Introduction
The introduction of a crop into a new region could create new problems such as mismatch between genotypes and environmental conditions. Among them, pre-harvest sprouting (PHS) is a common phenomenon occurring in various regions of the world, which causes great economic losses due to reductions in yield, industrial quality and seed viability (Paulsen and Auld, Reference Paulsen, Auld, Benech-Arnold and Sánchez2004; Gubler et al., Reference Gubler, Millar and Jacobsen2005; Kermode, Reference Kermode2005). PHS occurs due to environmental conditions surrounding the maternal plants that favour germination, such as a prolonged period of high humidity or rain, and adequate temperatures. PHS usually occurs when seeds are at the stages between physiological maturity and harvest. In temperate zones with iso-hygrous rainfall patterns (rainfall uniformly distributed over the year), such as the Argentinean pampas, the risk of PHS is potentially high. To predict PHS tolerance of seeds in certain genotypes or accessions, it is necessary to know their levels and patterns of dormancy during seed development and maturation. These patterns may vary depending on environmental conditions during seed development and maturation on the mother plant. Therefore, the influence of the environment on seed dormancy must also be considered for PHS issues.
Dormancy is defined as an internal condition that prevents seed germination under favourable water, temperature and gaseous conditions (Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000). Seeds with relatively low dormancy germinate in a wider range of conditions than those with relatively high levels of dormancy (Vegis, Reference Vegis1964). The decrease in dormancy over time is usually accompanied by an increase in the temperature range allowing seed germination. In spring–summer species, the broadening of the permissive temperature germination window occurs mainly by a decrease in the minimum temperature allowing germination, while in winter annual species it occurs by a gradual increase in the maximum permissive temperature for seed germination (Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000; Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007; Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2007).
In general, low temperatures and/or long photoperiods during seed development on the mother plant increase dormancy (Fenner, Reference Fenner1991; Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sánchez2004; Gualano and Benech-Arnold, Reference Gualano and Benech-Arnold2009a); although there are exceptions to this general pattern (Argel and Humphreys, Reference Argel and Humphreys1983; Fonseca and Sánchez, Reference Fonseca and Sánchez2000). Other factors affecting dormancy during seed development include water availability and nutrients (Sánchez et al., Reference Sánchez, Eyherabide and de Miguel1981; Benech-Arnold et al., Reference Benech-Arnold, Fenner and Edwards1991, Reference Benech-Arnold, Fenner and Edwards1995; Fenner, Reference Fenner1991; Gualano and Benech-Arnold, Reference Gualano and Benech-Arnold2009b). There are also complex interactions between these factors (Gualano and Benech-Arnold, Reference Gualano and Benech-Arnold2009b).
Cultivars producing seeds with high dormancy at harvest have a high tolerance to sprouting. However, seed dormancy has to be released during post-harvest storage in order to allow germination at the time of planting for the following crop season. During storage or under natural conditions on the ground after seed dispersal, temperature and its combination with moisture conditions are the main factors that modulate dormancy levels of a seed lot (Hilhorst, Reference Hilhorst1995; Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007). In spring–summer species, low winter temperatures generally favour dormancy release under humid conditions in the soil (Hilhorst, Reference Hilhorst1995; Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007). These conditions can be reproduced artificially as a method to break dormancy, commonly called cold-stratification. In contrast, high temperatures tend to accelerate dormancy release rate during seed storage at low seed moisture content (Karssen, Reference Karssen1970; Hilhorst, Reference Hilhorst1995).
Only a limited amount of information about seed dormancy in Chenopodium species is available. Some literature refers to seed coat importance in dormancy maintenance. Seed coats in quinoa include, from outside-in, the perigonium and pericarp as part of the fruit, and the episperm as cover of the true seed. An inverse correlation between the thickness of the seed covers (probably episperm) and germination has been found in C. bonus-henricus (Dorne, Reference Dorne1981). The environment surrounding the maternal plants exerts an influence on the morphology and composition of the seed coat, which is associated with the dormancy level in seeds of three Chenopodium species. In C. polyspermum and C. album, the photoperiod to which seeds are exposed during development modifies the seed coat thickness and significantly affects their viability (Jacques, Reference Jacques1968; Karssen, Reference Karssen1970; Pourrat and Jacques, Reference Pourrat and Jacques1975). In C. bonus-henricus, the high altitude at which plants develop increases the thickness and polyphenol contents of the seed coat, and is inversely correlated with germination percentage. Some of these reports, however, are not precise in relation to the tissue layers they consider ‘seed coat’ or ‘teguments’. Regarding storage conditions, there is evidence showing that high temperatures (37°C) combined with low seed water content provokes a rapid dormancy release of C. album seeds (Karssen, Reference Karssen1970).
Quinoa (C. quinoa) is a mostly autogamous plant (around 95% self-pollination) (Risi and Galwey, Reference Risi and Galwey1984), originating in the Andean region of South America, and is being introduced to England (Risi and Galwey, Reference Risi, Galwey, Wickens, Haq and Day1989), Denmark (Jacobsen and Stølen, Reference Jacobsen and Stølen1993), USA (Johnson and Ward, Reference Johnson, Ward, Janick and Simon1993) and India (Barghava et al., Reference Barghava, Shukla and Ohri2007) as a grain crop for its nutritional characteristics and production potential in adverse conditions. It has been used by the Andean people for over 5000 years as an important component of their diet (Tapia et al., Reference Tapia, Gandarillas, Alandia, Cardozo, Mujica, Ortiz, Otazu, Rea, Salas and Sanabria1979; Repo-Carrasco et al., Reference Repo-Carrasco, Espinoza and Jacobsen2003). Quinoa grain value is mainly in the quality of its proteins, which contain a balanced composition of essential amino acids with a high proportion of lysine, an amino acid deficient in cereal crops (Risi and Galwey, Reference Risi and Galwey1984; Ruales and Nair, Reference Ruales and Nair1992). Results of test production have indicated high adaptability of quinoa cultivars worldwide, including arid and high rainfall areas, hot or cold climate areas, tropical areas, altitudes over 4000 metres above sea level (m.a.s.l.) and at sea level (Bertero et al., Reference Bertero, de la Vega, Correa, Jacobsen and Mujica2004). Demand for quinoa in the USA, Europe and Asia is growing (Jacobsen, Reference Jacobsen2003). This has sparked interest in its cultivation in various regions beyond the traditional production areas inside and outside South America, including the Argentinean pampas. There are cultivars originating in the central region of Chile that show potential for adaptation and production, with good yields in the Argentinean pampas (Bertero and Ruiz, Reference Bertero and Ruiz2008). However, the majority of commercial cultivars have no dormancy, which limits the expansion of quinoa cultivation to non-traditional areas (Bertero and Benech-Arnold, Reference Bertero and Benech-Arnold2000). As shown in other species, selection of dormant cultivars would improve quinoa production in regions such as ‘the pampas’ where environmental conditions often favour sprouting. Previous suggestions of the occurrence of dormancy in quinoa were by Jacobsen et al. (Reference Jacobsen, Jørnsgård, Christiansen and Stølen1999) and Vergara et al. (Reference Vergara, Delatorre, Berti, Riquelme, Pinto, Delatorre, Salinas, Olave and Delfino2007).
There is almost no information about the mechanisms involved in the imposition of seed dormancy and the influence of storage conditions on the dynamics of dormancy release in this species. Two accessions exhibiting dormancy at harvest were recently identified as potential sources of resistance to PHS in quinoa. The objectives of the present work were: (1) to determine differences in dormancy levels of seeds in the two accessions of C. quinoa in response to the production environment; (2) to identify environmental factors responsible for differences in dormancy levels and in patterns of dormancy release; and (3) to determine differences in dormancy release patterns in the two accessions in response to storage temperature.
Materials and methods
Plant materials
Experiments were conducted using two quinoa accessions 2-Want and Chadmo, both of which exhibited some degree of seed dormancy at harvest. Chadmo originates from Chiloé, Chile (latitude 42°30′S, longitude 73°55′W, altitude 10 m.a.s.l.). There is no precise information about the origin of 2-Want, although it is presumed to originate from Bolivia (Christensen et al., Reference Christensen, Pratt, Pratt, Nelson, Stevens, Jellen, Coleman, Fairbanks, Bonifacio and Maughan2007). Both 2-Want and Chadmo were obtained from the Germplasm Bank at the US Department of Agriculture, National Plant Germplasm System, Beltsville, Maryland, USA, where they are identified as AMES 13737 and PI 614850, respectively.
Field experiments in the first year
Trials were conducted in the INTA (National Agricultural Research Center) experimental fields in Castelar, Buenos Aires (34°60′S, 58°65′W), Argentina. The crops were produced for both genotypes with three different sowing dates (S) (S1, 2 November 2005; S2, 27 December 2005; and S3, 13 March 2006) with appropriate irrigation and nutrient availability. These dates were chosen to expose developing seeds to environmental conditions prevailing between summer and autumn, which is considered to be optimum for quinoa production in the pampas (Bertero, Reference Bertero2001). However, due to excessive rain during February and early March in 2006, the third sowing date planned for mid-February was greatly delayed. Because of this drawback, the grain-filling period for that sowing date occurred during winter.
Flowering date (FD) for each crop was determined based on the presence of at least one open flower in 50% of the plants. Starting about 15 d after flowering (DAF), panicles were harvested every 5 d from four randomly sampled plants. Seeds were sampled from the middle third of each panicle separately and used for germination tests. Therefore, each replicate represents a single plant. Seed development and maturation (from 15 DAF to harvest) was characterized through the evolution of seed dry weight and moisture content, which were determined by weighing 50 seeds per replicate before and after drying at 105°C for 24 h in an oven (Ministério da Agricultura e Reforma Agrária, 1992). Physiological maturity (PM) was considered to be complete when dry weight levelled off. Harvest time (H) was determined based on a 20% seed moisture content, which is usual practice in the traditional production areas.
Germination tests in the first year
Seeds from each sowing date (S1, S2 and S3) were sampled and subjected to germination tests. Thirty-five seeds were placed on filter paper (Schleicher & Schuell 0859, Dassel, Germany) moistened with 4 ml of distilled water in plastic boxes of 85 mm diameter in four replicates, which were incubated under fluorescent light at 5, 10 or 25°C in an incubator with a temperature variation of ± 1°C. Visible radicle protrusion was used as germination criterion. Germination was recorded daily for 15 d, and time (d) of incubation required to reach 50% germination was considered. When necessary, seed viability was verified by the tetrazolium test (ISTA, 2005) with a modification adapted to this species (M. Castellión, personal communication). Briefly, the whole seeds were soaked in a 1% (w/v) tetrazolium solution in phosphate buffer, pH 6.5–7.5, and were kept at 30°C for 16–20 h to facilitate imbibition. For Chadmo, it was also necessary to puncture the pericarp to facilitate penetration of the chemical. After soaking, seeds were cut longitudinally through the embryo and were examined for staining.
Germination data during seed development was analysed on a thermal time (TT) scale. Accumulated TT was calculated as
where Tdm is the daily mean air temperature and Tb is the base temperature. Daily mean air temperature data were obtained from a meteorological station located about 300 m from the experimental site. Tb used for calculation was 3°C (Jacobsen and Bach, Reference Jacobsen and Bach1998).
For all assays, seeds were separated from their perigoniums. All germination tests described below were performed as described here, unless otherwise specified.
Field experiments in the second year
The crops of both genotypes were produced on two sowing dates (7 November 2006 and 8 February 2007), based on conditions and results observed in the first experimental year.
Germination tests in the second year
Germination tests in the second year were performed in essentially the same way as in the first year. However, based on results observed during the first year, the following modifications were introduced in the protocol: seeds were incubated at just two temperatures (10 and 25°C), three replicates of 40 seeds instead of four replicates of 35 seeds were used for each treatment, and germination was recorded daily for 10 d.
Experiments under controlled storage conditions
Seeds of both accessions harvested in each production period in the first-year experiments were dried to a 10% moisture content in a seed drying room (at approximately 15°C and 15% relative humidity), divided into two lots and stored at 5 or 25°C in heat-sealed airtight bags. Seeds were sampled at 20- to 40-day intervals and were subjected to germination tests. Chadmo from S3 was not included due to low seed production.
Seeds from the second experimental year were dried and stored at the same temperatures as in the first year. In this experimental year 2-Want from S2 was excluded for storage at 5°C due to low seed production. Seeds were sampled for germination tests every ~30 d.
Results and discussion
To understand differences in the environmental conditions to which plants and developing seeds were exposed during field experiments, mean daily temperature and photoperiod (including civil twilight) during the experimental periods were analysed (Fig. 1). Mean temperatures during seed development were 23.5, 18.8 and 12.4°C and mean photoperiods were 14.7, 12.9 and 10.5 h for the first, second and third sowing dates in the 2005/2006 experiments, respectively (Fig. 1A). For the 2006/2007 experiments, mean temperatures were 24.1 and 17.2°C and mean photoperiods were 14.7 and 11.5 h for the first and second sowing date, respectively (Fig. 1B).

Figure 1 Mean daily temperature (°C) and photoperiod (h d− 1) during the 2005/2006 (A) and 2006/2007 (B) experimental periods. Each data point represents an average of a 10-day period. S1, S2 and S3 indicate the first, second and third sowing dates. The horizontal black bars represent the duration of seed development (from the start of filling to physiological maturity). Data were obtained from a meteorological station located about 300 m from the experimental site (Climate and Water Institute, INTA).
Germination patterns during seed development
Seeds of both accessions displayed a relatively high dormancy level during the entire developmental period. For Chadmo, all seeds tested showed no germination for the entire period of development regardless of incubation temperatures, although high viability (over 95%) of the seeds was confirmed by the tetrazolium test (data not shown). For 2-Want, there was no germination for seeds incubated at 5 and 10°C throughout the entire developmental period; however, seeds started to germinate at 25°C at 27 DAF. This capacity of seed to germinate at 25°C increased sharply from PM onwards, exceeding 80% at harvest (Fig. 2).
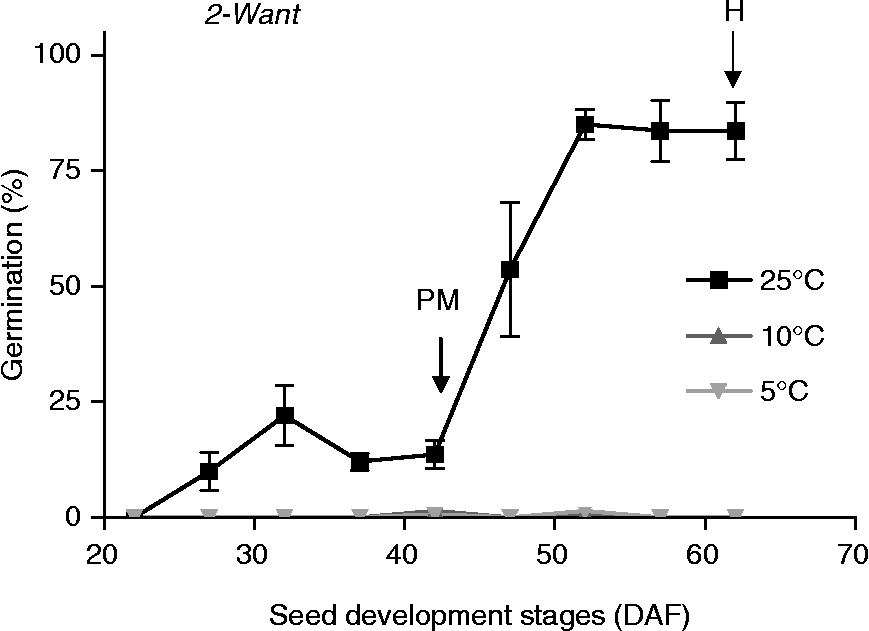
Figure 2 Germination of 2-Want quinoa seeds at different incubation temperatures (5, 10 and 25°C), collected at different times during seed development from a crop sown in November 2005 (S1). The arrows indicate the time of physiological maturity (PM) and harvest (H). Bars indicate the standard errors. DAF, days after flowering.
Variation in germination patterns in response to seed production environment
Developing 2-Want seeds exhibited germination when sampled and placed at 25°C (Fig. 2), which allowed us to evaluate the effects of sowing dates on the development of germinability. Clear differences were not observed in the developmental patterns of germinability between the S1, S2 and S3 (November, December and March sowings, respectively) of the first year of production, when germination percentages were plotted against DAF; although differences between sowing dates were observed in germination percentages obtained at PM (Fig. 3A). In contrast, the analysis of germination patterns based on TT units revealed clearer differences between production periods (Fig. 3B), in which S3 seeds presented an anticipated germinability at 25°C in comparison to that observed for the other sowing dates. For example, S3 seeds exhibited germinabiity after approximately 200°Cd, while S1 and S2 seeds required twice the TT to show some germination at 25°C. Although the Tb value used to calculate TT in the present study was originally determined for germination (Jacobsen and Bach, Reference Jacobsen and Bach1998), similar differences between sowing dates germination patterns were observed when a wide range of possible Tbs between − 5 and 4°C were examined (data not shown).

Figure 3 Effects of sowing date on the occurrence of germinability during seed development in 2-Want. Germination percentages at 25°C are plotted against days after flowering (DAF) (A) or thermal time (TT) after flowering [as degree days (°Cd) with a base temperature (Tb) = 3°C] (B). The arrows indicate the time of physiological maturity (PM) in each case. S1, S2 and S3 indicate first, second and third sowing dates in the 2005/2006 experiments, respectively. Bars indicate the standard errors.
This analysis of germination patterns based on TT suggests that early sowing (spring) dates could cause a higher dormancy level of seeds, at least the for 2-Want accession. Probably, daily temperature during production periods compensates these variations when data was plotted in DAF.
For the second year, although the amount of data was limited, patterns observed during the first experimental year were maintained (data not shown).
Dormancy release dynamics under different storage temperatures
Seeds produced in the first year exhibited around 100% germination after 180 d of storage, regardless of accessions, production timing, and storage or incubation temperature. Seeds produced during the second season, however, showed a higher level of dormancy, so this period was, in some cases, insufficient for a complete loss of seed dormancy (data not shown).
In the first sowing date of the first year, germinability of 2-Want seeds at 25°C, which had already been established during development (Fig. 2), was maintained at almost 100% germination during seed storage at 5°C (Fig. 4A). Germination of 2-Want seeds at 10°C and 5°C of incubation was observed only after 16 and 36 d of storage, respectively. Full germination (100%) at 10°C and 5°C did not occur until approximately 60 and 100 d of storage, respectively (Fig. 4A). A similar pattern of dormancy release (seeds acquire first the capacity to germinate at higher and then at lower temperatures) was observed for Chadmo, although the complete release from dormancy required a longer time period (~180 d) in this accession (Fig. 4B).

Figure 4 Final germination percentage during post-harvest storage at 5°C for seeds incubated at 5, 10 and 25°C. (A) 2-Want accession, (B) Chadmo accession, both from the S1 production (sown in November) in 2005. Bars indicate the standard errors.
It is common that dormancy is expressed at specific temperatures, and that the optimal temperature range for germination expands as dormancy is lost (Vegis, Reference Vegis1964). In winter species such as wheat and barley, dormancy is expressed at high temperatures, while dormancy is expressed at low temperatures in summer crops such as sorghum (Benech-Arnold et al., Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000; Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sánchez2004; Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007; Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2007).
Obtained germination data for stored seeds clearly showed that the permissive temperature range for germination was expanded towards low temperatures during seed storage, following the general pattern of summer species (Fig. 4). Moreover, this was also observed in germination tests of developing quinoa seeds, in which seeds germinate at high (25°C), but not at low temperatures (10 and 5°C).
Variation in post-harvest dormancy release patterns in response to the production environment
Germination tests at 5°C allowed a clearer distinction between sowing dates in terms of dormancy release patterns. For 2-Want, dormancy release in S3 was much faster (by ~60 d) compared to S1 or S2, which were similar to each other. There were no large differences between dormancy release rates for S1, S2 and S3 (Fig. 5A). For Chadmo, dormancy release in S2 was advanced (by 20 d) compared to that of S1. The dormancy release rates were similar between S1 and S2 (Fig. 5B).
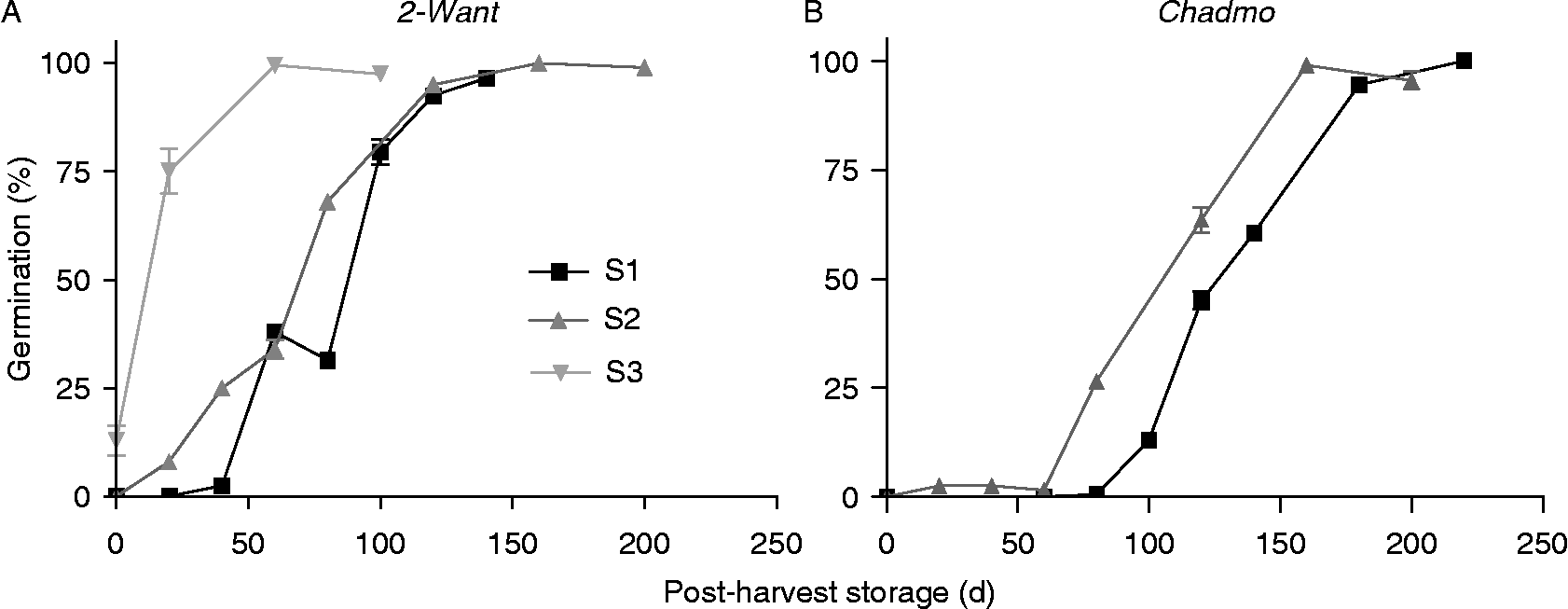
Figure 5 Effects of sowing date on dormancy release during post-harvest storage at 5°C. (A) and (B) are 2-Want and Chadmo accessions, respectively. S1, S2 and S3 are first, second and third sowing dates of the 2005/2006 experiments, respectively. Germination was tested at 5°C. Bars indicate the standard error of the mean.
For both accessions in the first year, the time to reach 90% germination at 5°C did not exceed 180 d under the experimental storage conditions (10% moisture content and a constant temperature of 5 or 25°C). This indicated that 6-month storage would be sufficient to make the seeds available for the next sowing even when temperatures of sowing are low. Tetrazolium tests indicated high viability (average 95 ± 5%) in 12-month-stored seeds from both accessions and storage temperatures (data not shown).
When comparing storage temperatures in both accessions and for all tested sowing dates, storage at 25°C resulted in an earlier and faster (i.e. higher dormancy release rate) exit from dormancy than that observed at 5°C. In addition, Chadmo displayed an advance of about 20 d in the time of germination onset. In S2 of the first year, the greatest difference in germination was slightly above 50% in 2-Want and about 70% in Chadmo (Figs 6A and B). In S1 the maximal difference was recorded at approximately 20 d later, while in S3 (for 2-Want only, see Materials and methods) was 20% and 40 d earlier (data not shown). Calculation of TT value [ = Σ(Tdm − Tb), see Materials and methods] for dormancy release under each storage condition indicated that seeds stored at 5°C required less TT for the release from dormancy than those stored at 25°C (data not shown).
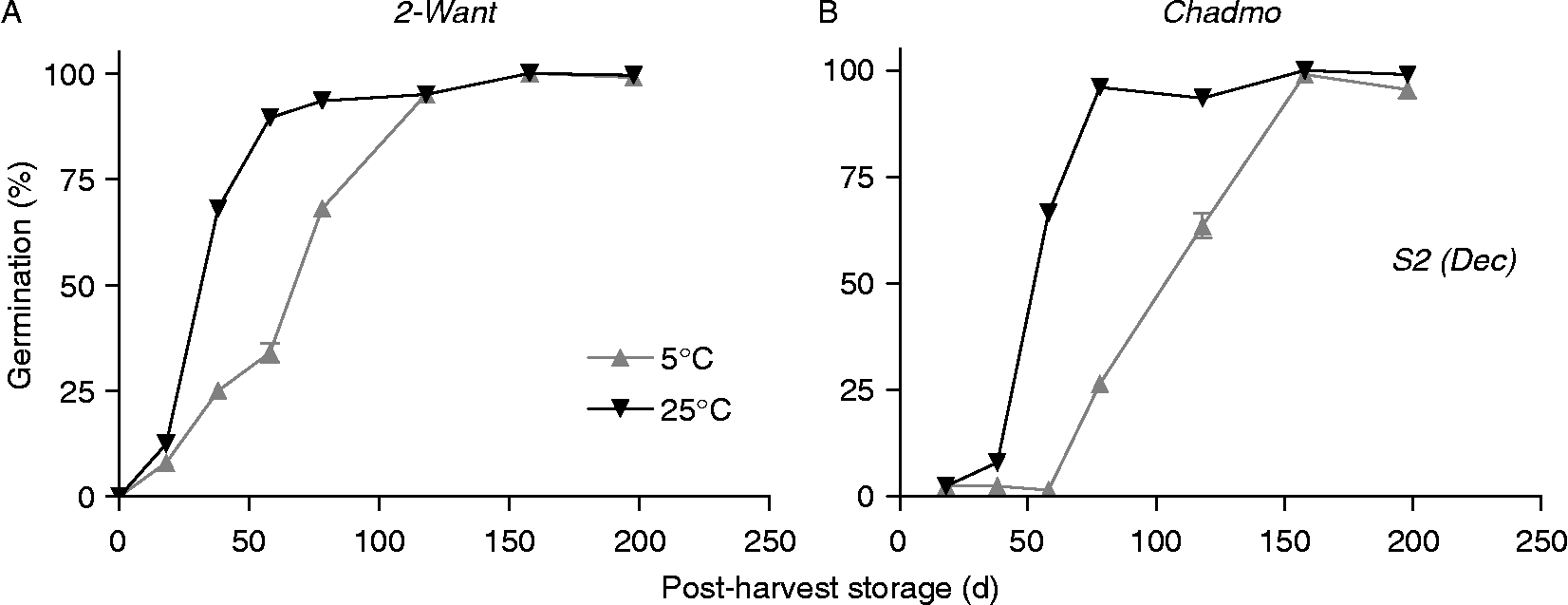
Figure 6 Effect of storage temperature (5 and 25°C) on germination of seeds from the S2 production (sown in December) in 2005 incubated at 5°C. (A) 2-Want, (B) Chadmo. Bars indicate the standard error of the mean.
In the second-year production, dormancy was deeper than in the first year. Patterns observed during the first year were maintained, moreover, higher temperatures (10°C) clearly distinguished between sowing dates due to the higher level of dormancy (data not shown). Differences between storage temperatures also showed the same pattern of response, and were greater than in the first year, notable even when the seeds were incubated at 25°C (data not shown).
The results indicate that it is possible to handle a quinoa crop in areas with frequent risk of PHS, ensuring a higher tolerance to this problem and a release from dormancy in time for the next planting in the field. In the site where experiments were conducted, early sowings (November) can be expected to maintain dormancy levels for longer, and a storage at relatively high temperature will favour dormancy release, without damage to seed viability.
Since crops were conducted in conditions of adequate irrigation and nutrient availability and in the same place to ensure similar soil characteristics, temperature and photoperiod can be expected to be the most influential environmental factors responsible for the observed effects. A regression analysis indicated the existence of a positive significant (P < 0.05) correlation between mean temperature or mean photoperiod experienced by developing seeds and the time (d) of seed dry storage required to reach 50% germination (T50) at 10°C of incubation (Fig. 7). Similar correlations were found for seeds incubated at 5 and 25°C (data not shown).
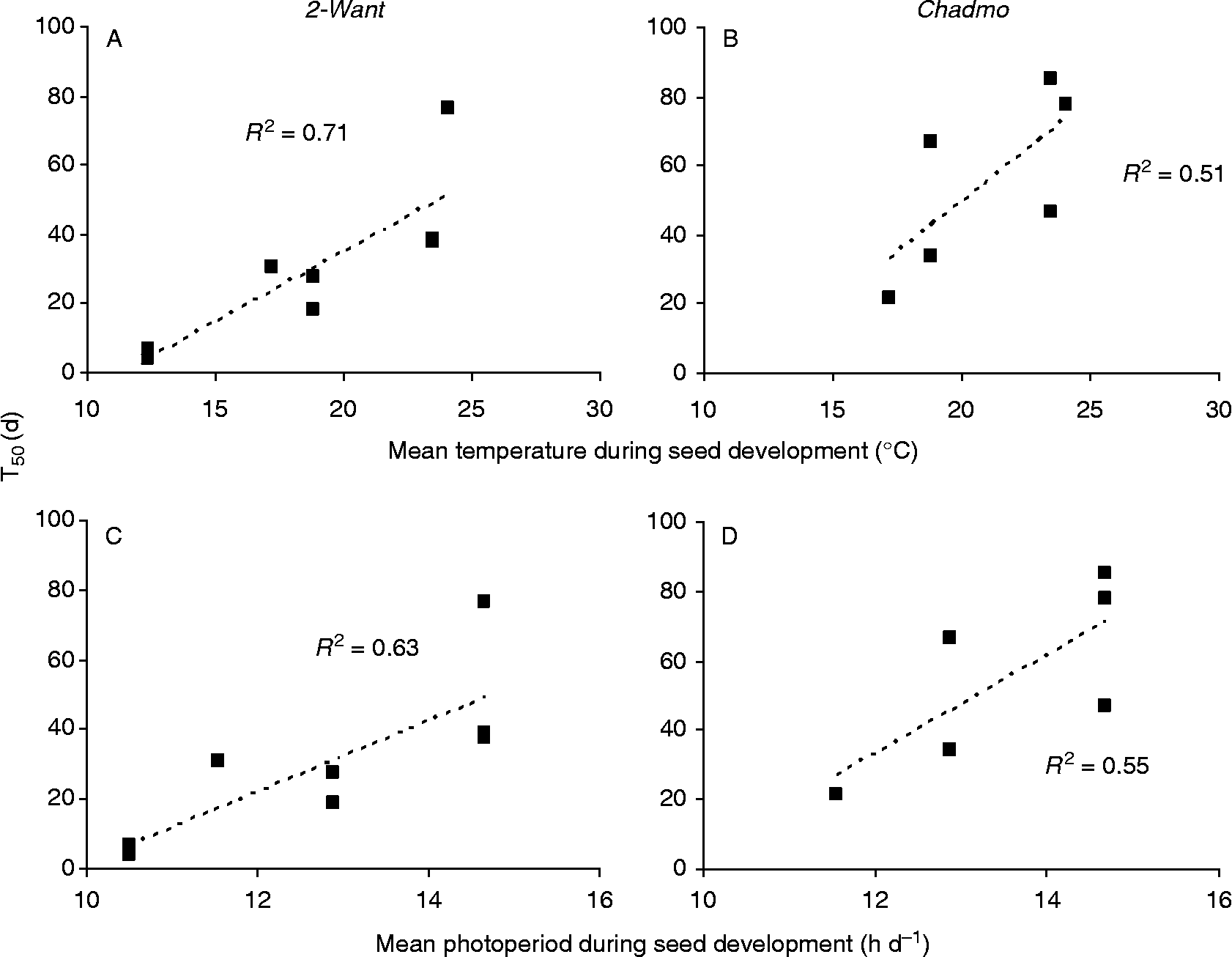
Figure 7 Correlation between time (d) of seed dry storage required to reach 50% germination (T50) and mean temperature (A and B) or photoperiod (C and D) during seed development. Data corresponding to different sowing dates and different storage temperatures (5 and 25°C) in the first- and second-year experiments were combined and analysed. Germination tests were performed at 10°C.
An additional experiment including both accessions conducted in a glasshouse under controlled photoperiod and temperature conditions confirmed the photoperiodic effect on dormancy but, due to technical drawbacks, failed to confirm a temperature effect, although a tendency consistent with Fig. 7 was observed (data not shown). Nonetheless, it is widely accepted that temperature is one of the most influential environmental factors affecting the level of dormancy (Fenner, Reference Fenner1991; Benech-Arnold, Reference Benech-Arnold, Benech-Arnold and Sánchez2004; Allen et al., Reference Allen, Benech-Arnold, Batlla, Bradford, Bradford and Nonogaki2007), so this point deserves further analysis.
This work provides a first description of responses to the environment of quinoa seed dormancy and recommends the accession Chadmo as a candidate to provide tolerance to PHS to the species, by including it in adaptive breeding programmes.
Acknowledgements
We thank Roberto Benech-Arnold and IFEVA (Insitute for Physiological and Ecological Reseach Applied to Agriculture– UBA – CONICET) for providing us with working facilities, Martín León for field and laboratory assistance, Teresa Boca and Alejandro Aparicio for statistical advice and INTA Germplasm Bank for help in experiments. Financial support was provided by a fellowship from INTA (National Institute of Agricultural Technology), Argentina.









