Introduction
Physiological quality traits (i.e. ability to germinate and desiccation tolerance) are important for the long-term conservation of germplasm as they affect storage longevity in genebanks (Hay and Smith, Reference Hay, Smith, Smith, Linington, Dickie, Pritchard and Probert2003). The Australian Grains Genebank's (AGG) pulse collection contains over 28,300 accessions, with lentil (Lens culinaris Medik.) accounting for approximately 5328. It is one of our mandate crops and we currently distribute around 700 accessions a year to research and breeding programmes. Lentil produces orthodox seeds (i.e. they can tolerate desiccation to low moisture levels required for long-term storage at sub-zero temperatures) which, although are expected to survive for a long period of time under genebank storage conditions (Walters et al., Reference Walters, Wheeler and Grotenhuis2005), their actual inherent longevity is still currently unknown. This makes it difficult for genebank managers to make decisions about managing their collections, including setting viability monitoring intervals and making timely regenerations.
Regeneration is one of the most crucial, and most expensive, components of genebank management (Ellis et al., Reference Ellis, Hong and Roberts1985; Kameswara Rao et al., Reference Kameswara Rao, Hanson, Dulloo, Ghosh, Nowell and Larinde2006; FAO, 2014), with many genebanks reporting to be regenerating their material more frequently than expected, based on the predictions. If regeneration is occurring too frequently, the risk of losing genetic diversity is high (FAO, 2014); thus, the frequency of regeneration should be minimized through the maximization of seed quality and subsequent seed storage longevity (Kameswara Rao and Jackson, Reference Kameswara Rao and Jackson1966). Other than inherent (genotypic) differences in longevity, the impact of the environment (humidity and temperature) during seed development also affects both the progression of seed quality traits through development and the end/maximum physiological quality (Ellis, Reference Ellis2019 and references therein; Zinsmeister et al., Reference Zinsmeister, Leprince and Buitink2020). Therefore, it is recommended that genebanks carry out studies to measure changes in seed quality to determine the optimal stage of maturity for harvest.
Seeds have evolved to be highly adapted to their natural environment, and the effects of a change in the maternal environment during seed development and maturation can affect the acquisition of physiological traits. Kochanek et al. (Reference Kochanek, Buckley, Probert, Adkins and Steadman2010) reported that low temperatures during the ripening phase of seed development had detrimental effects on longevity but had no effect on longevity if low temperatures coincided before seed set. Similarly, in rice, high temperatures have a more damaging effect on seed quality early on in seed development, and this effect reduces during late seed filling onwards (Ellis, Reference Ellis2011). Naturally, within a seed population, individual seeds vary in the timing of maturation due to variation in pollination, fertilization and the environment over the period from flowering to dispersal. However, ideally seeds should be harvested as close to peak maturity as possible to ensure they have reached maximum quality in terms of desiccation tolerance and longevity (Ellis et al., Reference Ellis, Hong and Roberts1987; Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a; Kameswara Rao and Jackson, Reference Kameswara Rao and Jackson1996). Plus, immature seeds generally have a lower initial viability and/or show a faster loss in viability (Ellis et al., Reference Ellis, Demir, Pieta-Filho, Côme and Corbineau1993a; Ellis and Hong, Reference Ellis and Hong1994; Hay and Probert, Reference Hay and Probert1995). The previous theory, suggested by Harrington (Reference Harrington and Kozlowski1972), that maximum quality is attained at physiological maturity (end of seed filling) and declines thereafter has been disputed by a multitude of studies, from a range of crops, that have shown quality to continue to increase after seed filling – reaching its peak towards the end of the maturation drying phase (Ellis, Reference Ellis2019 and references therein). This maturation drying phase begins at the end of seed filling when the abscission layer is formed which blocks the vascular connection with the mother plant (Galau et al., Reference Galau, Jakobsen and Hughes1991; Pepler et al., Reference Pepler, Gooding and Ellis2006; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017). As a result, the moisture status of the seeds is now determined by the ambient conditions; the seeds have become hygroscopic (Ellis and Hong, Reference Ellis and Hong1994). It has been well reported how seed quality, including longevity, increases during this maturation drying phase, with results from gene expression and metabolite studies showing seeds to be metabolically active in this phase (Angelovici et al., Reference Angelovici, Galili, Fernie and Fait2010). Chatelain et al. (Reference Chatelain, Hundertmark, Leprince, Le Gall, Satour, Deligny-Penninck, Rogniaux and Buitink2012) further suggested that the maturation drying phase should be divided into two, the first part being important for subsequent seed longevity; characterized by the accumulation of oligosaccharides and low molecular weight proteins (Sinniah et al., Reference Sinniah, Ellis and John1998b).
Harvesting seeds when they have reached optimum maturity is only part of ensuring that seeds are at their maximum physiological quality, and subsequent longevity, when placed into storage. As discussed, seed quality traits are not static, and delaying harvest beyond optimum maturity can result in changes (increase/decrease) to subsequent seed quality, including storage longevity, depending on the environmental conditions (Ellis, Reference Ellis2019). Change in seed quality can be attributed to the water relations in the seed which, in turn, determines which physiological reactions can occur. This means, as the moisture content of mature seeds is determined by the ambient conditions (temperature and relative humidity), the seeds are continuously undergoing wet-dry cycling, that is, therefore are constantly switching between ageing and repair. Continuous wetting and drying of barley seeds, for example, during maturation due to frequent periods of rainfall increased their subsequent storage longevity (Ellis and Pieta Filho, Reference Ellis and Pieta Filho1992). Furthermore, wheat seed quality was shown to be restored, following rainfall during maturation and development, if seeds were left to redry on the plant (Yadav and Ellis, Reference Yadav and Ellis2016). Therefore, not all rain events can be detrimental to seed quality, as long as seeds can repair all the damage that has been accumulated, seed quality will be restored/maintained. Furthermore, rehydration levels that push the seed to almost functional capacity [98% equilibrium relative humidity (eRH)] can allow the continuation of maturation processes, such as the accumulation of protectants which are involved in increasing the storability of seeds and can lead to further improvements in seed quality (Powell et al., Reference Powell, Yule, Jing, Groot, Bino and Pritchard2000; Butler et al., Reference Butler, Hay, Ellis, Smith and Murray2009). However, it is important to note that the beneficial effect of rehydration/drying cycles on seeds depends on the level of damage the seeds have already accumulated; that is, if the seeds have suffered a substantial amount of damage, they are not able to reach the same quality that was previously attained (Butler et al., Reference Butler, Hay, Ellis, Smith and Murray2009). Similarly, improvements in seed quality are also not infinite and presumably, there is a ‘maximum longevity’ that any developing cohort of seeds can attain (Whitehouse, Reference Whitehouse2016). For example, pre-germination of barley seeds induced by rain close to harvest increased the seeds’ initial quality (rapid and uniform germination) but reduced their potential longevity (Gualano et al., Reference Gualano, Del Fueya and Benech-Arnold2014). To conclude, it is the net changes in seed quality traits (improvement vs deterioration processes during development and maturation) in planta that determine seed quality at harvest.
We report here an investigation that delves into the genotype-by-environment interaction during lentil seed development. Seeds of lentils were grown across four different regeneration environments, which vary in the level of temperature and relative humidity control and/or exposure to the ambient environment. We aimed to measure the effect of temperature, relative humidity and/or rainfall on seeds’ progression through development and subsequent end seed physiological quality (i.e. ability to germinate and survive air-dry storage); testing the following hypotheses: (1) higher temperatures, following mass maturity (MM), will have a beneficial effect on subsequent seed longevity and (2) seed storage longevity will be lower in seeds that dry quickly in planta. The results of this study will directly be used to optimize our future regeneration procedure for lentil at the AGG to ultimately ensure the availability of high-quality seeds for future utilization.
Materials and methods
Regeneration environments
The AGG has access to four, controlled/semi-controlled, facilities which are all used for regeneration (glasshouse, big igloo, green igloo and cage). The glasshouse is a fully protected, polycarbonate, growing environment. It has built-in evaporative heating and cooling with a set temperature of 25 ± 3°C. Plants are grown in pots under natural daylight with full irrigation and fertigation control. This environment is the coolest, most stable, environment of the four with typical average day temperatures between 21 and 26°C and night temperatures between 17 and 20°C, throughout the growing season. The relative humidity in this environment is not controlled.
The big igloo is a UV stabilized high-density polyethylene greenhouse. There is no stringent temperature or relative humidity control, rather the environment is reliant on passive cooling through natural ventilation and in-built fans, which are used during the summer months when temperatures reach above 30°C. Plants are sown in pots and exposed to natural daylight, with the use of an automatic shade structure (42% shade) when ambient temperatures exceed 25°C, full irrigation and fertigation control. This environment is the hottest of the four, with temperatures capable of exceeding 45°C.
The green igloo structure is a shade cloth tunnel (42% shade), exposing plants to ambient conditions (temperature/relative humidity/rainfall). Plants are grown in pots with supplementary irrigation and fertigation, as required. Although semi-protected, the environmental conditions are entirely dependent on the ambient conditions but, generally, this environment is hotter than the glasshouse but cooler than the big igloo.
Like the green igloo, the cage is also a semi-protected shade cloth structure (42% shade) exposed to ambient conditions (temperature/relative humidity/rainfall) and natural daylight. The plants, however, are soil bed grown. Supplementary irrigation and fertigation are applied, as required.
Plant material
Seeds of two lentil accessions (L. culinaris Medik.), both elite breeding lines, were sampled from the AGG long-term storage collection, after only a few months of storage. The newly acquired material (accessions 76072 and 76080), which had undergone seed multiplication the previous year following their release from quarantine, were high yielding and highly viable (98% viability).
Seeds were prepared for sowing, following standard AGG protocols and practices (Street et al., Reference Street, Rukhkyan, Ismail, Dulloo, Thormann, Jorge and Hanson2008), in the four regeneration environments described above (glasshouse, big igloo, green igloo and the cage). A total of 30 seeds from each accession were sown into 8-inch pots (5 per pot) for regeneration in the glasshouse, big igloo and green igloo with seeds sown directly into plots (30 per plot) in the cage. Sowing commenced first in the green igloo (30 May 2019) and was followed by the glasshouse (5 June 2019), the big igloo (13 June 2019) and, lastly, the cage (26 June 2019). Standard production practices and routine plant protection mechanisms were followed. A data logger was placed, hanging directly above the pots/plots, in each of the regeneration environments to monitor and record changes in temperature and humidity of the surrounding environment, at 15-min intervals. A sample of seeds was first harvested at 21 days after 50% anthesis (DAA) with harvests continuing at 14-d intervals thereafter until 63 DAA, after which sampling increased to once or twice per week. Sampling terminated at 130 DAA unless seed quality and/or quantity diminished earlier due to temporal variation in seed quality development and maturation between the different environments.
For each harvest, 400–500 pods were collected, at random across all 30 plants sown within each accession, and immediately threshed by hand. A subsample was placed inside a 3.2 ml sample holder in the measuring chamber of an HC2-AW-USB water activity station (Rotronic, Bassersdorf, Germany), and the eRH was measured at ambient temperature (21.5°C) once the reading had stabilized. The estimated seed moisture content (eMC), on a fresh weight basis, was then calculated using the seed viability constants menu on the Seed Information Database (SID; Royal Botanic Gardens Kew, 2020) assuming a seed oil content of 0.80 (Earle and Jones, Reference Earle and Jones1962). A subsample of 130 seeds was then set aside: 40 for germination testing (fresh germination) and 3 × 30 seeds for dry weight determination, using the low-temperature oven method according to ISTA (103°C for 17 h; ISTA, 2020). The remaining seeds were transferred into a labelled paper envelope and placed in the drying room, set at 15°C and 15% RH, for 6weeks, or until the moisture content had declined to 6–7%. Following drying, the eRH was checked, using the same method as previously, before another subsample of 40 seeds was removed for germination testing to determine desiccation tolerance (i.e. % germination of dried seed). The remaining seeds were then packed and sealed into an aluminium foil bag and transferred to the AGG's long-term storage facility, at −20°C, until all harvests had been completed and the seed storage experiments (SEEs) began.
Seed germination
The ability to germinate was estimated with 2 replicates of 20 seeds, sown on two layers of wetted Whatman No.1 paper in 90 mm Petri dishes. They were incubated at a constant temperature of 21°C (16-h light and 8-h dark cycle). Germination was scored at 2, 5, 7 and 14 d. Any non-germinated, hard seeds that remained after the 14-d incubation period were dehulled and tested for a further 10 d before final scoring. Seeds were classified as germinated when the radicle had emerged by at least 2 mm.
Longevity determination (SSE)
Due to the large quantity of seeds for experimental analysis, it was decided to split the SSEs according to the regeneration environments. Furthermore, to reduce the workload, only a subset of samples (63, 70, 84, 91, 105, 112, 118, 121, 124 and 130 DAA) were selected (seed permitting) for analysis. Therefore, for each of the four SSEs, seeds from each of the accessions, which were harvested at different intervals, were removed from cold storage and left to equilibrate in the drying room before opening. The sample was cleaned, removing any damaged, broken or unfilled seeds, before it was split into, a minimum of, 8 subsamples, each containing 40 seeds. Each subsample was then placed into 30 mm diameter open Petri dishes and held over a non-saturated lithium chloride (LiCl2) solution (60% RH) in a sealed electrical enclosure box at ambient temperature until seeds reached equilibrium. After 7 d, the RH of the solution, and the eRH of the seeds, was checked using the water activity measuring equipment described above. The solution was adjusted by adding distilled water, stirring and left to equilibrate before re-checking, as and when required. When the seeds had equilibrated, reaching an approximate moisture content of 13% (fresh weight basis), a subsample was immediately used to determine the initial germination (prior to experimental storage) and the remaining packets were sealed inside aluminium foil packets before being transferred to an incubator, set at 45°C. One packet per accession × harvest interval (DAA) was removed after 4, 7, 18, 35, 60, 90 and 105 d for germination testing. As the inherent longevity of lentil is currently unknown, this sampling schedule was adjusted (increasing/decreasing in frequency and/or terminating before or extending beyond 105 d) according to the rate seed lots appeared to be losing viability. The eRH of a sample of seeds was determined mid-way through and at the end of the experiment.
Statistical analysis
All analyses were carried out using GenStat for Windows, Version 18 (VSN International Ltd., Oxford, UK) and fitted using OriginLab 2019, graphing software (Hearne Scientific Software Pty Lt., Victoria, Australia).
Longevity determination
Seed survival (ability to germinate after different periods of experimental storage) curves were fitted by probit analysis, fitting the Ellis and Roberts viability equation (equation 1). The period (days) for viability to fall to 50% (p 50) was estimated and used as the measure of longevity (Ellis and Roberts, Reference Ellis and Roberts1980).
where v is the ability to germinate (v) in normal equivalent deviation (NED) of a seed lot stored for period p (days), K i is the initial viability (NED) and σ (days) is the standard deviation of the normal distribution of seed deaths in time. For those seed lots showing loss in dormancy during (early) storage, that is, afterripening, a probit model combining loss in dormancy with loss in viability was applied (equation 2; Kebreab and Murdoch, Reference Kebreab and Murdoch1999).
where g is the ability to germinate in NED, p, K i and σ are the same as in equation (1), F is the cumulative normal distribution function, K d is the initial proportion of non-dormant seeds (NED) and β1 is the probit rate of loss of dormancy. For those seed lots that showed a reduced initial viability, asymmetry in the survival data (not symmetrical about 50%) and a systematic pattern of residuals when fitting equation (1), the ‘control mortality’ parameter (‘immunity’ in GenStat) was included in the probit analysis. The control mortality parameter is the estimate of the proportion of ‘non-responding’ seeds (i.e. dead or empty) within the population (Mead and Gray, Reference Mead and Gray1999) that is they are not part of the ageing (responding) population when placed under experimental storage. Probit analysis was carried out for all seed lots simultaneously, fitting the full model (different estimates for all parameters).
Temporal changes in seed quality traits
The four remaining datasets collected [seed dry weight, eMC, ability to germinate (pre- and post-desiccation)], for each accession grown in the different regeneration environments, were plotted against DAA (harvest interval). A four-parameter logistic model (equation 3) was applied and fitted to each of the datasets individually. In the instances where a logistic s-shaped or inverse s-shaped curve could not be fitted (i.e. due to a decline in quality), a probit model was applied and fitted to the data.
Results
Acquisition of physiological traits
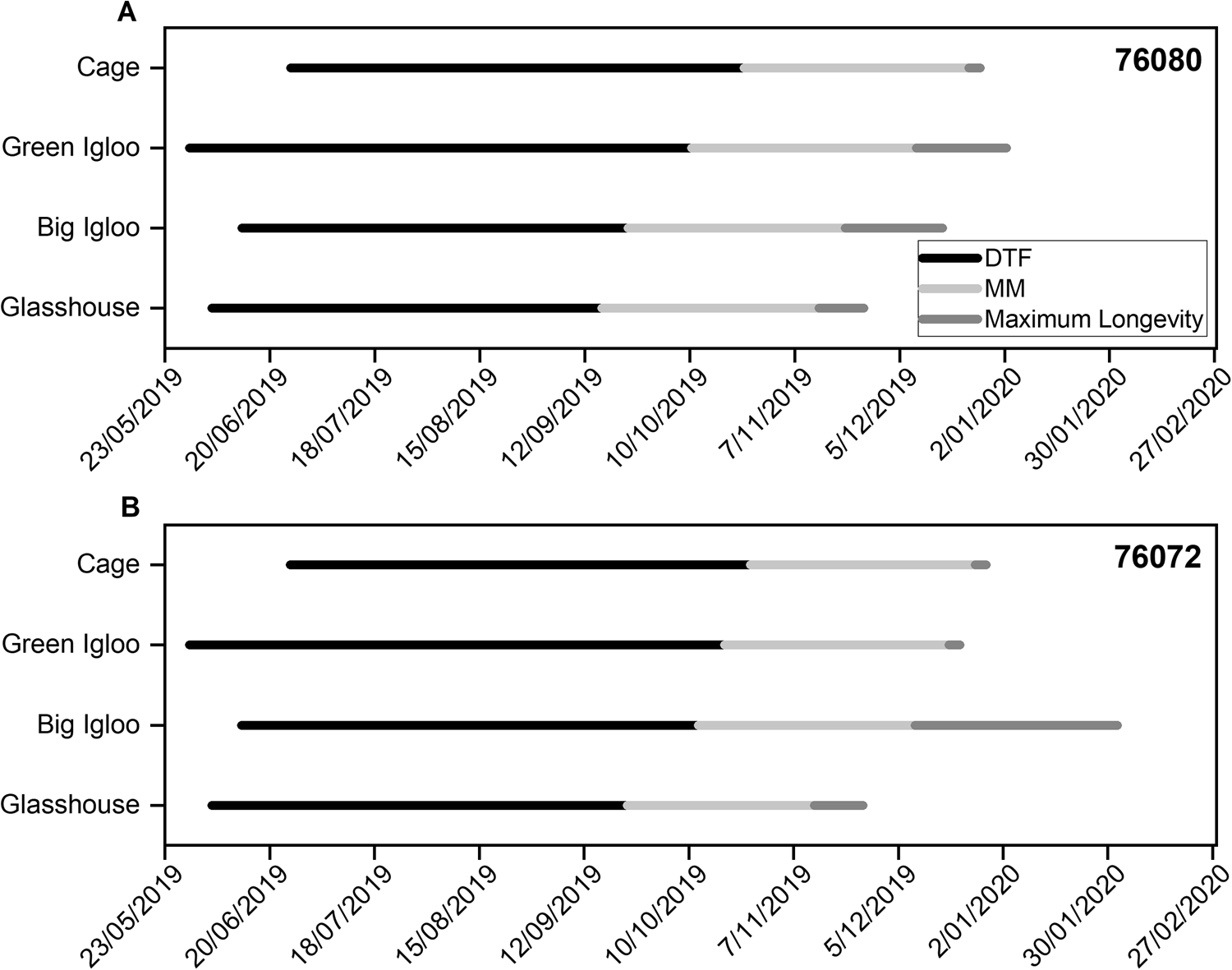
A summary of the key developmental events, in relation to time (from sowing to flowering, MM and maximum seed longevity) is depicted in Fig. 1. The estimated time from sowing to 50% flowering (DTF) varied between accessions, with accession 76080 progressing more quickly compared with accession 76072 in each of the regeneration environments (Fig. 1). Progression through development, between sowing and DTF, was the slowest when seeds from both accessions were sown in the green igloo, occurring after 143 and 134 d for accessions 76072 and 76080, respectively, with flowering occurring first in the glasshouse and the big igloo (approximately 32 and 31 d earlier). An increase in seed dry weight occurred quickly following the onset of flowering, with all seeds reaching a maximum dry weight of approximately 45 mg seed−1 (Fig. 2), signifying MM. Despite these temporal differences between accessions and accessions × growth environment during early development, the timing from DTF (days after 50% anthesis; DAA) to MM appeared to be consistent between accessions, occurring after approximately 58 DAA in the big igloo and after approximately 60 DAA in the green igloo and in the cage (Figs 1 and 2). Only the glasshouse environment resulted in variation between the accessions, with accession 76072 reaching MM approximately 1 week earlier compared with accession 76080 (Figs 1 and 2a, e).
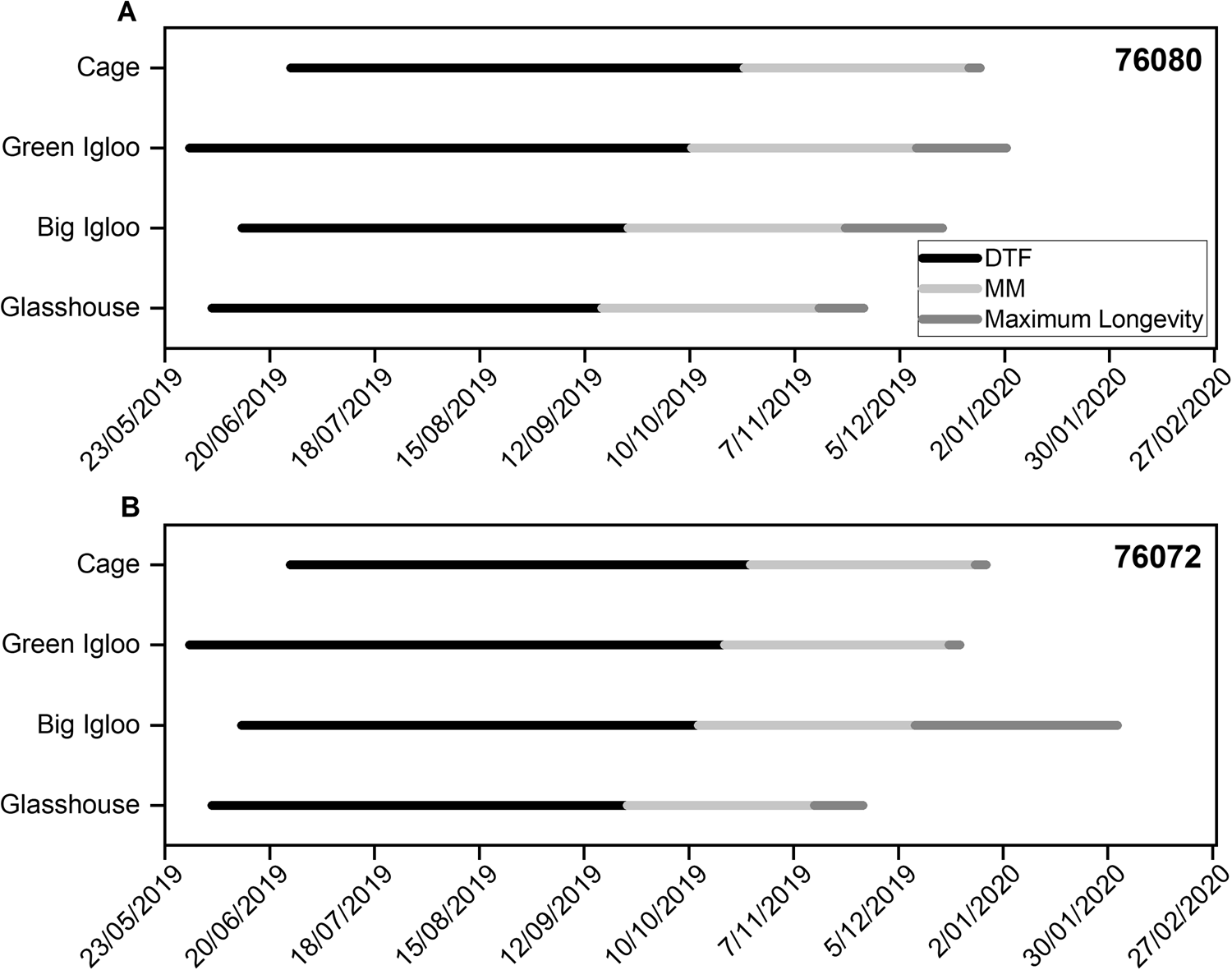
Fig. 1. Timeline of key stages during development when seeds of accessions 76080 (a) and 76072 (b) were grown in each of the four different regeneration environments at the AGG. Time (in days) between sowing and 50% flowering (DTF; black), between 50% flowering and MM (light grey) and between MM and maximum longevity (dark grey). The longevity (p 50) of each seed lot was quantified (Fig. 3), with maximum longevity referring to the seed lot with the highest estimate of p 50 (Supplementary Tables S1 and S2).
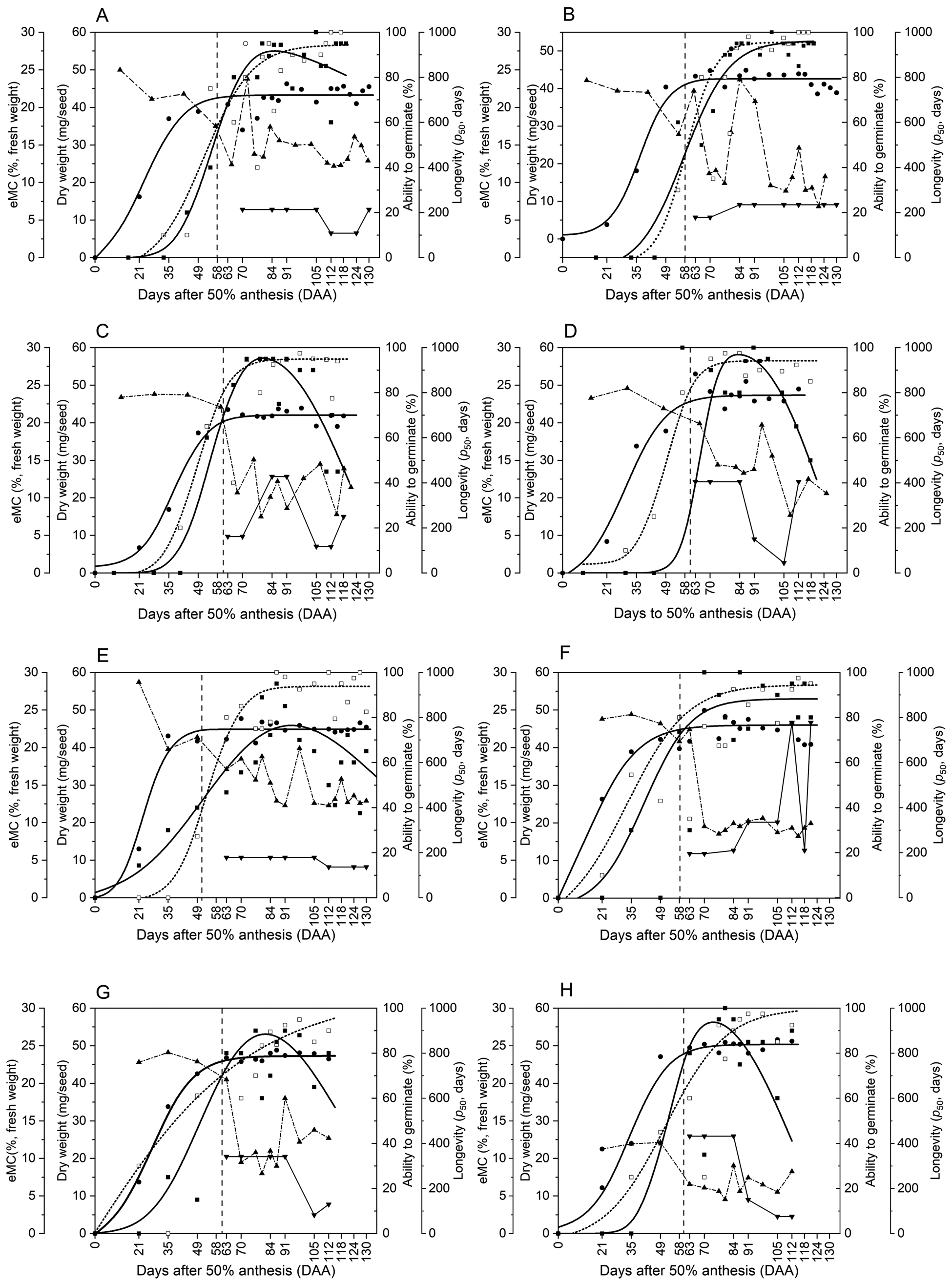
Fig. 2. The physiological changes which occur during the development of two accessions of lentil, 76080 (a–d) and 76072 (e–h), when grown in four different regeneration environments [glasshouse (a,e), big igloo (b, f), green igloo (c,g) and cage (d,h)] at the AGG. The vertical dashed line symbolizes MM, when seeds reach their maximum dry weight (black circles). Seeds start to acquire the ability to germinate (fresh; white squares) and desiccation tolerance (black squares) before MM and continue to increase thereafter, during the maturation drying phase when seeds begin to decline in moisture content (fresh weight; black triangles). Estimates of storage longevity (p 50) were determined after MM, from 63 d after 50% anthesis (DAA) to a maximum of 130 d (black inverted triangles). The optimum time of harvest, within each zone, would be when all these physiological traits have reached their maximum.
Maximum quality is defined when all physiological traits (ability to germinate, desiccation tolerance and potential longevity) have peaked. Although almost all seeds had acquired the ability to germinate (fresh) by the first sampling interval, at 21 DAA, germination did not peak until after MM, the timing of which differed between the regeneration environments (Fig. 2). Seeds from both accessions all reached a high maximum germination (>95%) when grown in the different environments which was maintained until sampling terminated. By the time seeds had reached MM, they had started to show a natural decline in moisture content. On average, before MM, seeds from accession 76080 ranged between 21.8 and 23.7% moisture content (fresh weight), estimated at 49 DAA, and declined between 16.4 and 22.2% moisture content by 63 DAA (Fig. 2a–d). Similar values were also seen in accession 76072, with seeds ranging between 21.4 and 24.3% at 49 DAA and between 13.1 and 22.4% at 63 DAA (Fig. 2e–h). Both accessions, and in all environments, showed a further decline in moisture content as development progressed further into the maturation drying phase. It is during this maturation drying phase that seeds usually acquire desiccation tolerance, which continued to increase until seeds had reached equilibrium with the environment. According to the data, accessions did not start to show desiccation tolerance to low moisture contents until between 35 and 49 DAA but continued to increase thereafter peaking at different times depending on the regeneration environment (Fig. 2). In both accessions, desiccation tolerance peaked much earlier following MM (approximately between 77 and 84 DAA) when grown in the cage and green igloo (Fig. 2c, d, g, h), compared with the glasshouse and big igloo (Fig. 2a, b, e, f), but with a sharp decline thereafter. Seeds grown in the glasshouse also showed a decline in desiccation tolerance following its peak, albeit the rate of loss was slower (Fig. 2a, e), with the big igloo being the only environment where seeds did not show a loss in this physiological quality trait (at least until 130 DAA) (Fig. 2b, f). Subsequent storage longevity (discussed in detail in the next section), although was seen to fluctuate throughout development in each of the environments, generally peaked at approximately the time desiccation tolerance reached its maximum (Fig. 2). In summary, progression from MM to maximum storage longevity occurred the fastest in the cage, with both accessions peaking in quality shortly after MM, followed by the glasshouse 10–11 d after. Contrastingly, in the other environments, there were varietal differences in progression following MM, with accession 76072 peaking in longevity earlier when grown in the green igloo compared with the big igloo, and vice versa for accession 76080 (Fig. 1).
Seed longevity
Some seed lots, at certain stages of maturity, showed dormancy when grown in specific environments that were lost during early experimental storage; and all seed lots showed a loss in viability (Fig. 3). Longevity estimates varied between each accession and accession × growth environment, with significant differences in survival curves (P < 0.05) between some, but not all, seed lots harvested at different maturity stages within each accession × growth environment (Fig. 3). Overall, there were several patterns of within-accession variation in longevity development across the different regeneration environments (Fig. 2). For example, there was evidence of seeds increasing in longevity during development that, once peaked, was either maintained for some time thereafter (Fig. 2b) or showed a subsequent decline (Fig. 2c, f). Furthermore, there were other instances where seeds failed to show a continuation in longevity accumulation as time progressed during development, as longevity was already at its maximum at the start of sampling. This level was maintained for some time before either declining (Fig. 2e, h) or declining before increasing again (Fig. 2a, d, g).
Fig. 3. The ability to germinate during storage at 45°C and 60% relative humidity for seeds of two lentil accessions, 76080 (a–d) and 76072 (e–h), harvested from each of the four regeneration environments [glasshouse (a,e), big igloo (b,f), green igloo (c,g) and cage (d,h)] at different stages of maturity between 21 and 130 d after 50% anthesis (DAA) and dried in the dry room at 15°C and 15% relative humidity until equilibrium. The combined loss in dormancy and loss in viability model were applied to those seed lots which showed dormancy during early storage, with all survival curves being fitted using the Ellis and Roberts (Reference Ellis and Roberts1980) viability model. Seed lots which showed a reduced initial viability, an additional parameter was applied to probit analysis to determine the proportion of responding seeds within the population (Mead and Grey, Reference Mead and Gray1999). The dashed lines correspond to seed lots which could be constrained to a single curve (P > 0.05). Survival curves are quantified in Supplementary Tables S1 and S2.
Across the four regeneration environments, seeds of accession 76080 achieved the greatest maximum longevity when grown in the semi-protected environments of the green igloo and the cage, showing p 50 values of 427.6 and 405.1 d, respectively (Figs 2c, d and 3c, d). Seeds grown in the green igloo increased in longevity during development, reaching their maximum value at 84 DAA (Fig. 2c). The quality of seeds was maintained for 1 week, thereafter, with both seed survival curves (84 and 91 DAA) being constrained to a common line without a significant reduction (P > 0.05) in the residual deviance (Fig. 3c). Following its peak, seeds declined in longevity, reaching their lowest estimate of p 50 of 117.1 d at 105 DAA. This seed lot, although had a high proportion of responding seeds (shown by the high K i value), seeds were not able to maintain viability for long in storage, hence showed a steepened subsequent slope, and resulted in a difference in longevity by −310.5 d (Fig. 3c; Supplementary Table S1). When seeds of accession 76080 were grown in the cage, on the other hand, they showed a reduction in both K i and σ the longer they remained on the plant, resulting in fewer viable seeds at the beginning of the storage and a faster rate of loss in viability as time progressed (Fig. 3d; Supplementary Table S1). This meant seeds were at their greatest longevity early on in development, at 63–84 DAA, compared with when seeds were grown in the big igloo which did not peak in longevity until 84 DAA but seemed to maintain this quality thereafter (at least up to 130 DAA) (Fig. 3a, d). Finally, the environment that produced the lowest quality seeds, in terms of maximum longevity (p 50), was the glasshouse (Fig. 3a), with a maximum estimated p 50 value half of that achieved when seeds of accession 76080 were grown in the green igloo (213.8 d) (Fig. 3c). In this environment, seeds were at their maximum longevity at the start of the sampling period (70 DAA) and were able to maintain this quality up to 105 DAA – shown by the common line (Fig. 3a). However, harvesting seeds later, at 112 and 124 DAA, resulted in a significant reduction in longevity, in comparison. Although a larger proportion of seeds were ‘responding’ at the start of storage in these later seed lots (shown by the higher K i value), they lost viability at a faster rate, compared with when seeds were harvested earlier (Supplementary Table S1).
For seeds of accession 76072 grown across the same four regeneration environments, and sown at the same time, as seeds of the accession 76080, the greatest longevity was achieved in the big igloo (777.6 d), followed by the cage (432.2 d), green igloo (341.2 d) and, lastly, the glasshouse (178.3 d) (Fig. 2e–h; Supplementary Table S2). The difference in longevity between the two accessions grown in the big igloo was large, with longevity increasing with the length of time seeds of accession 76072 remained on the plant, peaking at 112 DAA, with a p 50 value more than two times greater than that of accession 76080 (Figs 2b, f and 3b, f). Although the proportion of viable seeds within each seed lot reduced with time (DAA), the seeds maintained viability for longer during storage, shown by the reduction in σ−1 (Supplementary Table S2). The temporal patterns of longevity development, when seeds of accession 76072 were grown in the more open environments of the green igloo and the cage, were similar (Fig. 2g, h; Supplementary Table S2). In both environments, longevity was at its greatest at the beginning of sampling (at 63 DAA) and was maintained for some time thereafter. There was no significant difference in the longevity (P > 0.05) of seeds harvested between 63 and 91 DAA when grown in the green igloo (Fig. 3g), and between 63 and 84 DAA when grown in the cage (Fig. 3h). However, following 91 and 84 DAA in each of the respective environments, longevity declined. In the green igloo, this decline was sharp, as seeds appeared to already be at ‘tipping point’ at the start of storage (shown by high K i and σ−1) (Supplementary Table S2). In the cage, on the other hand, the decline was more gradual with p 50 values declining from 432.2 d at 84 DAA to 149.7 d at 91 DAA and then to 44.8 d at 105 DAA (Fig. 2h; Supplementary Table S2). This decline in longevity was a result of both a reduction in K i that is the proportion of viable seeds in the seed lot, and an increase in the rate of viability loss (σ−1). Finally, as with accession 76080, accession 76072 showed the lowest maximum longevity when grown in the glasshouse, compared with the other environments (Figs 2e and 3e). The longevity of seeds grown in this environment reached its peak at 63 DAA and remained at this level until 105 DAA, after which it declined. Between 105 and 112 DAA, the value of p 50 dropped by 42 d, with very little difference in K i and a slight steepening of the slope (σ−1) (Fig. 3a; Supplementary Table S2). Once longevity had declined at 112 DAA, it was maintained until the end of the sampling, as survival curves for seeds harvested at 112, 124 and 130 DAA could be constrained to a common line without a significant increase in residual deviance (Figs 2e and 3e).
Discussion
To maximize seed longevity, it is important to harvest seeds at optimal maturity. The optimum time to harvest is when seeds have acquired maximum physiological quality before deterioration begins. Harrington's hypothesis that seed quality peaks at the end of seed filling (physiological maturity) and declines thereafter has long been disputed by a wealth of evidence that shows the continuation of quality throughout development and into the maturation drying phase (when the vascular connection with the mother plant has been terminated) (Ellis, Reference Ellis2019 and references therein). Although true, it is not possible to ascertain a single point in time, during development in planta whereby seeds, of a particular species/variety, reach maximum quality as the pre-harvest environment can restrict seeds progression through development and through maturation drying, in particular (Whitehouse et al., Reference Whitehouse, Hay and Ellis2018a). Furthermore, there is evidence that seed quality can continue to accrue ex planta in immature seeds by mimicking the conditions experienced in planta (Probert et al., Reference Probert, adams, Coneybeer, Crawford and Hay2007) − typically during the maturation drying phase (Hong et al., Reference Hong, Gedebo and Ellis2000) − but also in mature seeds by stimulating the same physiological processes that occur within the seed during maturation drying (Whitehouse et al., Reference Whitehouse, Hay and Ellis2015, Reference Whitehouse, Hay and Ellis2017, Reference Whitehouse, Hay and Ellis2018a,Reference Whitehouse, Owoborode, Adebayo, Oyatomi, Olaniyan, Abberton and Hayb). We are becoming increasingly aware of the different temporal patterns in seed quality development and decline as a result of the genotype-by-environment interaction which ultimately affects ‘when’ maximum quality is attained and for how long it is maintained in planta, thereafter (Ellis, Reference Ellis2019). In this current study, we mapped the variation in temporal patterns of lentil seed quality development when grown in four different regeneration environments at the AGG to assess the effect of temperature, relative humidity and rainfall, particularly during the maturation drying phase, on end seed physiological quality, including subsequent storage longevity.
Physiological quality traits were acquired during development, with total developmental time (from sowing to maximum longevity) varying between accessions and the regeneration environment (Fig. 1). In general, prior to MM, accession 76080 progressed faster within each of the regeneration environments, compared with accession 76072 (Fig. 1). Despite the different sowing dates and different durations to flowering between accessions × regeneration environments, duration from flowering to MM was consistent in accession 76080 (58–60 DAA; Fig. 2a–d) and fairly consistent in accession 76072 (50–60 DAA; Fig. 2e–h). This very little variation in time indicating timing to MM is heavily influenced by genotype, as opposed to environmental conditions. However, following MM the abscission layer is formed which blocks the vascular connection with the mother plant (Galau et al., Reference Galau, Jakobsen and Hughes1991; Leprince et al., Reference Leprince, Pellizzaro, Berriri and Buitink2017). As a result, the seeds are now hygroscopic (Ellis and Hong, Reference Ellis and Hong1994) and the temporal pattern of progression through the maturation drying stage, as well as the accumulation in physiological quality (desiccation tolerance and longevity), becomes very much dependent upon all environmental elements/conditions, as well as genotype. This accounts for the within-accession variation seen in seed quality development in this study, when seeds were grown in different environments (Figs 2 and 3).
The ability to germinate, both fresh and dry seeds, was acquired early in development, but did not reach its maximum until after MM, as seen in a range of other crops (Kameswara Rao et al., Reference Kameswara Rao, Appa Rao, Mengesha and Ellis1991; Pieta Filho and Ellis, Reference Pieta Filho and Ellis1991a,Reference Pieta Filho and Ellisb; Ellis and Pieta Filho, Reference Ellis and Pieta Filho1992; Demir and Ellis, Reference Demir and Ellis1992a,Reference Demir and Ellisb, Reference Demir and Ellis1993; Ellis et al., Reference Ellis, Demir, Pieta-Filho, Côme and Corbineau1993a; Ellis and Hong, Reference Ellis and Hong1994; Zanakis et al., Reference Zanakis, Ellis and Summerfield1994; Hay and Probert, Reference Hay and Probert1995; Sanhewe and Ellis, Reference Sanhewe and Ellis1996; Hay et al., Reference Hay, Probert and Smith1997; Sinniah et al., Reference Sinniah, Eliis and John1998a; Hay et al., Reference Hay, Smith, Ellis and Butler2010; Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017; Basso et al., Reference Basso, Hoshino-Bezerra, Sartori, Buitink, Leprince and de Silva2018). Fresh germination peaked between 84 and 112 DAA in accession 76080 (Fig. 2a–d) and between 87 and 98 DAA in accession 76072 (Fig. 2e–g), depending on the environment, and was maintained thereafter. Desiccation tolerance (measured by the ability to germinate following drying to low moisture contents), on the other hand, peaked slightly later compared with fresh germination but was quickly lost in certain environments (Fig. 2). For example, seeds from both accessions quickly lost desiccation tolerance following its peak, which occurred around 80 DAA, in the cage and the green igloo (Fig. 2c, d, g, h). This is thought to be a result of high moisture due to the onset of rain, which began in January (Fig. 4). When dry seeds reach high moisture levels, close to full hydration, seeds switch into the germination phase and they quickly begin to lose desiccation tolerance (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). Imbibition of water results in an irreversible change to the physical properties of the membrane lipid bilayer which makes the cellular membranes susceptible to injury upon subsequent desiccation (Leprince et al., Reference Leprince, Deltour, Thorpe, Atherton and Hendry1990; Buitink et al., Reference Buitink, Leprince and Hoekstra2000; Hoekstra et al., Reference Hoekstra, Golovina and Buitink2001). In the glasshouse (Fig. 2a, e) however, seeds from both accessions also lost desiccation tolerance, albeit at a slower rate compared with seeds in the cage (Fig. 2d, h) and the green igloo (Fig. 2c, g). This loss cannot be explained by rain exposure as seeds grown in this environment are protected from the external environment. The temperature control of this environment maintained relatively stable conditions, with an average temperature of 23.5°C and 51.7% RH following MM (Fig. 4), equating to an eMC of 11.6% (calculated using the SID). The seeds, therefore, did not show a large decline in moisture content once the abscission layer had formed between the seed and the parent plant, compared with when seeds were grown in the other environments, where the temperature was higher (Fig. 4). It has been well reported how seed quality, including longevity, increases during this maturation drying phase, with results from gene expression and metabolite studies showing seeds to be metabolically active in this phase (Angelovici et al., Reference Angelovici, Galili, Fernie and Fait2010).
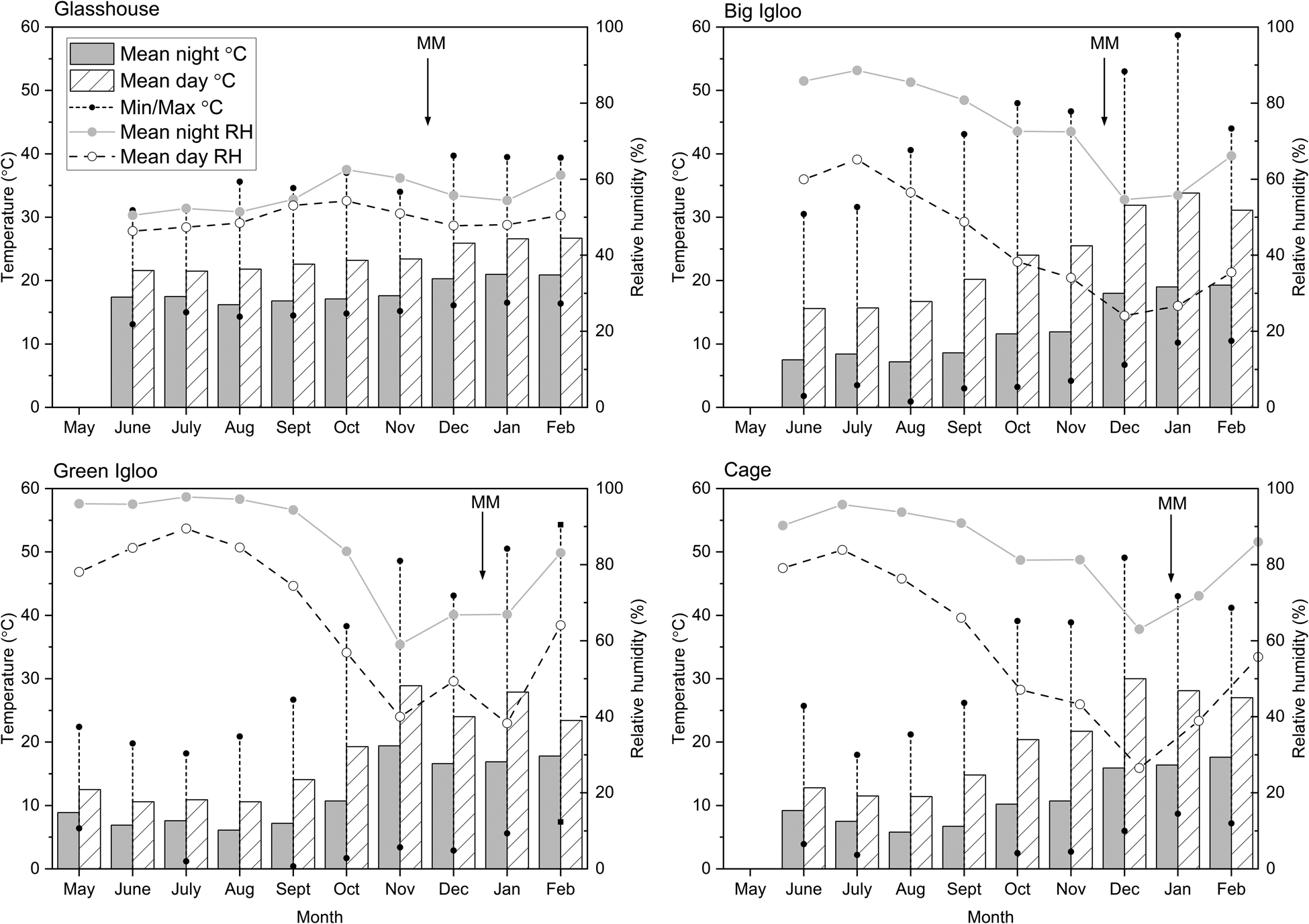
Fig. 4. Temperature and relative humidity data collected and downloaded from data loggers from each of the four regeneration environments (glasshouse, big igloo, green igloo and cage). The data loggers recorded at 15-min intervals and averages calculated for each month during development. Also shown on each of the graphs are approximates of when MM was acquired.
Longevity determination was carried out on all seeds that had already reached MM; therefore, they were in the desiccation phase of seed development where seed quality traits can still be accrued (Chatelain et al., Reference Chatelain, Hundertmark, Leprince, Le Gall, Satour, Deligny-Penninck, Rogniaux and Buitink2012). Variation was seen both between and within accessions grown across the different regeneration environments, in regard to when ‘maximum’ longevity was attained, its value (i.e. measured by p 50) and how long it was maintained for (Figs 2 and 3). Lentils are sensitive to high temperatures (>30°C) during the reproductive phase, causing a reduction in grain yield and quality (Gaur et al., Reference Gaur, Saminen, Krishnamurthy, Kumar, Ghane, Beebe, Rao, Chaturvedi, Basu, Nayyar, Jayalakshmi, Babbar and Varshney2015). However, like in wheat (Nasehzadeh and Ellis, Reference Nasehzadeh and Ellis2017) and rice (Ellis, Reference Ellis2011), high temperatures later on in development, and throughout maturation, appear to have a positive effect, with longevity estimates generally reaching greater maximum values when seeds were exposed to higher temperatures (big igloo, green igloo and cage), particularly following MM, compared with the cooler temperatures experienced in the glasshouse (Figs 2 and 3). Within this glasshouse environment (Figs 2a, e and 3a, e), both accessions showed their greatest longevity at the start of sampling which, although was able to be maintained for a maximum of 42 d, further accumulation in longevity did not occur with developmental time. This is thought to be a result of the glasshouses’ controlled, lower, temperature conditions – particularly following MM, during the maturation drying phase. Seeds are sown at the end of Autumn, before the frosts, to ensure the final stages of development coincide with the hot, dry months of summer – as it is only when seeds experience some drying that a substantial accumulation of longevity is activated. It is likely that the moderate average temperature (25.7°C), and humidity conditions (49.3% RH) of the growing environment following MM (November onwards), is responsible for holding seeds in the pre-desiccation state where increases in longevity are limited (Fig. 4). The loss in moisture is a critical factor controlling the maturation processes, by inducing the stress response and other protective mechanisms (Radawan et al., Reference Radawan, Hara, Kleinwächter and Selmer2014). Chatelain et al. (Reference Chatelain, Hundertmark, Leprince, Le Gall, Satour, Deligny-Penninck, Rogniaux and Buitink2012) suggested that the maturation drying phase should be divided into two, with the first part being important for subsequent seed longevity, characterized by the accumulation of oligosaccharides and low molecular weight proteins (Sinniah et al., Reference Sinniah, Ellis and John1998b). Considering this, therefore, it is likely that the greater storage longevity observed in seeds grown in the big igloo, the green igloo and the cage is a result of a stress response initiated by high-temperature exposure during development and maturation (when seeds are still metabolically active). It is known that the accumulation of sugars and heat-stable proteins during development is associated with desiccation tolerance and longevity (Sinniah et al., Reference Sinniah, Ellis and John1998b) and maybe; it is the enhanced metabolism of such protectants, and other metabolic pathways, that help aid the stabilization of seed during desiccation and survival in air-dry storage (Whitehouse et al., Reference Whitehouse, Hay and Ellis2017, Reference Whitehouse, Hay and Ellis2018a). There also did not appear to be a critical temperature limit whereby there was a negative effect on subsequent longevity, as longevity was either maintained (Fig. 2b) or continued to increase (Fig. 2f) despite temperatures in the big igloo far exceeding that of the ambient environment (30–40°C during peak summer) – reaching maximum values between 46.7 and 57.7°C (Fig. 4).
Furthermore, not only did the higher temperature environments lead to higher estimates of subsequent storage longevity, but also combining a high temperature with lower, ambient, RH conditions (typical in the green igloo and the cage) resulted in an earlier peak in longevity (Fig. 2c, d, g, h), compared with when seeds were exposed to slightly hotter, more humid conditions (big igloo; Fig. 2b, f). Other studies have also reported similar findings; for example, a warm seed production environment can bring forward the time when maximum seed quality is attained. Similarly, a systematic study on winter wheat showed the progressive effect of temperature on seed quality development, with potential longevity increasing at a greater rate the warmer the ambient temperature, despite the warmer conditions reducing the duration of seed development and maturation (Sanhewe et al., Reference Sanhewe, Ellis, Hong, Wheeler, Batts, Hadley and Morison1996). In some species, this can result in an improvement in seed quality, as it enables development to progress further before seeds are shed (Daws et al., Reference Daws, Lydall, Chmielarz, Leprince, Matthews, Thanos and Pritchard2004), but others show a reduction in seed quality as the enhanced progression through development results in seeds not fully acquiring maximum seed quality (Ellis et al., Reference Ellis, Hong and Jackson1993b). When seeds dry too quickly in planta that is to the point where they have become developmentally fixed, it can halt the continuation of accruement in longevity. This could explain why longevity did not increase further following MM (60 DAA) when accession 76072 was grown in the green igloo and the cage, as seeds had already dried on the plant to low moisture levels (<12%; Fig. 2g, h).
The green igloo and the cage differed from the other environments in that they were exposed to the ambient conditions, including rainfall. As discussed earlier, rainfall exposure was shown to cause a loss in desiccation tolerance (Fig. 2c, d, g, h). As seeds need to be able to tolerate desiccation in order to survive air-dry storage, it is therefore not surprising that seeds which showed a loss in desiccation tolerance also showed a decline in longevity (Fig. 2). For example, longevity accumulation of seeds of accession 76080 grown in the green igloo (Fig. 2c) followed the expected pattern, whereby accruement increased with developmental time (DAA) before peaking, at 84 DAA. Maximum longevity was then held for 1week before being lost. This decline in storage longevity coincided with the onset of rain, which occurred at the end of January and into February, and caused a spike in ambient RH (Fig. 4) and thus, subsequently, seed moisture content (Fig. 2c). Although the K i was higher in subsequent seed lots (105 and 112 DAA), which indicates there was a larger proportion of highly viable seeds compared with 84 DAA, they were not able to maintain viability for long during storage (Supplementary Table S1). This is because, prior to this, seeds had already equilibrated to low moisture contents (<15% RH) and therefore are likely to have already entered the final stage of maturation drying whereby they become developmentally ‘fixed’. As a result, rain exposure is likely to have ‘over-advanced’ the higher quality seeds causing them to lose desiccation tolerance and subsequent quality; similar to what is seen when priming mature, high-quality seeds (Powell et al., Reference Powell, Yule, Jing, Groot, Bino and Pritchard2000; Śilwińska and Jendrzejczak, Reference Śliwińska and Jendrzejczak2002). Furthermore, the increase in moisture content, due to rain, pushed seeds to the part of the moisture isotherm (~70% RH) just below where seeds become metabolically active (>80% RH), and although seeds do not have to be fully hydrated to initiate repair processes, it is unlikely that they are fully functional below 98%. In addition to this, the high ambient temperature (>25°C) during these later stages is likely to have been detrimental to seed quality, with moist, mature seeds accumulating damage (Fig. 4). This scenario was also observed in the other accession, 76072, when grown in the green igloo as well as in the cage, whereby seeds were also exposed to the onset of rain (Fig. 2g, h). However, research has suggested that not all rainfall events are detrimental to end seed quality. For example, wheat seed quality, which is usually damaged by rainfall events during maturation and development, was consistently shown to be restored following the redrying of seeds in planta (Yadav and Ellis, Reference Yadav and Ellis2016). Evidence of this was also seen in this study, with seeds of both accessions increasing in longevity, following its decline (Figs 2 and 3). This is because seeds, following MM, are continuously undergoing wet-dry cycling and, therefore, are constantly switching between ageing and repair processes. Generally, if seeds can repair all the damage that has been accumulated, seed quality will be restored/maintained (Ellis and Pieta Filho, Reference Ellis and Pieta Filho1992). But if the seeds have suffered a substantial amount of damage, they are not able to reach the same quality that was previously attained, which is why, in this study, although we saw evidence of repair, longevity was also not fully restored (Figs 2a, c, g and 3a).
To conclude, the results of this study provide further confirmation that the quality of seeds cannot be accurately predicted post-MM with respect to developmental time (DAA) as the pre-harvest environment (ambient temperature and humidity) can restrict seeds progression through development and through maturation drying in particular (Whitehouse et al., Reference Whitehouse, Owoborode, Adebayo, Oyatomi, Olaniyan, Abberton and Hay2018b). Further to this, it is not clear from the results whether there is a ‘maximum longevity’ that any developing cohort of seeds can attain, rather the ‘value’ was a direct consequence of the net changes in seed quality (improvement vs deterioration) governed by the environmental conditions. If the seeds were showing a typical developmental response, for example, one would assume longevity would continually increase before plateauing and, although seeds grown in different environments would increase in longevity at different rates (due to slight variations in the environmental conditions), they would all, eventually, reach the same level. This was not observed in this study. Further research is required to conclude whether end seed quality can be optimized further in each of the environments to potentially reach its maximum. Nevertheless, the results presented here will help optimize, and tailor, the regeneration protocols at the AGG for seeds of non-shattering crops that are regenerated in each individual growing environment to ensure the highest maximum quality, and subsequent storage longevity is achieved under subsequent genebank storage. Relative longevity estimates from SEEs are expected to be comparable to that under conventional genebank storage conditions (i.e. short-lived seeds identified in the storage experiment will also lose viability quickly under genebank storage) as the seeds in this experiment were stored at a temperature and moisture content where the effects of changes in these variables are well defined by seed viability equations (Probert et al., Reference Probert, Daws and Hay2009; Hay et al., Reference Hay, Valdez, Lee and Sta. Cruz2019). If one was to make recommendations, for regenerating crops, based on the observations thus far, the glasshouse seems the least ideal environment, producing the lowest maximum longevity estimate in both accessions of lentil. The remaining three environments, where seeds were exposed to higher temperatures, particularly following MM while seeds are still metabolically active, appear to be beneficial for subsequent seed storage longevity. However, large increases in moisture content thereafter (i.e. when seeds have already dried in planta), generally due to rainfall, have a negative impact on seed physiological quality. Considering this evidence, it may be beneficial for seeds to be sown earlier (March/April) to avoid the onset of rain in the open environments of the cage and the green igloo, and/or the extreme heat, combined with high RH, in the big igloo during the late summer months. It is important to note that this research, thus far, is only relevant to domesticated crops as they are resistant to shattering and therefore remain on the plant following full maturation. Wild species readily disperse their seed, usually during the first part of the desiccation phase when the seed starts to equilibrate with the ambient conditions (Hay and Probert, Reference Hay and Probert2013), and, therefore, may not have acquired their full storage potential when collected. Further research is required to assess the management of wild species; however, considering the observations presented here, wild seed may benefit from restricting the rate of early drying to allow maturation processes to progress further before seeds are shed.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S0960258521000313.
Acknowledgement
We would like to thank the staff at the AGG, especially Nicole Sawyer, for technical assistance.
Financial support
This research was supported by funding from the Grains Research and Development Corporation (GRDC), grant number DAV1707-001BLX to SLN, through the AGG project – Phase 3, 2017–2022.
Conflicts of interest
The authors declare none.