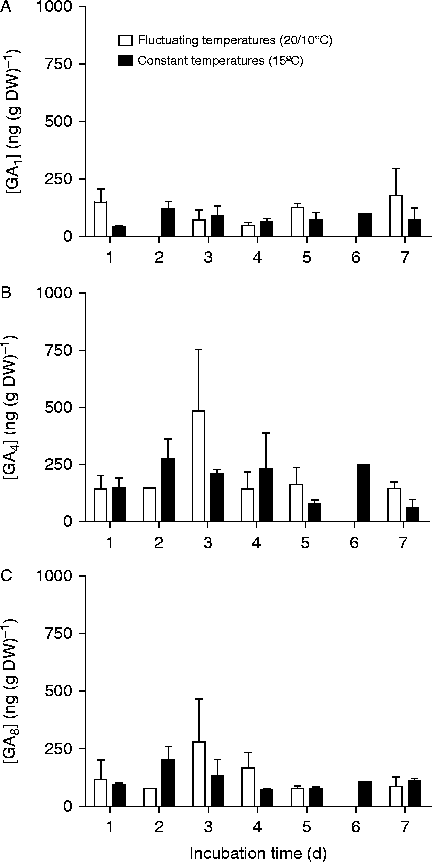
Figure 2 Embryonic content of GA1 (A), GA4 (B) and GA8 (C) as a function of incubation time for Cynara cardunculus seeds exposed to fluctuating (20°C, 12 h/10°C, 12 h) (solid bars) or constant (15°C, 24 h) (open bars) temperatures. Values represent the mean of three biological replicates ± SEs.

Figure 3 Embryonic content of GA1, GA3, GA4, GA8 and GA34 in dry seeds (DS, grey bars) or after 5 d of incubation at fluctuating (FT, closed bars) and constant temperatures (CT, open bars). Data are means of three biological replicates ± SEs.
Effect of fluctuating temperatures on seed sensitivity to GAs
Sensitivity of seeds to GA3 at fluctuating or constant temperature was determined in two independent tests through incubation in GA3 solutions at different concentrations. Sensitivity to GA3 was determined as its effectiveness to overcome the inhibitory effect imposed on germination by a solution of Trinexapac-ethyl (TE, 750 μM) (test 1) or ABA (250 μM) (test 2), both at fluctuating and constant temperatures. In both tests, the two main effects, thermal treatment (20/10°C and 15°C) and seed responses to increasing doses of GA3, differed, as well as their interaction (P< 0.05) (Fig. 4A and B ). In test 1, germination did not differ between 20/10°C and 15°C when seeds were incubated in the presence of GA3 (10, 50, 250, 500 and 1500 μM). Germination was similar between water at 20/10°C and 1500 μM of GA3 at 20/10°C or 15°C. Germination in TE at fluctuating or constant temperatures or water at constant temperatures was low. Likewise, in test 2, germination was also similar between 20/10°C and 15°C when seeds were treated with GA3 (10, 50, 250, 1000 and 1500 μM) (Fig. 4B). Germination in all ABA+ GA solutions was less than that scored in water at 20/10°C or in GA3 at constant or fluctuating temperatures. Total germination in water at 15°C or ABA (250 μM) at 20/10°C and 15°C was scarce. These results indicate that the effect of fluctuating temperatures is not through an increase in sensitivity to GA.

Figure 4 Final germination percentages of Cynara cardunculus seeds incubated at fluctuating temperatures (20°C, 12 h/10°C, 12 h) (closed bars) or constant (15°C, 24 h) (open bars) temperatures incubated in the indicated solutions. Vertical bars indicate the SEs. Similar letters at the top of each bar indicate no differences according Tukey's test (P < 0.05).
Identification of Cynara genes encoding putative ABA and GA metabolism and signalling enzymes
In order to study the effect of both thermal treatments on the expression of genes that are involved in ABA and GA synthesis, inactivation and signalling, degenerate primers matching highly conserved nucleotide sequences were designed for NCED, CYP707A2, ABI5, GA3ox, GAI and RGL2. A total of six cDNA fragments belonging to putative candidate genes (CycaNCED, CycaCYP707A2, CycaABI5, CycaGA3ox, CycaGAI and CycaRGL2) were isolated. The PCR products (size ranged between 189 and 620 bp) were purified, cloned and sequenced to confirm their putative identity. The obtained sequences are shown in Supplementary Figure S1 (available online). Sequences were compared to all known proteins in the GenBank database using BLASTx. Results of bioinformatics analysis are shown in Supplementary Table S1 (available online). Each of the six sequences identified in C. cardunculus appeared as most closely related to the proposed candidate proteins, supporting their expected identities.
Expression of ABA and GA metabolism and signalling genes during incubation at fluctuating or constant temperatures
In order to evaluate if fluctuating temperatures alter ABA and GA metabolism and signalling in relation to constant temperatures acting at the level of gene transcription, the expression pattern of CycaNCED, CycaCYP707A2 and CycaABI5 (ABA synthesis, inactivation and signalling, respectively), and CycaGA3ox, CycaRGL2 and CycaGAI (GA synthesis the former, and GA signalling the remainder) was analysed during incubation under fluctuating and constant temperatures. Incubation under fluctuating temperatures reduced expression of genes involved in ABA synthesis and signalling (Fig. 5A and C) in relation to that observed under constant temperatures. Indeed, CycaNCED expression could not be detected beyond 1 d of incubation when it was performed at fluctuating temperatures. In contrast, at constant temperatures, the expression of CycaNCED was detected throughout 5 d of incubation (Fig. 5A). Fluctuating temperatures reduced the expression of CycaABI5 in relation to that observed at constant temperatures (Fig. 5C). Differences were just found on day 5 of incubation (P= 0.017). The expression of CycaCYP707A2 was not affected by the thermal treatment (P= 0.26, 0.92 and 0.11 for days 1, 3 and 5, respectively) (Fig. 5B). These results are in full agreement with our previous results reporting ABA content and ABA sensitivity reductions as a result of incubation under fluctuating temperatures that terminate dormancy (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010). In contrast, fluctuating temperatures did not modify the expression of GA synthesis (CycaGA3ox) and signalling (CycaRGL2 and CycaGAI) genes in relation to that observed at constant temperature (Fig. 5D–F). Transcript expression for CycaGA3ox was detected by 3 d after incubation and did not differ between 20/10°C and 15°C (P= 0.20 and 0.77, for days 3 and 5 respectively). Likewise, transcript expression for CycaRGL2 and CycaGAI was similar among treatments (P= 0.26, 0.07 and 0. 34 for days 1, 3 and 5, respectively, and P= 0.15 and 0.97 for days 1 and 5, respectively). These results are also in agreement with results reported in this paper showing that stimulation of germination by fluctuating temperatures is neither through an enhancement of GA synthesis, nor through an increase in GA sensitivity (Figs 2–4).

Figure 5 Expression analysis relative to actin mRNA of CycaNCED (A), CycaCYP707A2 (B), CycaABI5 (C), CycaGA3ox (D), CycaGAI (E) and CycaRGL2 (E) in embryos isolated from achenes on 0, 1, 3 and 5 d of incubation. RT-PCR was performed as described in the Materials and methods section. Mean values from three biological replicates are shown. Bars indicate SEs.
Discussion
Fluctuating temperatures were proposed as an environmental signal involved in the termination of seed dormancy, together with light and nitrates (Bewley and Black, Reference Bewley and Black1994; Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000; Finch-Savage and Footitt, Reference Finch-Savage and Footitt2012). Furthermore, for seeds buried in the soil seed bank, the perception of fluctuating temperatures is a most relevant signal since it provides information about the spatial position of seeds (Thompson and Grime, Reference Thompson and Grime1983; Ghersa et al., Reference Ghersa, Benech-Arnold and Martinez-Ghersa1992). Despite the fact that seed responses to fluctuating temperatures are widespread, knowledge about physiological mechanisms underlying such responses is still insufficient (Hu et al., Reference Hu, Huang and Wang2012). The termination of seed dormancy is associated with changes in hormone contents (i.e. a low ABA/GA ratio), as well as in hormone sensitivity (i.e. high GA sensitivity and/or low ABA sensitivity) (Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005; Finkelstein et al., Reference Finkelstein, Reeves, Ariizumi and Steber2008; Graeber et al., Reference Graeber, Nakabayashi, Miatton, Leubner-Metzger and Soppe2012). Several studies reported changes in ABA/GA ratio and sensitivity in response to light or nitrate (e.g. Toyomasu et al., Reference Toyomasu, Tsuji, Yamane, Nakayama, Yamaguchi, Murofushi, Takahashi and Inoue1993, Reference Toyomasu, Kawaide, Mitsuhashi, Inoue and Kamiya1998; Sawada et al., 2008a, b, c; Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009). In contrast, changes in ABA/GA ratio and hormone sensitivity as a result of exposure to fluctuating temperatures that terminate dormancy have been partially assessed. In a previous paper (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010), we have reported that fluctuating temperatures terminate C. cardunculus dormancy through a reduction in ABA content and sensitivity. To account for a lower ABA content and sensitivity in seeds exposed to fluctuating temperatures, the expression of genes involved in ABA synthesis, catabolism and signalling should be different between both thermal treatments, so long as the effect of the stimulatory thermal regime is exerted at the level of gene transcription. Fluctuating temperatures reduced mRNA abundance of CycaNCED from day 1 on and CycaABI5 at day 5 (Fig. 5A and C). In contrast, mRNA abundance of CycaCYP707A2 did not differ between treatments (Fig. 5B). These results suggest that fluctuating temperatures terminate seed dormancy by means of a negative regulation of the transcription of ABA synthesis and signalling genes (i.e. putative CycaNCED and CycaABI5). This is consistent with the results of Argyris et al. (Reference Argyris, Dahal, Hayashi, Still and Bradford2008) and Toh et al. (Reference Toh, Imamura, Watanabe, Nakabayashi, Okamoto, Jikumaru, Hanada, Aso, Ishiyama, Tamura, Iuchi, Kobayashi, Yamaguchi, Kamiya, Nambara and Kawakami2008) who found a negative regulation of ABA synthesis genes at temperatures permissive for germination. Also, and similar to our results, both studies did not show changes in the expression of ABA catabolism genes (e.g. LsABA8ox4). On the other hand, the inhibition of germination by constant (Fig. 1) or supraoptimal temperatures reported by Argyris et al. (Reference Argyris, Dahal, Hayashi, Still and Bradford2008), Toh et al. (Reference Toh, Imamura, Watanabe, Nakabayashi, Okamoto, Jikumaru, Hanada, Aso, Ishiyama, Tamura, Iuchi, Kobayashi, Yamaguchi, Kamiya, Nambara and Kawakami2008) and Huo et al. (Reference Huo, Dahal, Kunusoth, McCallum and Bradford2013), seems to be exerted through a positive regulation of the expression of ABA synthesis genes. Fluctuating temperatures down-regulated the expression of the ABA-signalling gene CycaABI5 from day 3 onwards. ABI5 is a positive regulator of ABA signalling (Lopez-Molina et al., Reference Lopez-Molina, Mongrand, McLachlin, Chait and Chua2002). The low expression of CycaABI5 under fluctuating temperatures, might determine reduced protein abundance, thus resulting in low sensitivity to ABA inhibitory action. This result agrees with the results of our physiological approach where fluctuating temperatures reduced ABA sensitivity of germination (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010). Therefore, it appears that the stimulation of germination by fluctuating temperatures operates, at least in part, through the modulation of the expression of ABA synthesis and signalling genes. Seed sensitivity to ABA is related to ABI5 content, and a close relationship exists between ABA content and ABI5 protein (Lopez-Molina et al., Reference Lopez-Molina, Mongrand and Chua2001). Therefore, environmental signals that lead to a reduction of ABA content (i.e. light, nitrates and fluctuating temperatures in this case) would also reduce the expression of ABI5. Nevertheless, ABI5 content and stability are not modulated by ABA content only. Lopez-Molina et al. (Reference Lopez-Molina, Mongrand, Kinoshita and Chua2003) and Stone et al. (Reference Stone, Williams, Farmer, Vierstra and Callis2006), reported that AFP (ABI5 binding protein) and KEG (Keep On Going) target ABI5 for subsequent degradation by the ubiquitine–proteosome system. Although the reduction in ABA content triggered by incubation under fluctuating temperatures also occurs with other signals that terminate dormancy, such as light and nitrates, the regulatory site for ABA content is not always a reduced rate of ABA synthesis. For instance, Lactuca sativa light-treated seeds exhibit two regulatory sites at the level of gene transcription to control ABA content (Sawada et al., Reference Sawada, Aoki, Nakaminami, Mitsuhashi and Tatematsu2008a, Reference Sawada, Aoki, Nakaminami, Mitsuhashi, Tatematsu, Kushiro, Koshiba, Kamiya, Inoue, Nambara and Toyomasub): a negative regulation of two ABA synthesis genes LsNCED2 and LsNCED4 and a positive regulation of the ABA catabolism gene LsABA8ox4. In turn, for nitrate-treated seeds, the regulatory site for ABA content is only an up-regulation of the ABA catabolism gene CYP707A2 (Matakiadis et al., Reference Matakiadis, Alboresi, Jikumaru, Tatematsu, Pichon, Renou, Kamiya, Nambara and Truong2009). Recently, Foley et al. (Reference Foley, Chao, Dogramaci, Horvath and Anderson2012) analysed the trancriptome of Euphorbia esula seeds exposed to fluctuating and constant temperatures and found that fluctuating temperatures did not modify the transcription of ABI3 and ABI5 after 3 d of seed exposure to an alternating thermal regime, a time at which E. esula seeds are still far from the onset of germination (Foley and Chao, Reference Foley and Chao2008). Transcriptional changes at times closer to germination onset were not provided, thus precluding a more direct comparison with our results.
In a previous paper we have shown that the manipulation of the seed hormone balance through the use of exogenously applied ABA, GA and their respective synthesis inhibitors, under fluctuating and constant temperatures, allows for the modification of seed germination behaviour (Huarte and Benech-Arnold, Reference Huarte and Benech-Arnold2010). From these results, it was observed that those experimental conditions that imposed a low ABA/GA ratio (i.e. fluridone and GA3 application) were as effective as fluctuating temperatures to terminate dormancy. One explanation for these results was that fluctuating temperatures stimulate germination through an enhancement of GA synthesis and/or sensitivity in addition to the observed reduction in ABA content. The contents of GA1 and GA4 did not differ throughout incubation under fluctuating or constant temperatures (Figs 2 and 3). These results show that GA synthesis was equally active at both thermal regimes. It appears, then, that at constant temperatures, a continuous ABA synthesis is the mechanism responsible for dormancy maintenance (i.e. high ABA/GA ratio). Therefore, using fluridone in the incubation medium impaired ABA synthesis and, accordingly, reduced ABA/GA ratio, since GA synthesis is active regardless of the thermal treatment. For this reason, seeds treated with fluridone and incubated under a constant temperature behaved as if they had been exposed to fluctuating temperatures. Conversely, fluctuating temperatures reduced ABA content without affecting GA synthesis, resulting in a low ABA/GA ratio that allowed for the termination of dormancy. The presence of paclobutrazol in the incubation medium when seeds were incubated at fluctuating temperatures reduced GA content and reset the ABA/GA ratio to that imposed by incubation under constant temperatures. Hence, paclobutrazol-treated seeds incubated under fluctuating temperatures behaved as if they had been incubated under constant temperatures. In each one of these scenarios GA de novo synthesis appeared as a requisite for germination of seeds from this species to occur. The determination of active GA content was consistent with the expression level of CycaGA3ox (Fig. 5D). Selection of expression of CycaGA3ox among other genes involved in GA synthesis was due to the fact that GA 3-oxidase gene, coding the enzyme committed to the final inter-conversion into the bioactive GA4 or GA1, is the gene up-regulated by Pfr, the active form of phytochrome (Yamaguchi, Reference Yamaguchi2008). Fluctuating temperatures did not enhance seed sensitivity to exogenous GA3 either (Fig. 4A, B), thus suggesting that an increase in GA sensitivity is not part of the physiological mechanism through which fluctuating temperatures terminate seed dormancy. This is further supported by the fact that fluctuating temperatures did not down-regulate the expression of GA signalling genes (GAI and RGL2) (Fig. 5E and F).
These results, together with those reported by Huarte and Benech-Arnold (Reference Huarte and Benech-Arnold2010), allow us to give an explanation of the mechanisms underlying the termination of dormancy by fluctuating temperatures in C. cardunculus and to denote the hormonal nature of the process. Fluctuating temperatures terminate seed dormancy by a reduction of ABA synthesis and sensitivity with no changes in GA synthesis, catabolism and sensitivity.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0960258514000051
Acknowledgements
We are grateful to Natalia Ilina and Jessica Barneto for their guidance of H.R.H. in molecular experiments, Eduardo Fernandez for his help with the statistical analyses and Oscar Masciarelli for measurements of gibberellins.
Financial support
This work was supported by Universidad Católica Argentina grant 2010-2012 to H.R.H.
Conflicts of interest
None.








