Introduction
In fire-prone ecosystems, seeds germinate predominantly after fire (Bell et al., Reference Bell, Vlahos and Watson1987; Tyler, Reference Tyler1995), and smoke is an important component of fire that promotes germination (De Lange and Boucher, Reference De Lange and Boucher1990). The major germination-stimulating chemical in smoke water is 3-methyl-2H-furo[2,3-c]pyran-2-one (karrikinolide; KAR1) (Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2004, Reference Baker, Steadman, Plummer and Dixon2009; Van Staden et al., Reference Van Staden, Jäger, Light and Burger2004). In addition to the presence of other structurally related karrikins in smoke (Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2009), glyceronitrile (2,3-dihydroxypropanenitrile) is another germination-promoting chemical that has recently been isolated from smoke water (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011). There are possibly additional chemical(s) that promote germination in smoke water, since some smoke-responsive species do not germinate with either KAR1 or glyceronitrile (Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013).
Smoke water has stimulated the germination of many species, primarily from fire-prone regions including south-western and eastern Australia (Roche et al., Reference Roche, Dixon and Pate1997; Read et al., Reference Read, Bellairs, Mulligan and Lamb2000), California (Keeley and Fotheringham, Reference Keeley and Fotheringham1998a), the Western Cape of South Africa (Brown et al., Reference Brown, Van Staden, Daws and Johnson2003) and the Mediterranean Basin (Pérez-Fernández and Rodríguez-Echeverría, Reference Pérez-Fernández and Rodríguez-Echeverría2003; Çatav et al., Reference Çatav, Küçükakyüz, Akbaş and Tavşanoğlu2014). These taxa are from a wide range of families (Pausas and Keeley, Reference Pausas and Keeley2009) including the Haemodoraceae (Roche et al., Reference Roche, Dixon and Pate1997; Brown et al., Reference Brown, Van Staden, Daws and Johnson2003; Brown and Botha, Reference Brown and Botha2004). Examples of genera from this family that are stimulated by smoke water include Wachendorfia from South Africa (Brown and Botha, Reference Brown and Botha2004) and Anigozanthos and Conostylis from Australia (Roche et al., Reference Roche, Dixon and Pate1997). Conostylis is the largest genus in the Haemodoraceae family, with over 45 species (Macfarlane et al., Reference Macfarlane, Hopper, Purdie, George and Patrick1987; Hopper et al., Reference Hopper, Chase and Fay2006). Conostylis taxa that are promoted by smoke water include C. aculeata (Roche et al., Reference Roche, Dixon and Pate1997; Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2004; Norman et al., Reference Norman, Plummer, Koch and Mullins2006), C. candicans (Lloyd et al., Reference Lloyd, Dixon and Sivasithamparam2000; Turner et al., Reference Turner, Merritt, Renton and Dixon2009), C. neocymosa (Dixon et al., Reference Dixon, Roche and Pate1995; Tieu et al., Reference Tieu, Dixon, Meney and Sivasithamparam2001), C. setigera (Tieu et al., Reference Tieu, Dixon, Sivasithamparam, Plummer and Sieler1999) and C. setosa (Dixon et al., Reference Dixon, Roche and Pate1995).
Both Conostylis and Anigozanthos are in the same subfamily (Conostylidoideae), and both genera are endemic to fire-prone south-western Australia (Macfarlane et al., Reference Macfarlane, Hopper, Purdie, George and Patrick1987; Cowling et al., Reference Cowling, Rundel, Lamont, Arroyo and Arianoutsou1996; Hopper and Gioia, Reference Hopper and Gioia2004). Most species known to germinate in response to smoke water are responsive to KAR1 (Chiwocha et al., Reference Chiwocha, Dixon, Flematti, Ghisalberti, Merritt, Nelson, Riseborough, Smith and Stevens2009). However, many Anigozanthos taxa germinate in response to smoke water but not KAR1 (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013, Reference Downes, Light, Pošta, Kohout and Van Staden2014). Instead, these Anigozanthos taxa germinate in response to glyceronitrile, another smoke-derived chemical (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013, Reference Downes, Light, Pošta, Kohout and Van Staden2014). To date, only two genera, Anigozanthos (Haemodoraceae) and Rhodocoma (Restionaceae), are known to contain one or more taxa that are glyceronitrile- but not KAR1-responsive (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013, Reference Downes, Light, Pošta, Kohout and Van Staden2014). Conversely, at least two Conostylis taxa, C. aculeata and C. candicans, are promoted to germinate by both smoke water and KAR1, and the latter also responds to glyceronitrile (Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2004, Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011). Additional Conostylis taxa require examination to determine whether both KAR1- and glyceronitrile-responsiveness is more widespread in this genus.
Increases in light and soil nitrate are other features of the post-fire environment that could potentially influence Conostylis germination, apart from smoke. Fire can alter the quantity and type of light that seeds receive in the post-fire period through the removal of the canopy and litter, and through soil disturbance (Thanos and Rundel, Reference Thanos and Rundel1995). Inorganic nitrate has been dismissed as a major germination-promoting chemical in smoke water because many smoke-responsive species are not nitrate responsive (Keeley and Fotheringham, Reference Keeley and Fotheringham1998b). Furthermore, the concentration of nitrate in smoke water is too low to promote germination (Lloyd, Reference Lloyd2001). There is approximately 0.01 mM nitrate in a 1:10 dilution of ‘Seed Starter’ (Lloyd, Reference Lloyd2001), the optimum dilution of smoke water for germinating a number of species (Tieu et al., Reference Tieu, Dixon, Sivasithamparam, Plummer and Sieler1999; Baker et al., Reference Baker, Steadman, Plummer and Dixon2005a), and 10 mM nitrate is often required to promote germination of nitrate-responsive seeds (Thanos and Rundel, Reference Thanos and Rundel1995; Plummer et al., Reference Plummer, Rogers and Bell2001; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b). Although nitrate is not a major component of smoke water, soil nitrate levels can be enhanced following fire through the nitrification of ammonium in ash (Christensen, Reference Christensen1973; Stock and Lewis, Reference Stock and Lewis1986; Baldwin and Morse, Reference Baldwin and Morse1994). Some species from fire-prone environments are promoted to germinate by nitrate (Thanos and Rundel, Reference Thanos and Rundel1995; Pérez-Fernández and Rodríguez-Echeverría, Reference Pérez-Fernández and Rodríguez-Echeverría2003; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b), and so it is possible that nitrate may influence Conostylis germination.
It is unknown whether Conostylis seeds respond to any fire-related cues when freshly collected, or whether they first require a period of dormancy release. Conostylis seeds are water permeable and have a small embryo, and according to Turner et al. (Reference Turner, Steadman, Vlahos, Koch and Dixon2013), C. candicans has either physiological or morphophysiological dormancy. Across all plant families, non-deep physiological dormancy is the most common form of seed dormancy (Baskin and Baskin, Reference Baskin and Baskin2004), and in such seeds, a period of soil burial may increase the sensitivity of seeds to light, nitrate and/or smoke water (Bouwmeester and Karssen, Reference Bouwmeester and Karssen1993; Derkx and Karssen, Reference Derkx and Karssen1993; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b, Reference Baker, Steadman, Plummer, Merritt and Dixonc). A number of species from south-western Australia require burial or show enhanced levels of germination following soil burial, including C. aculeata, before becoming responsive to smoke water in autumn, the start of the Mediterranean climate rainy season (Roche et al., Reference Roche, Dixon and Pate1997; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b, Reference Baker, Steadman, Plummer, Merritt and Dixonc).
The primary aim of this study was to determine whether the seeds of eight Conostylis taxa are promoted to germinate by smoke water, and whether they respond to KAR1 and/or glyceronitrile. The relative effectiveness of these chemicals will assist in understanding the evolutionary development of responses to different chemicals in smoke water within the Haemodoraceae subfamily, Conostylidoideae. This study also aimed to determine whether light and nitrate, which are other germination stimulants associated with post-fire environments, influenced germination of these Conostylis taxa. A further aim was to determine whether seeds respond to any of these stimulants when freshly matured or whether seeds require a period of dormancy release prior to germination stimulation.
Materials and methods
Seed collection
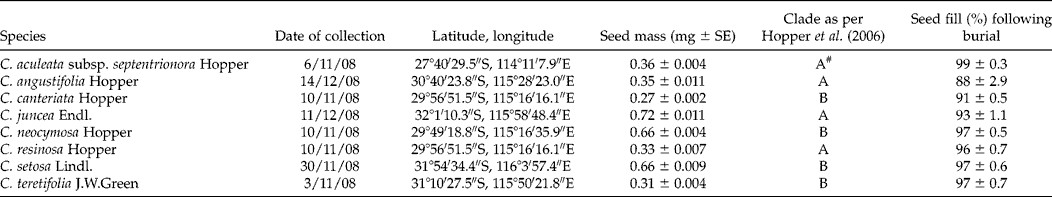
Seeds of eight Conostylis taxa were collected at maturity in November and December 2008 from natural populations between Perth and Kalbarri, Western Australia (Table 1). All eight taxa are rhizomatous perennial grass-like herbs, and all have a tufted growth form, except for C. aculeata subsp. septentrionora which is shortly proliferous (Western Australian Herbarium, 1998–). Dried, pressed plant specimens were submitted to Perth Herbarium. Each of the two major clades in this genus are represented by four taxa (Table 1; Hopper et al., Reference Hopper, Chase and Fay2006). Following collection, seeds were aspirated through a vacuum separator (Kimseed International, Osborne Park, Perth, Australia) to remove lighter seeds and chaff. Each time lighter seeds were removed they were dissected, and the aspiration process repeated until all removed seeds were filled. It was then assumed that the remaining seeds, which were used in all subsequent trials, had high seed fill. These filled seeds were also examined under a binocular microscope and any damaged seeds were removed. To determine the mean seed mass for each species, four replicates of 50 seeds (except for C. setosa, where there were only sufficient seeds for four seeds per replicate) were weighed on a five-decimal-place balance and mean mass per seed calculated (Table 1).
Table 1 Collection details for the eight Conostylis taxa examined, including the date of collection and latitude and longitude of the source populations, as well as the seed mass and taxonomic clade for each of the taxa. The mean percentage of seeds that were filled in each of the 12 bags (4 per replicate site) following 1 year of burial, as determined by visual inspection under a microscope and aspiration of lighter seeds using a vacuum separator, is also provided. Note that only filled seeds were used in the post-burial germination tests

# This taxon was not included in the Hopper et al. (Reference Hopper, Chase and Fay2006) analysis, but other C. aculeata subspecies in the analysis were in this clade.
Germination of fresh seeds in response to nitrate, smoke water or KAR1 under light or dark conditions
Germination trials were established within 1 month of seed collection to determine whether fresh seeds were dormant (cf. Baskin et al., Reference Baskin, Thompson and Baskin2006). To minimize fungal contamination, seeds were surface sterilized in 2% sodium hypochlorite with a drop of Tween 80 (polyoxyethylene sorbitan mono-oleate). Seeds were placed in the hypochlorite under vacuum for 5 min, returned to normal air pressure for 5 min and placed under vacuum for a further 5 min. Seeds were then transferred to a laminar flow bench and rinsed at least twice with sterile deionized water. Thereafter, 50 seeds were placed on two pieces of Whatman No. 1 filter paper in 9-cm Petri dishes (three replicates for each treatment). Three 4-cm2 pieces of ‘Vileda’ sponge were placed under the filter paper to hold sufficient test solution.
For each species, seeds were incubated with one of the following test solutions: sterile deionized water (control), 10 mM potassium nitrate, a 1:10 (v/v) dilution of ‘Seed Starter’ smoke water or 0.1 μM KAR1. The ‘Seed Starter’ was purchased from Kings Park and Botanic Garden, Perth, Western Australia in 2003 and stored at 4°C prior to use. Before dilution the ‘Seed Starter’ was filtered through a 0.2 μm Acrodisc syringe filter. Note that ‘Seed Starter’ does not contain added gibberellins. The KAR1 (99% purity) was isolated from smoke water (which did not contain added gibberellins) according to the methods of Van Staden et al. (Reference Van Staden, Jäger, Light and Burger2004). To each Petri dish, 10 ml of the relevant test solution was added. Petri dishes were sealed with Parafilm and incubated in either continuous light (provided by cool white fluorescent tubes), or continuous darkness (wrapped in aluminium foil) at 15°C. This temperature is optimal for the germination of many species from south-western Australia, including C. neocymosa, since it coincides with the onset of winter rainfall when seedlings have the greatest chance of establishment and subsequent survival (Bellairs and Bell, Reference Bellairs and Bell1990; Bell, Reference Bell2001). Germination was scored after 4 weeks.
Germination of seeds after 1 year of burial in response to nitrate, smoke water or KAR1 under light or dark conditions
Since a period of seed burial enhanced the germination response to smoke water in C. aculeata subsp. septentrionora and a number of other species from south-western Australia and elsewhere (Roche et al., Reference Roche, Dixon and Pate1997; Keeley and Fotheringham, Reference Keeley and Fotheringham1998a; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b, Reference Baker, Steadman, Plummer, Merritt and Dixonc), burial experiments were also undertaken. Prior to burial, seeds were stored in an air-conditioned laboratory at approximately 22°C. For each of the eight Conostylis taxa examined in this study, four nylon mesh bags (each containing 150 seeds) were buried at each of the three different sites (replicates) within the respective source populations in autumn (between 13 and 23 May 2009). The mesh bags enabled the seeds to be exposed to changes in soil temperature and moisture, and assisted in seed retrieval. Seeds were buried approximately 1–2 cm beneath the soil surface, since the majority of soil-stored seeds occur at this depth (Tacey and Glossop, Reference Tacey and Glossop1980). Seeds were exhumed the following autumn (between 2 and 10 April 2010) as previous studies have demonstrated that dormancy is overcome in some south-western Australian species when seeds are buried in autumn and exhumed 1 year later (Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b, Reference Baker, Steadman, Plummer, Merritt and Dixonc). Upon retrieval, the seeds were examined and passed through a vacuum separator to determine the number that had either commenced germinating, as indicated by the presence of an emerging radicle, or were empty, indicating that they had either germinated or were non-viable. This method, however, does not verify the total loss of viability since the proportion of germinated versus non-viable seeds is unknown, and it is possible that some of the retrieved seeds may have been non-viable, though filled.
Following exhumation, seeds were surface sterilized and placed in Petri dishes as described above for the fresh seeds. Seeds were incubated with one of the following test solutions: sterile deionized water (control), 10 mM potassium nitrate, a 1:10 (v/v) dilution of ‘Seed Starter’ smoke water or 0.1 μM KAR1 (isolated) - which were prepared as for the fresh seeds above. Three replicates (burial locations) of 50 seeds (comprised of 12–13 seeds from each of the four bags) were incubated in each of these test solutions for 4 weeks at 15°C in continuous light or continuous darkness.
Germination of laboratory-stored seeds in response to smoke water, KAR1 or glyceronitrile in the dark
Conostylis seeds collected in 2008, and not used in the initial germination or burial trials, were sealed in aluminium foil bags and stored at 15°C. In late August 2012, additional germination trials were undertaken using these seeds. Seeds were surface-sterilized as outlined above and the following treatments were undertaken: sterile deionized water (control); 0.1 μM KAR1 (synthesized); 0.1 μM KAR1 (isolated); 10, 50 or 100 μM glyceronitrile; or a 1:10 (v/v) dilution of ‘Seed Starter’ smoke water. KAR1 (>95% purity) was synthesized as per Flematti et al. (Reference Flematti, Ghisalberti, Dixon and Trengove2005), or isolated (>99% purity) from smoke water as described in Van Staden et al. (Reference Van Staden, Jäger, Light and Burger2004), and stored at 5°C. The effects of synthesized or isolated KAR1 were compared since the use of isolated, as opposed to synthesized KAR1, has been questioned (Chiwocha et al., Reference Chiwocha, Dixon, Flematti, Ghisalberti, Merritt, Nelson, Riseborough, Smith and Stevens2009). Glyceronitrile (>95% purity) was synthesized as a racemic mixture according to the method of Kopecký and Šmejkal (Reference Kopecký and Šmejkal1984). The pH of the solutions were as follows: deionized water (6.4); KAR1 0.1 μM synthesized (6.3); KAR1 0.1 μM isolated (6.2); glyceronitrile 10 μM (6.0), 50 μM (6.2), 100 μM (6.3); and smoke water (4.7). All solutions, apart from the deionized water, were diluted from stock solutions of higher concentration and used within a week of dilution.
Statistical analysis
For each species, for freshly collected seeds and seeds after 1 year of burial, a two-way ANOVA was performed to compare chemical treatments (water, nitrate, smoke water and KAR1) as one factor, and light versus dark as the other. A one-way ANOVA was performed on each species to compare the effect of chemical treatments on the laboratory-stored seeds. Germination percentage data were converted to a value between zero and one, and arcsine square-root transformed before analysis (Zar, Reference Zar2014). A Tukey test was undertaken for multiple comparison testing (Zar, Reference Zar2014). Statistical analyses were conducted in Genstat 17 (VSN International, Oxford, UK).
Results
Germination of fresh seeds in response to nitrate, smoke water or KAR1 under light or dark conditions
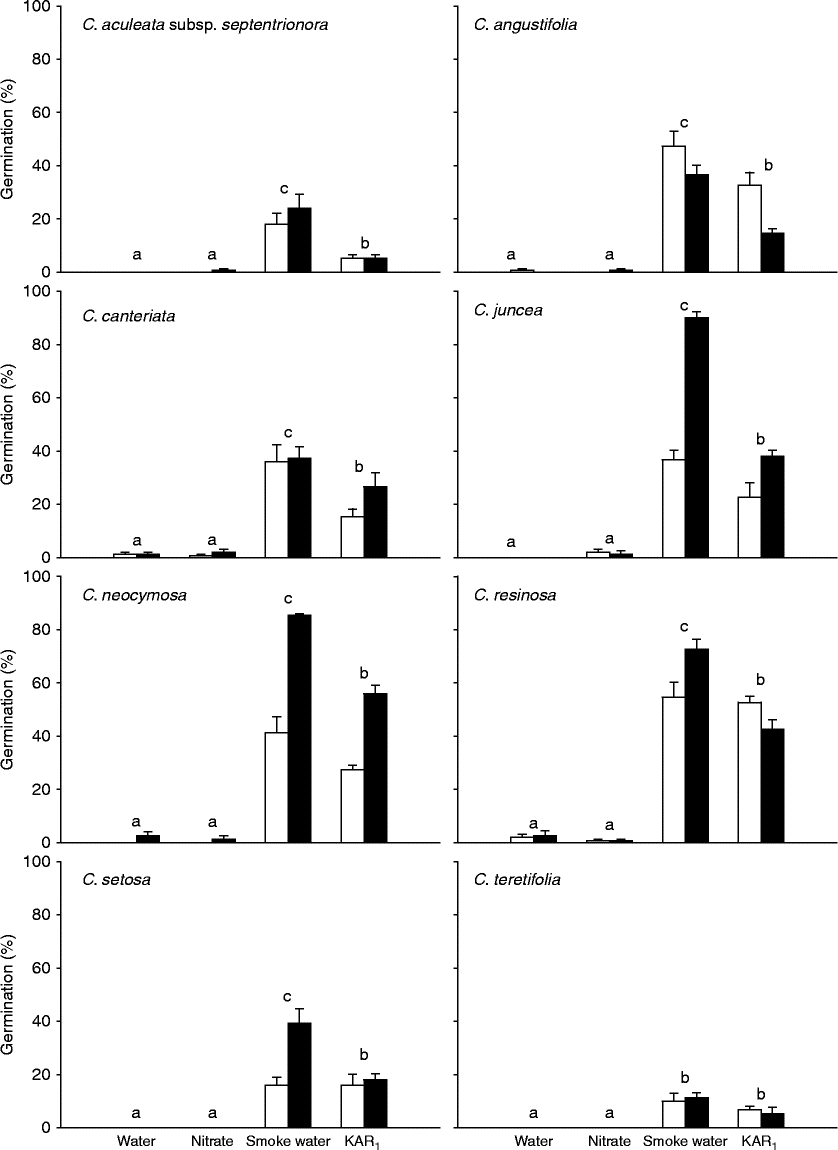
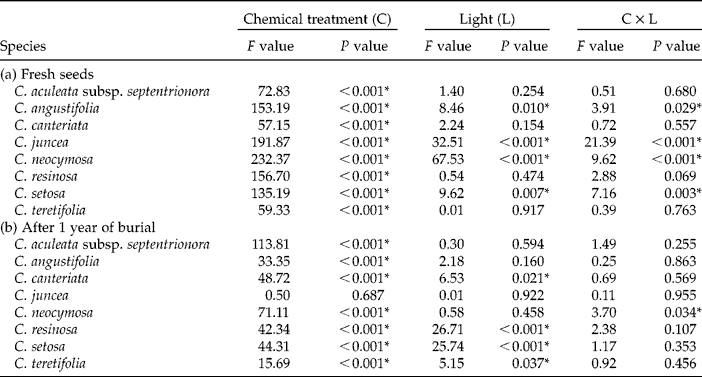
Freshly collected seeds of all eight Conostylis taxa germinated in response to both smoke water and KAR1 (Fig. 1). The level of smoke-stimulated germination ranged from 10 ± 3.0% to 90 ± 2.3% in C. teretifolia and C. juncea, respectively. In both the water and nitrate treatments, germination of fresh seeds in all Conostylis species tested was negligible ( < 3%).
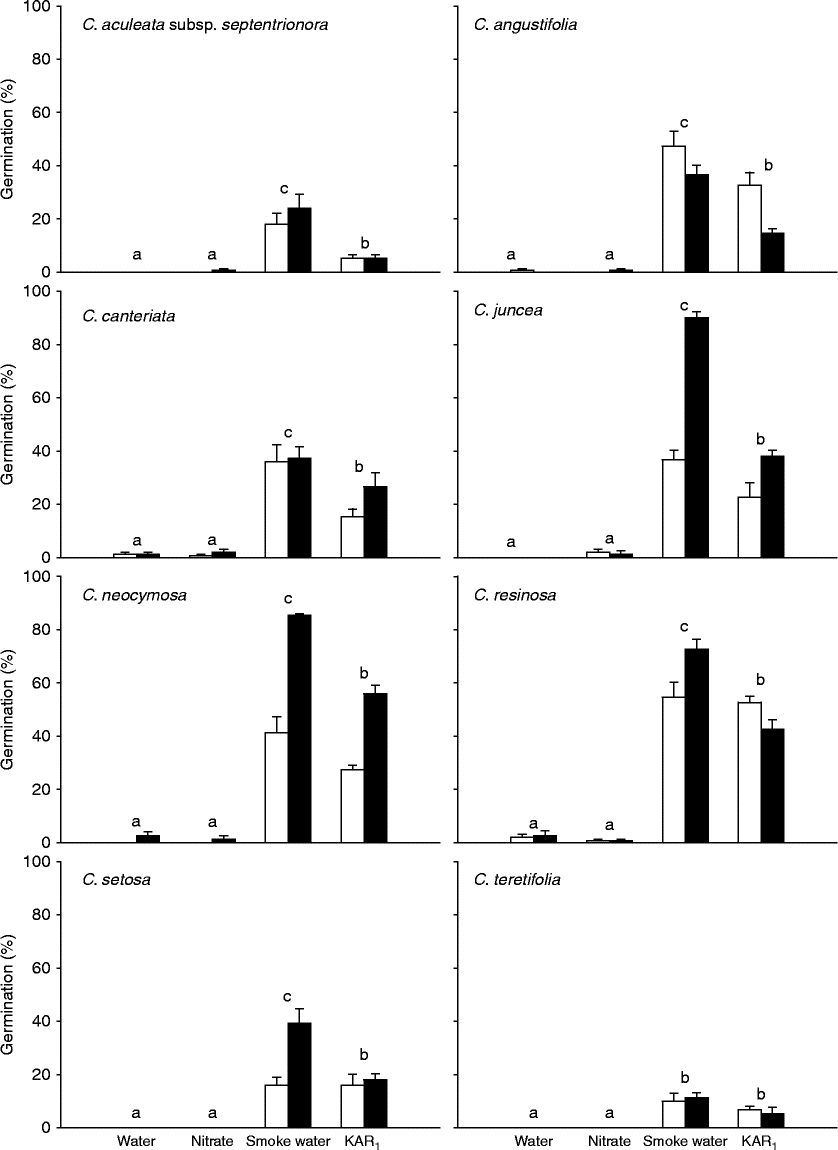
Figure 1 Germination (%, mean ± SE) of freshly collected seeds of eight Conostylis taxa in response to water, 10 mM potassium nitrate, a 1:10 (v/v) dilution of ‘Seed Starter’ smoke water or 0.1 μM KAR1 after incubation for 4 weeks at 15°C in continuous light (white bars) or continuous dark (black bars). Different letters indicate significant differences between treatments (P < 0.05).
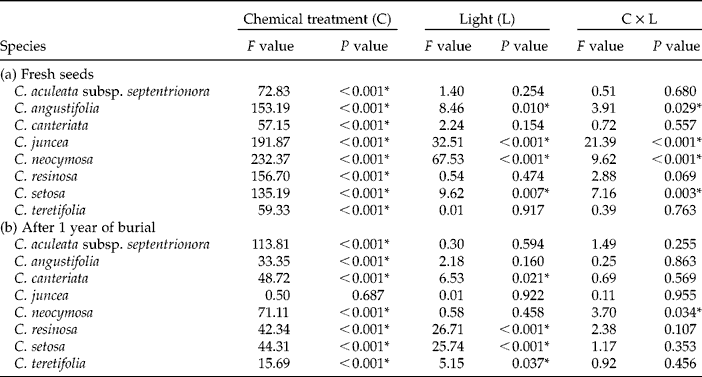
In four of the eight species there was a significant effect of the light conditions on germination levels (Fig. 1, Table 2a). For three of these species (C. juncea, C. neocymosa and C. setosa) germination was higher in the dark than the light for the smoke water and KAR1 treatments. Conversely, germination of C. angustifolia was higher in the light than the dark in these two treatments. For these four taxa light conditions had no effect on the water or nitrate treatments as germination in both the light and dark was negligible ( < 3%).
Table 2 F and P values of two-way ANOVAs, with chemical treatments (C; water, nitrate, smoke water and KAR1) as one factor and light conditions (L; light versus dark) as the other, for the germination of eight Conostylis species when (a) freshly collected and (b) after 1 year of burial. P values < 0.05 are considered significant and are highlighted with an asterisk*
Germination of seeds after 1 year of burial in response to nitrate, smoke water or KAR1 under light or dark conditions
Upon exhumation following burial, no seeds were observed to have commenced germinating recently. However, three C. neocymosa seedlings had grown through the bags, suggesting earlier germination of some of these seeds. This represented less than 0.2% of the seeds of this taxon that were buried. The percentage of seeds buried that were empty upon retrieval, indicating either germination or loss of viability, ranged from 1 to 12% in C. aculeata subsp. septentrionora and C. angustifolia, respectively (Table 1).
Overall, germination was generally higher in all treatments after up to 6 months of laboratory storage at 22°C followed by 1 year of burial, than in freshly collected seeds (Figs 1 and 2). After 1 year of burial, both smoke water and KAR1 stimulated germination to higher levels than water alone in seven of the eight Conostylis species (Fig. 2, Table 2b). The exception was C. juncea, which germinated to similar levels across all treatments. The nitrate treatment did not promote higher levels of germination than the water control in any of the species.
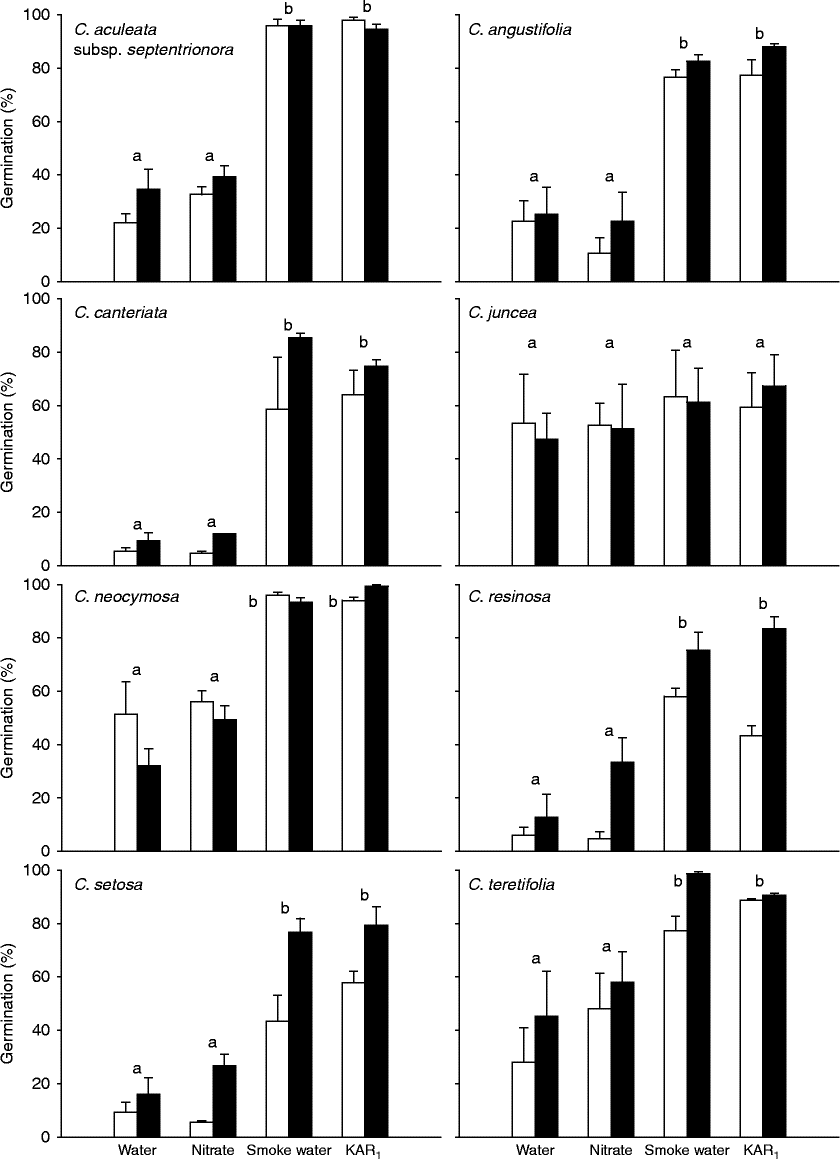
Figure 2 Germination (%, mean ± SE) of seeds of eight Conostylis taxa following 1 year of burial, in response to water, 10 mM potassium nitrate, a 1:10 (v/v) dilution of ‘Seed Starter’ smoke water or 0.1 μM KAR1 after incubation for 4 weeks at 15°C in continuous light (white bars) or continuous dark (black bars). Different letters indicate significant differences between treatments (P < 0.05).
Four taxa, C. canteriata, C. resinosa, C. setosa and C. teretifolia, germinated to higher levels in the dark than the light following burial (Fig. 2, Table 2b). In C. neocymosa the effect of light was variable between chemical treatments. In water, germination was higher in the light than the dark, whereas germination with KAR1 was higher in the dark. In the remaining three taxa germination was not significantly influenced by the light conditions.
Germination of laboratory-stored seeds in response to smoke water, KAR1 or glyceronitrile in the dark
Following 44–45 months of laboratory storage at 15°C, only three of the eight Conostylis species germinated to >10% in water alone (Fig. 3). These three species (C. juncea, C. neocymosa and C. setosa) also had the heaviest seeds of the eight taxa (Table 1). C. neocymosa, had high germination ( ≥ 75%) across all treatments. The germination of the other seven Conostylis species examined was significantly enhanced in response to both smoke water and KAR1. There was no difference in germination levels between seeds treated with synthesized or isolated KAR1.

Figure 3 Germination (%, mean ± SE) of seeds of eight Conostylis taxa following 44–45 months of storage at 15°C in response to water, 1:10 (v/v) dilution of ‘Seed Starter’ smoke water, 0.1 μM KAR1 (synthesized), 0.1 μM KAR1 (isolated), and 10, 50 or 100 μM glyceronitrile after 4 weeks' incubation at 15°C in the dark. Different letters indicate significant differences between treatments (P < 0.05).
Of the seven species that were smoke-water responsive, four (C. aculeata subsp. septentrionora, C. canteriata, C. juncea and C. resinosa) were stimulated by at least one of the glyceronitrile concentrations to germinate to higher levels than the water control. Three of these taxa are from clade A and one is from clade B (Table 1). Of the glyceronitrile concentrations tested, only 50 μM promoted C. canteriata germination, whereas 50 and 100 μM were most effective at promoting C. aculeata subsp. septentrionora germination. In the remaining two taxa, there was no difference in germination levels between the three concentrations (10, 50 or 100 μM) of glyceronitrile tested. Generally, germination levels in glyceronitrile were lower than those in smoke water in the seven smoke-responsive species. The exceptions were C. aculeata subsp. septentrionora and C. juncea where germination levels in one or more of the glyceronitrile concentrations tested were similar to those in smoke water. Of the four species where glyceronitrile stimulated germination above the water control, the level of germination was as high as that promoted by KAR1 in C. aculeata subsp. septentrionora and C. juncea, but lower than that promoted by KAR1 in C. canteriata and C. resinosa.
Discussion
In south-western Australia, many taxa germinate following fire. Various Conostylis, Anigozanthos and Blancoa taxa, all in the Haemodoraceae subfamily Conostylidoideae, are endemic to this region and are promoted to germinate by smoke or smoke water (Dixon et al., Reference Dixon, Roche and Pate1995; Roche et al., Reference Roche, Dixon and Pate1997; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2014). In this study, smoke water stimulated the germination of fresh seeds (tested within a month of collection) of all eight Conostylis taxa examined. Similarly, the germination of 1-month-old C. candicans (Turner et al., Reference Turner, Merritt, Renton and Dixon2009) and C. neocymosa (Tieu et al., Reference Tieu, Dixon, Meney and Sivasithamparam2001) seeds were promoted by smoke water. Some Blancoa canescens seeds also respond to smoke water when fresh (Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2014). Blancoa is the sister genus of Conostylis, and was previously deemed by some to be synonymous with Conostylis (Hopper et al., Reference Hopper, Chase and Fay2006, Reference Hopper, Smith, Fay, Manning and Chase2009). A proportion of Conostylis and Blancoa seeds are, therefore, either characteristically responsive to smoke water when fresh, or afterripen rapidly to become smoke responsive. Thus, in the Mediterranean climate of south-western Australia, should a fire occur in the summer when seeds of these taxa are shed, some seeds could germinate in response to the smoke water that permeates the soil when the next rains occur.
The germination of some fresh Conostylis seeds in response to smoke water contrasts with that of many Anigozanthos taxa, which have highly dormant seeds when shed (Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2014). This might be linked to differences in ecology between these two genera. In many Conostylis taxa, seed production is not primarily limited to a few years post-fire as in many Anigozanthos taxa. Interestingly, no Conostylis taxa are documented as having fire-stimulated flowering, in contrast to a number of Anigozanthos taxa (Lamont and Downes, Reference Lamont and Downes2011). Furthermore, whereas no Conostylis taxa are reported as fire ephemerals, a number of Anigozanthos species are fire ephemerals, including A. gabrielae, A. kalbarriensis, A. onycis and A. preissii (Hopper, Reference Hopper1993; Pate and Hopper, Reference Pate, Hopper, Schulze and Mooney1993). Fire ephemerals are taxa that germinate predominantly after fire, are short lived and persist only in the soil seed bank until a subsequent fire (Pate and Hopper, Reference Pate, Hopper, Schulze and Mooney1993; Baker et al., Reference Baker, Steadman, Plummer and Dixon2005a). Although both Anigozanthos and Conostylis seeds may be stimulated by smoke water, some Anigozanthos taxa are classified as fire ephemerals because plants are only present in the above-ground flora for a year or two following fire (Hopper, Reference Hopper1993; Pate and Hopper, Reference Pate, Hopper, Schulze and Mooney1993), whereas all of the Conostylis taxa examined are longer-lived rhizomatous perennials (Western Australian Herbarium, 1998–). Hence, there is less selection pressure for Conostylis seeds to be maintained in a long-lived seed bank. Whether Conostylis seeds persist for fewer years in the soil seed bank than Anigozanthos seeds is currently unknown.
The germination of Conostylis seeds was higher in response to smoke water following up to 6 months of laboratory storage and then a year of burial, than in fresh seeds. Higher germination after a period of burial is common in many species with soil-stored seed banks (Roche et al., Reference Roche, Dixon and Pate1997; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005c; Newton et al., Reference Newton, Bond and Farrant2006; Ooi et al., Reference Ooi, Auld and Whelan2006). Following burial, some Conostylis seeds germinated in water alone, although to lower levels than with smoke water. Hence, it is possible for some Conostylis seeds to germinate under suitable conditions in the absence of fire. This would provide fitness benefits if the fire interval were to exceed seed longevity. However, conditions for seedling establishment are generally more amenable soon after a fire than in the inter-fire period, due to increased water, light and nutrient availability post-fire, and reduced competition from established plants. Consequently, the survival of seedlings of many taxa in the inter-fire period is low (Bell, Reference Bell1999). Since neither the longevity of Conostylis seeds in the soil seed bank, nor the extent that Conostylis seedlings can survive following inter-fire germination, has been specifically studied, these areas require further investigation.
As seeds were stored in the laboratory for up to 6 months at 22°C prior to burial, it is possible that some dormancy was alleviated before seeds were buried. This is quite probable since seeds stored in the laboratory for 44–45 months at 15°C germinated to higher levels (Fig. 3) than freshly collected seeds (Fig. 1), particularly in the smoke water and KAR1 treatments. Also, Tieu et al. (Reference Tieu, Dixon, Meney and Sivasithamparam2001) reported dormancy release in C. neocymosa during laboratory storage. Dormancy alleviation during dry storage in these Conostylis species has implications for the use of seeds in conservation, horticulture or restoration purposes. In contrast to certain other species that co-occur with some of these Conostylis taxa, such as the fire ephemeral Tersonia cyathiflora (Gyrostemonaceae; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b), Conostylis seeds do not have an obligate requirement for burial to alleviate dormancy.
Storage at 22°C for up to 6 months prior to burial exposed the seeds to lower temperatures than if they were buried in the soil immediately, since seeds were collected in early summer and soil temperatures during this period are usually much higher (Tieu et al., Reference Tieu, Dixon, Meney and Sivasithamparam2001; Merritt et al., Reference Merritt, Turner, Clarke and Dixon2007). Seeds generally afterripen faster at higher temperatures (Steadman et al., Reference Steadman, Crawford and Gallagher2003; Baker et al., Reference Baker, Steadman, Plummer, Merritt and Dixon2005b) and, therefore, it may be possible that more seeds might have germinated at the start of the rainy season during burial had the seeds been buried sooner. Possibly, germination levels following burial might have been higher if the seeds had been buried immediately after collection, due to exposure of the seeds to an extra summer in the soil.
The eight Conostylis taxa examined are phylogenetically diverse, with four taxa from each of the two clades (Table 1; Hopper et al., Reference Hopper, Chase and Fay2006). As all eight taxa were promoted by both smoke water and KAR1, it suggests that this response may be typical for a range of Conostylis taxa. Blancoa, the sister genus to Conostylis (Hopper et al., Reference Hopper, Smith, Fay, Manning and Chase2009), is also responsive to both smoke water and KAR1 (Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2014). Interestingly, however, this is in contrast to seeds of many Anigozanthos taxa, which do not germinate in response to KAR1 even when responsive to smoke water (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013, Reference Downes, Light, Pošta, Kohout and Van Staden2014).
When glyceronitrile was tested on the Conostylis seeds following laboratory storage, four of the seven smoke-water responsive taxa were stimulated to germinate by one or more of the glyceronitrile concentrations tested, in addition to KAR1 (Fig. 3). Three of these taxa are from clade A and one is from clade B (Table 1). Whether there is any relationship between clade and glyceronitrile response is inconclusive and requires further analysis, possibly with a larger number of species. Glyceronitrile is another chemical that has been identified in smoke water that can promote the germination of certain taxa (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011). Since several Anigozanthos taxa respond to glyceronitrile and not KAR1 (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013, Reference Downes, Light, Pošta, Kohout and Van Staden2014), and some Conostylis taxa respond to both KAR1 and glyceronitrile, there are possibly different smoke-responsive pathways in at least some Conostylis taxa. Glyceronitrile generally stimulated fewer seeds to germinate than KAR1, indicating that KAR1 is a relatively more important signalling molecule than glyceronitrile in Conostylis germination post-fire. In contrast, seeds of a number of Anigozanthos taxa germinate to similar levels in response to glyceronitrile and smoke water, and their germination is not promoted by KAR1 (Flematti et al., Reference Flematti, Merritt, Piggott, Trengrove, Smith, Dixon and Ghisalberti2011; Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2013, Reference Downes, Light, Pošta, Kohout and Van Staden2014). It has been hypothesized that the KAR1 mechanism may have been lost in the Anigozanthos lineage (Downes et al., Reference Downes, Light, Pošta, Kohout and Van Staden2014) as this mechanism is evolutionarily ancient and phylogenetically widespread (Waters et al., Reference Waters, Nelson, Scaffidi, Flematti, Sun, Dixon and Smith2012; Flematti et al., Reference Flematti, Waters, Scaffidi, Merritt, Ghisalberti, Dixon and Smith2013). If so, perhaps the absence of a KAR1 response in Anigozanthos resulted in a greater selection pressure for a glyceronitrile germination response in Anigozanthos relative to Conostylis in the fire-prone environment in which both taxa radiated (Hopper and Gioia, Reference Hopper and Gioia2004; Hopper et al., Reference Hopper, Smith, Fay, Manning and Chase2009).
Nitrate and light are other potential germination cues that have been linked to the post-fire environment (Thanos and Rundel, Reference Thanos and Rundel1995; Bell, Reference Bell1999; Luna and Moreno, Reference Luna and Moreno2009). In this study, nitrate was not an important germination stimulant in the Conostylis taxa examined, under the conditions tested. Likewise, nitrate was not found to be an important germination cue among over 50 species from fire-prone areas of the western Mediterranean (Luna and Moreno, Reference Luna and Moreno2009). The response to light versus darkness was not consistent across the Conostylis taxa examined, and sometimes differed between fresh and buried seeds even in the same species. Light only promoted the germination of fresh C. angustifolia seeds when treated with smoke water and KAR1, and C. neocymosa seeds in the water control following burial. The remaining species were either promoted by darkness or there was no effect of the light conditions on germination. A number of other smoke-responsive taxa from fire-prone areas, such as Stylidium affine (Stylidiaceae) and Tersonia cyathiflora (Gyrostemonaceae), also display either higher germination in the dark with smoke water or no difference between light versus dark conditions (Downes et al., Reference Downes, Lamont, Light and Van Staden2010). This is in contrast to various taxa that are not from fire-prone ecosystems or do not only germinate post-fire, such as Arabidopsis thaliana (Brassicaceae) which only responds to KAR1 in the light (Nelson et al., Reference Nelson, Riseborough, Flematti, Stevens, Ghisalberti, Dixon and Smith2009) and numerous Asteraceae taxa, including lettuce (Lactuca sativa cv. Grand Rapids) (Drewes et al., Reference Drewes, Smith and Van Staden1995) and Podolepis canescens (Merritt et al., Reference Merritt, Kristiansen, Flematti, Turner, Ghisalberti, Trengove and Dixon2006), for which KAR1 and smoke water at least partly overcome a light requirement.
Results from this study clearly indicate that smoke is an important germination cue for the eight phylogenetically diverse Conostylis taxa examined from fire-prone south-western Australia. It is, therefore, likely that seeds of other Conostylis taxa may respond similarly. Smoke water stimulated some germination of fresh seeds, but higher levels of germination were observed following 1 year of burial. Although two different chemicals in smoke water, glyceronitrile and KAR1, both stimulated germination of Conostylis seeds, KAR1 elicited higher levels of germination at the concentrations tested.
Acknowledgements
Seeds were collected under licence and with Regulation 4 Authority from the Department of Environment and Conservation (now the Department of Parks and Wildlife), Western Australia.
Financial support
This research was undertaken with financial assistance from a Centre for Ecosystem Diversity and Dynamics Small Grant, Curtin University. The support of the University of KwaZulu-Natal and the National Research Foundation, Pretoria, South Africa, is gratefully acknowledged.
Conflict of interest
None.