Introduction
Cultivated soils contain large amounts of buried weed seeds, many of them maintaining their viability for an extended period of time until they receive the stimulus for germination. Seeds of several weed species detect light as an environmental germination-promoting factor during soil disturbances associated with tillage operations (Scopel et al., Reference Scopel, Ballaré and Radosevich1994). In this situation even an exceedingly small amount of photons can be perceived by phytochrome A (phyA), which induces germination through a very low fluence response (VLFR) (Scopel et al., Reference Scopel, Ballaré and Sánchez1991, Botto et al., Reference Botto, Sánchez and Casal1998).
Datura ferox seeds are extremely dormant at maturity, but after a period in the soil seed bank, primary dormancy is reduced and germination can be promoted by a low fluence response (LFR) through phytochrome B (phyB) and other stable phytochromes (Casal and Sánchez, Reference Casal and Sánchez1998). Germination promotion by VLFR requires a greater decrease in dormancy than LFR. When seeds remain buried in the soil for an extended period of time (months), light sensitivity is notably induced as dormancy decreases (Scopel et al., Reference Scopel, Ballaré and Sánchez1991; Gallagher and Cardina, Reference Gallagher and Cardina1998).
Field experiments have demonstrated that germination of buried photoblastic seeds can be induced by exposures to milliseconds of sunlight and by Pfr/Ptot ratios (per cent of phytochrome in its Pfr active form/total phytochrome chromoprotein amount) as low as 0.01% (Scopel et al., Reference Scopel, Ballaré and Sánchez1991, Reference Scopel, Ballaré and Radosevich1994; Gallagher and Cardina, Reference Gallagher and Cardina1998). The response is highly dependent on the depth of burial and the vegetation cover above ground (Botto et al., Reference Botto, Sánchez and Casal1998). This acquisition of light-sensitivity can be reproduced in laboratory conditions by treating the seeds for some time (days or weeks) in a water-saturated atmosphere (WSA) at constant temperature in darkness (de Miguel, Reference de Miguel1980). Therefore, a process that can take months or years in natural environments, takes only days or weeks in laboratory conditions.
In the final steps of the process, VLFR and LFR lead to germination by finely modulating both micropylar endosperm weakening (EW) and embryo growth potential (EGP) (Bewley and Black, Reference Bewley and Black1994; Casal and Sánchez, Reference Casal and Sánchez1998). As in LFR, VLFR induces EW after light stimulation (de Miguel and Sánchez, Reference de Miguel and Sánchez1992). Endosperm softening is led by the degradation of the main cell wall component, a 4-linked mannose (β-1,4-mannan), by endo-β-mannanase and β-mannosidase activities (Sánchez et al., Reference Sánchez, Sunell, Labavitch and Bonner1990). In addition, this is correlated with DfMan1 and DfExpa1 gene expression in the micropylar endosperm of (weakly dormant) D. ferox seeds that are highly sensitive to very low fluence (VLF) of photons (Arana et al., Reference Arana, Burgin, de Miguel and Sánchez2007). EGP is also induced by VLFR after light stimulation, as was observed for LFR (de Miguel and Sánchez, Reference de Miguel and Sánchez1992; Mella et al., Reference Mella, Burgin and Sánchez2004), but in contrast to the EW, there is no correlation between EGP and expression in the embryo of two expansin genes, DfExpa1 and DfExpa2, when germination is promoted by a VLFR in extremely sensitive D. ferox seeds (Arana et al., Reference Arana, Burgin, de Miguel and Sánchez2007).
Even though VLFR-promoted germination increases the ecological success of the weed, the process of physiological adaptation by which the seeds acquire a VLFR state, thus facilitating germination to occur in the right time and place, is poorly understood. This paper describes the effect of dormancy breakage and acquisition of extreme light-sensitivity on EW and EGP in VLFR-promoted germination of D. ferox seeds.
Materials and methods
Source of seeds and general incubation condition
Datura ferox seeds were collected from plants invading soybean fields near San Miguel del Monte (35.440114°S, 58.81 927°W), Nueve de Julio (35.453958°S, 60.884871°W) and Balcarce (37.847477°S, 58.244047°W), Province of Buenos Aires, Argentina. After harvest, the seeds were stored in dark glass jars at room temperature. In order to obtain different sensitivities to light and dormancy levels, seeds were maintained in a water-saturated atmosphere (WSA) in complete darkness at 25°C for 7–21 d (see supplementary Fig. S1, available online; de Miguel and Soriano, Reference de Miguel and Soriano1974). Then, seeds were sown on cotton wool saturated with distilled water (5 ml) in clear plastic boxes and exposed to the different light treatments. After light exposure, the plastic boxes were wrapped in black plastic sheets and incubated in darkness at alternating temperatures, 15 h at 20°C and 9 h at 30°C (20/30°C).
Light sources and treatments
Immediately after treatment in WSA, seeds were exposed to a 45-min far-red light pulse (FR; 15 μmol m− 2s− 1), a 45-min red light pulse (R; 150 μmol m− 2s− 1), or kept in darkness. FR treatments were provided by 125 W incandescent internal reflector lamps filtered through a RG9 Schott glass filter (Mainz, Germany) and a 10 cm water filter. R was provided by a LED set with a light-emitting maximum at 660 nm (Cavadevices, Buenos Aires, Argentina). The calculated Pfr/Ptot ratios were 0.03 and 0.87 for the FR and R sources, respectively (Casal et al., Reference Casal, Sánchez, Di Benedetto and de Miguel1991). After light treatments, seeds were kept in darkness at alternating temperatures until germination counting or sampling for enzyme activities, embryo growth potential and gene expression measurements.
Germination tests
Seeds with different levels of dormancy and sensitivity to light were classified according to their final germination response to a saturating FR pulse, indirectly indicating their dormancy level (de Miguel and Soriano, Reference de Miguel and Soriano1974). The seeds were treated in a WSA for different periods (7–21 d) and then irradiated with FR or R pulses, or kept in darkness. Germination was recorded 7 d after light treatments and seeds were classified according to their VLFR status into the following groups: (a) low sensitivity to FR (LS) and high dormancy level, with a final germination response between 0 and 30% of the total number of seeds; (b) medium sensitivity (MS) with medium dormancy breakage, final germination between 30 and 60%; and (c) high sensitivity (HS), weakly dormant seeds germinating above 60% (see Table 1). The results shown are the mean values of 25-seed samples in at least three biological replicates ± standard error.
Table 1 Final germination response of Datura ferox seeds after water-saturated atmosphere (WSA) and light treatments. Seeds were classified in groups according to their dormancy level and acquired sensitivity to a FR pulse as follows: (a) low sensitivity to FR (LS), with a final germination response between 0 and 30%; (b) medium sensitivity (MS), final germination between 30 and 60%; and (c) high sensitivity (HS), seeds germinating above 60%. Data are the mean values of 25 seeds per sample and at least three biological replicates per sensitivity group ± SE

FRp, far-red pulse; Rp, red pulse; VLFR, very low fluence response.
Enzyme activity measurements
Mannan-degrading activities were measured separately on 20 seeds per biological replicate for each group. As endosperm softening is confined to the micropylar region in D. ferox seeds (Sánchez and de Miguel, Reference Sánchez and de Miguel1997), endo-β-mannanase and β-mannosidase activities were measured on the 0.5 mm conical endosperm portion that encloses the radicle tip prior to protrusion. Forty-five hours after the beginning of the incubation on water and the light treatments, seeds were dissected under white light and the endosperm cones were collected. The extraction and determination of enzyme activities were carried out as described in Sánchez and de Miguel (Reference Sánchez and de Miguel1997). The results were plotted as a bar graph and two-way ANOVA with Bonferroni multiple comparison post-tests were used to test for effects due to sensitivity on enzyme activity means.
Embryo growth potential measurements
Seeds were treated in a WSA to obtain the different groups of sensitivity. Forty hours after light treatments, seeds were decoated and the tips removed by excising the 0.5 mm conical micropylar end with a surgical blade under green dim light. Dissected seeds were returned to incubation in darkness under alternating temperatures until the embryo length measurements were performed, 24 h later. The EGP can be associated with the length of the embryo when the tissues impeding its growth are removed. The EGP was measured as described in de Miguel and Sánchez (Reference de Miguel and Sánchez1992). In total, 20 embryos of at least three biological replicates per sensitivity group were measured. Graphs were drawn to show the embryo length frequency distributions for every group and treatment adjusted to a non-linear curve (Gaussian distribution, R2 values are shown in supplementary Table S1, available online) using GraphPad Prism (GraphPad Software, San Diego, California, USA). A one-way ANOVA, using the mean length values, was used to examine differences between groups in every treatment.
Total RNA isolation and semi-quantitative PCR (sqRT-PCR) conditions
Seeds with different levels of dormancy and light sensitivity were dissected and the embryo extracted immediately following incubation in WSA (t= 0), and 24 h after exposure to FR or kept in darkness. RNA extraction of at least three biological replicates from 40 embryos was performed with the NucleoSpin RNA Plant Kit (Macherey-Nagel, Germany) according to the manufacturer's instructions. RNA concentrations were determined in a UV-visible spectrophotometer. Reverse transcription reactions were performed with SuperScript III (Invitrogen, Brazil) using 500 ng of total RNA in a final volume of 20 μl. Polymerase chain reactions (PCR) were done with DNA Taq polymerase (Invitrogen) using 2 μl of a half-sample dilution in a final volume of 25 μl. Specific primers for DfExpa1 and DfExpa2 (see supplementary Table S2, available online) were designed with FastPCR software version 4.0.27 (Kalendar et al., Reference Kalendar, Lee and Schulman2009) with sequence data previously obtained and available from GenBank (accession numbers: DfExpa1, AF442773; and DfExpa2, AF442772; Mella et al., Reference Mella, Burgin and Sánchez2004). The number of PCR cycles for each gene was determined such that the amount of product was in the linear range of amplification. Primer sequences and specifications can be found in supplementary Table S2 (available online). Quantification was done by semi-quantitative reverse transcriptase PCR (sqRT-PCR) and performed as described in Auge et al. (Reference Auge, Perelman, Crocco, Sánchez and Botto2009). PCR amplifications were separated in a 2% agarose gel and transferred to Hybond N+ Nylon Membrane (GE Healthcare Life Sciences Corporation, Buckinghamshire, UK) using a Trans-Blot SD Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, Hercules, California, USA). A specific probe for each gene was generated and hybridized with the AlkPhos Direct Labelling and Detection System Kit (GE Healthcare Life Sciences Corporation). A chemifluorescent signal was generated using the ECF™ substrate reaction for c. 4–6 h, detected using a Storm 840 scanner (Molecular Dynamics, GE Healthcare Life Sciences Corporation) and analysed by the ImageQuant 5.2 program (Molecular Dynamics). The results are expressed as the ratio of DfExpa1 and DfExpa2 to Actin and normalized to 1 for the FR 24 h-HS sample values. An ANOVA followed by a Bonferroni post-test was performed to assess statistical differences between groups. All kits and reagents were used according to the manufacturers' instructions.
Results
FR-promoted germination through VLFR is dependent on the dormancy level in Datura ferox seeds
To study the influence of the dormancy level in laboratory conditions on FR-promoted germination, D. ferox seeds were pretreated in a water-saturated atmosphere (WSA) for various time periods, thus obtaining different sensitivity groups classified according to their final germination response to a FR pulse (Pfr/Ptot = 0.03, Casal et al., Reference Casal, Sánchez, Di Benedetto and de Miguel1991). The final dormancy level, and, therefore, the sensitivity to very low fluence (VLF) of photons, was correlated with the time the seeds were maintained in WSA (Table 1). A short treatment time in WSA resulted in a low dormancy breakage and response to a FR-inductive pulse; while with increasing time in WSA, such a reduction in dormancy level is obtained that enables most of the seed population to germinate after the FR stimulus. Therefore, high dormancy levels are correlated with low sensitivity to light, while seeds with low dormancy are extremely sensitive to very low fluence of photons. To further explore the VLFR-mediated promotion of germination, seeds pretreated in WSA and then subjected to a FR pulse were grouped as follows: (a) low sensitivity to FR (LS), with a final germination response between 0 and 30% of the total number of seeds; (b) medium sensitivity (MS), final germination between 30 and 60%; and (c) high sensitivity (HS), germination above 60% (Table 1). The results in Table 1 show that dormancy level and final response to a saturating FR pulse can be modulated in the laboratory (weeks) in a similar way as under natural conditions (months or years) (Scopel et al., Reference Scopel, Ballaré and Sánchez1991, Reference Scopel, Ballaré and Radosevich1994; Gallagher and Cardina, Reference Gallagher and Cardina1998). Even though the role of abscisic acid (ABA) in the regulation of seed dormancy is well known (Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010), we did not find a correlation between ABA levels and the final germination response after an FR pulse in the sensitivity groups (data not shown).
Effect of acquisition of VLF sensitivity on endosperm weakening
VLFR-mediated induction of germination in D. ferox seeds with the lowest dormancy level depends on both endosperm weakening (EW) and promotion of embryo growth potential (EGP) after the FR stimulus, in a similar way as through an LFR (de Miguel and Sánchez, Reference de Miguel and Sánchez1992; Arana et al., Reference Arana, Burgin, de Miguel and Sánchez2007). Activities of endo-β-mannanase and β-mannosidase are highly correlated with EW after an FR pulse in seeds that are highly sensitive to VLF of photons (Arana et al., Reference Arana, Burgin, de Miguel and Sánchez2007), but the relationship between enzyme activity and dormancy level was not previously explored in detail.
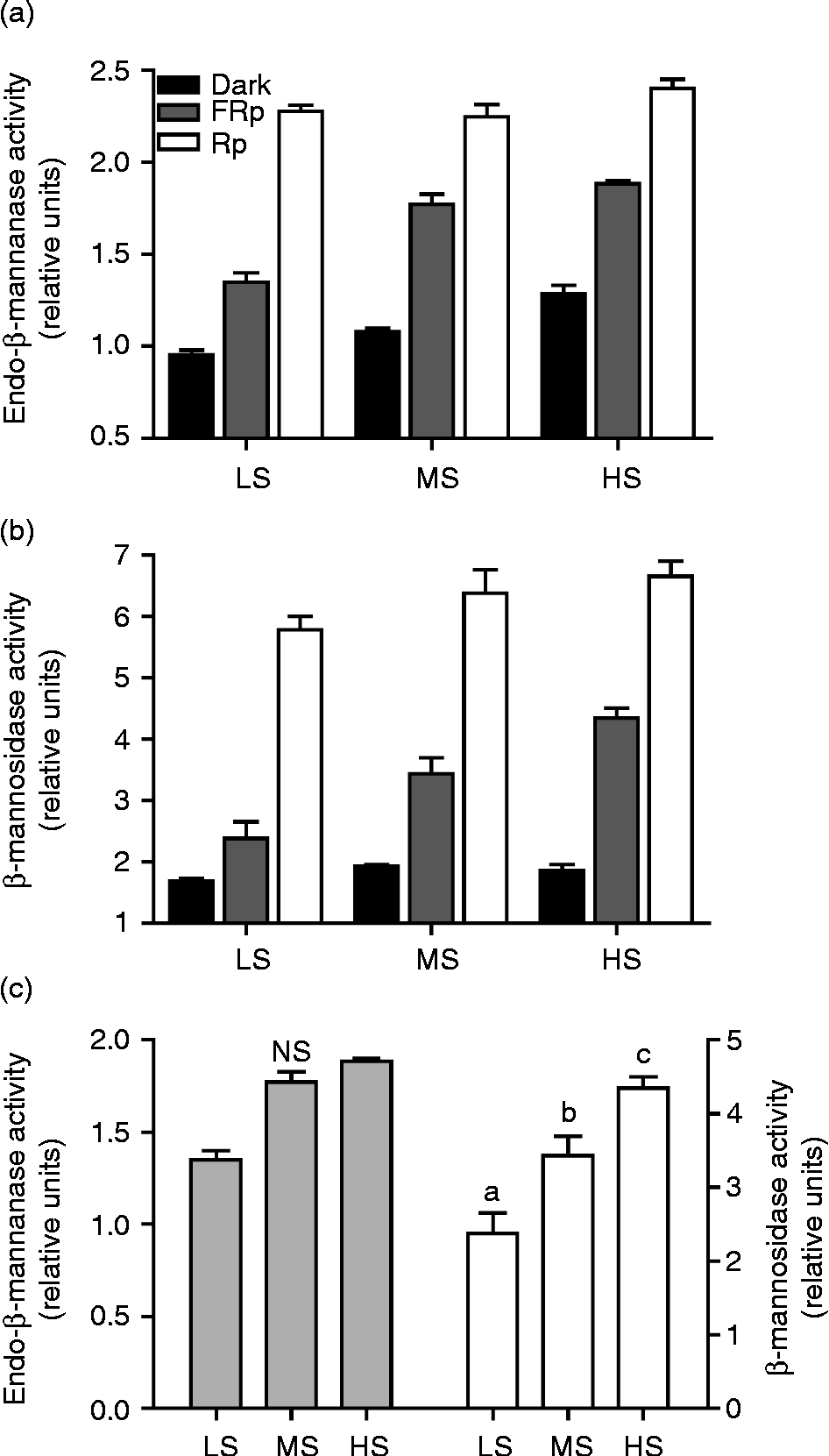
Endo-β-mannanase and β-mannosidase activities were studied in the micropylar endosperm of seeds pretreated in WSA and then subjected to FR or R stimulation, or kept in darkness. Enzyme activities increased proportionally with increasing sensitivity to FR and decreasing dormancy level (Fig. 1a and b). For both activities, the response is maximal in the micropylar endosperm of seeds treated with an R pulse (LFR-mediated germination). After the FR pulse (Fig. 1c), β-mannosidase activity was significantly different between groups (P< 0.01), while the endo-β-mannanase activity response was not (P>0.05). These results suggest that the regulation of EW by the VLFR is limited by β-mannosidase activity.

Figure 1 Endo-β-mannanase and β-mannosidase activities during acquisition of sensitivity to VLFR in D. ferox seeds.Twenty endosperm cones were assayed in at least three biological replicates per sensitivity group. Graphs show the endo-β-mannase (a) and β-mannosidase (b) activities measured after red pulse (Rp) and far-red pulse (FRp) irradiation or incubation in darkness prior to radicle protrusion. (c) Comparison of the enzyme activities after FRp irradiation for every sensitivity group. Bars represent the mean value of at least three independent biological replicates ± SE. Different letters show significant differences (P< 0.01, ANOVA).
VLFR stimulation of EGP is dependent on FR sensitivity and is correlated with expansin gene expression
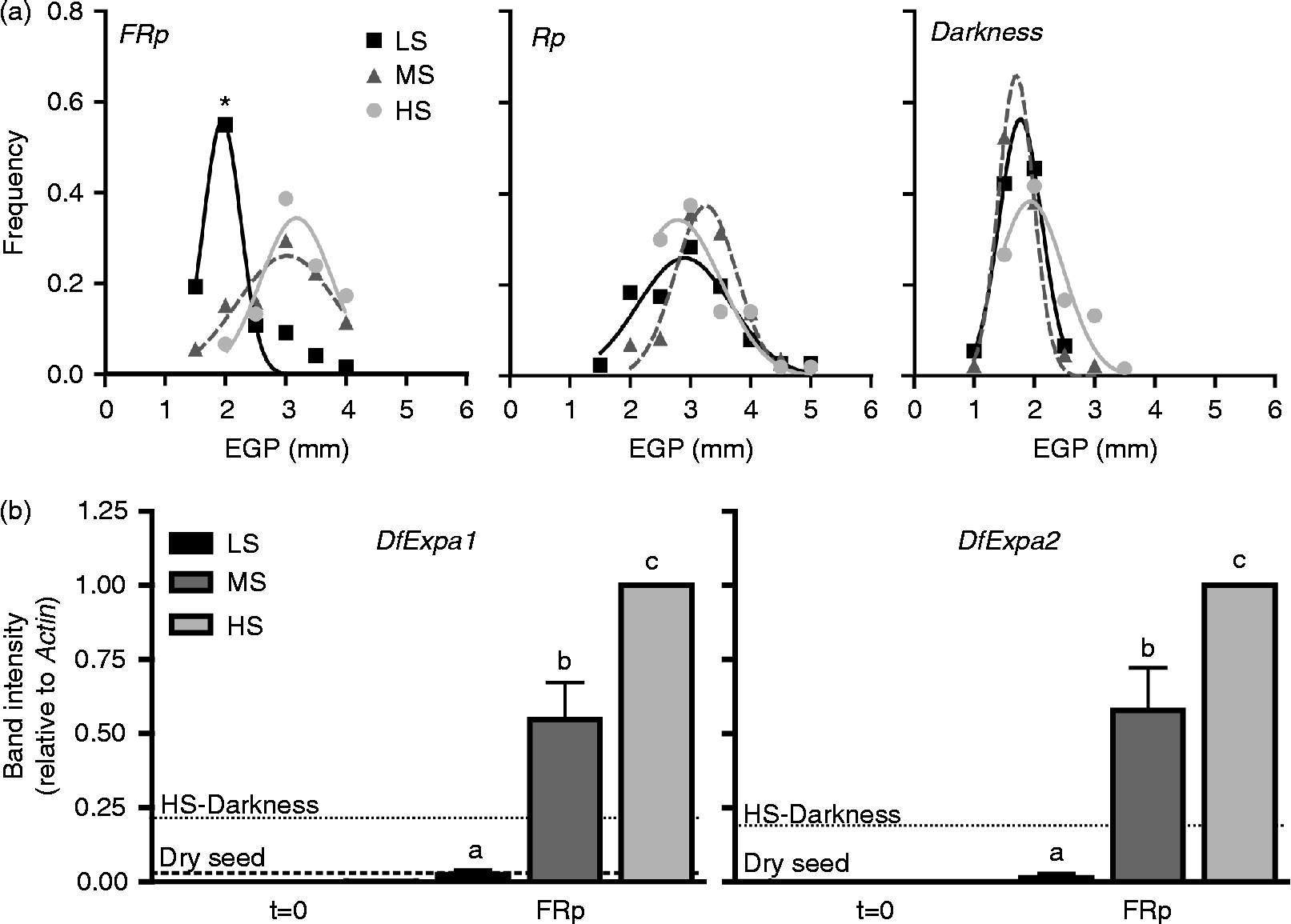
VLFR-promoted germination is correlated with a high EGP in VLF-sensitive seeds (Arana et al., Reference Arana, Burgin, de Miguel and Sánchez2007). However, the correlation between the different dormancy levels and the EGP response to a saturating FR pulse was not previously explored. The EGP response was studied in seeds pre-treated in WSA and then subjected to FR or R pulses, or kept in darkness. Although there was no difference between groups when seeds were irradiated with an R pulse or kept in darkness (Rp and Darkness in Fig. 2a), embryos from FR-treated seeds with higher dormancy level and low acquired sensitivity behaved in a similar way to embryos from seeds kept in darkness (FRp and Darkness in Fig. 2a, respectively). In contrast, the response of the embryos from seeds with a higher dormancy breakage and sensitivity to FR (MS and HS groups) was similar to that of the embryos treated with an R pulse. These results strongly suggest that, unlike the final germination response and the cell wall degrading activity correlating with EW (Table 1 and Fig. 1), it is not necessary to reach the highest sensitivity to VLF of photons to reach the maximum response of EGP.

Figure 2 Embryo growth potential and expansin gene expression in the embryo during VLFR. (a) Embryo length frequency distributions measured after light treatments (FR, R or darkness) for every sensitivity group described in Table 1 (LS, black squares; MS, dark grey triangles; and HS, light grey circles). The asterisk shows significant differences between the means of every group in a given treatment (P< 0.001, ANOVA). (b) DfExpa1 and DfExpa2 transcript accumulation after WSA treatment (t= 0) and 24 h after the FR pulse for every sensitivity group as indicated in Table 1 (LS, black; MS, dark grey; and HS, light grey); dashed and dotted lines in both graphs indicate the average expression level of each gene in embryos from dry seeds and seeds from the HS group kept in darkness for 24 h (HS-Darkness), respectively. Bars represent the mean value of at least three independent biological replicates ± SE normalized using HS-FR values as 1. Different letters indicate significant differences (P< 0.01, ANOVA).
The ability of the embryo to expand after the light stimulus is dependent on the extensibility of the tissue cell walls (primary cell wall). There is a strong correlation between EGP and expansin gene expression in LFR-promoted germination in D. ferox seeds (Mella et al., Reference Mella, Burgin and Sánchez2004), but the influence of the dormancy level and sensitivity to FR during VLFR-promoted germination has not been explored so far. The increase of the EGP response during the acquisition of sensitivity to the saturating FR pulse, is associated with an increase in DfExpa1 and DfExpa2 transcript accumulation in D. ferox embryos (Fig. 2b). This response is dependent on the light stimulus, since the expression in embryos from seeds with the lowest dormancy level and highest VLF state kept in darkness was lower than in MS and HS samples after FR stimulation (Fig. 2b). In addition, the WSA pretreatment by itself did not have any detectable effect on expansin gene expression in any sensitivity group (t= 0 in Fig. 2b). It is worth noting that the expression of both genes in the embryos of the HS group is higher than the expression observed for the MS group. Unlike the response detected for EGP for those groups, these results indicate that in these conditions expansin gene expression is limited by some other factor that is not limiting for the EGP response.
Discussion
VLFR germination promotion of weakly dormant D. ferox seeds is associated with an increase in EW and EGP after FR light irradiation (Arana et al., Reference Arana, Burgin, de Miguel and Sánchez2007). In this work we report the effect of progressive dormancy breakage, leading to the acquisition of a very high sensitivity to VLF of photons of FR light of micropylar endosperm weakening and expansion capacity of the embryo during VLFR-promoted germination. In seeds with coat-imposed dormancy, the expansion capacity of the embryo and the resistance imposed by the covering layers (seed coat or testa and endosperm) play a major role in the completion of germination (Bewley and Black, Reference Bewley and Black1994). The weakening of the endosperm, probably involving several types of hydrolytic enzymes, is required in these seeds to allow radicle emergence (Bewley, Reference Bewley1997; Sánchez and de Miguel, Reference Sánchez and de Miguel1997; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Iglesias-Fernández et al., Reference Iglesias-Fernández, Rodríguez-Gacio, Barrero-Sicilia, Carbonero and Matilla2011). Previous reports have shed light on the contribution of several cell wall modifying enzymes in endosperm weakening (EW), including mannanases, endo-β-1,3-glucanases, chitinases, peroxidases, expansins and other proteins (Bewley, Reference Bewley1997; Leubner-Metzger and Meins, Reference Leubner-Metzger and Meins2000; Wu et al., Reference Wu, Leubner-Metzger, Meins and Bradford2001; Li et al., Reference Li, Jones and McQueen-Mason2003; Penfield et al., Reference Penfield, Li, Gilday, Graham and Graham2006; Linkies et al., Reference Linkies, Müller, Morris, Turecková, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage and Leubner- Metzger2009; Müller et al., Reference Müller, Job, Belghazi, Job and Leubner-Metzger2010; Iglesias-Fernández et al., Reference Iglesias-Fernández, Rodríguez-Gacio, Barrero-Sicilia, Carbonero and Matilla2011). In D. ferox seeds, the micropylar endosperm offers a high resistance to radicle protrusion. This tissue mainly consists of 4-linked mannose polysaccharides, and endosperm weakening during germination is highly correlated with endo-β-mannanase and β-mannosidase activities in the endosperm cap (Sánchez et al., Reference Sánchez, Sunell, Labavitch and Bonner1990; Sánchez and de Miguel, Reference Sánchez and de Miguel1997; Sánchez et al. Reference Sánchez, de Miguel, Lima and de Lederkremer2002). Our results show that as dormancy breakage proceeds and sensitivity to VLF of FR photons increases (Table 1), the final germination response is correlated with the endo-β-mannanase and β-mannosidase activities in the micropylar end after FR stimulation (Fig. 1). Regulation of both activities is more likely to be independent for each enzyme, as the β-mannosidase activity response is highly correlated with the acquisition of VLFR by the seeds (Fig. 1c). In turn, EGP stimulation by FR is maximal when the seeds have a medium sensitivity to VLF of photons (Fig. 2a). This EGP response is accompanied by an increase in expansin gene expression after light-stimulation (Fig. 2b). However, transcript accumulation was not saturated when the seeds acquired a medium sensitivity level as was observed for EGP, suggesting that, although expansins are involved in cell wall extensibility, they are not restricting EGP from reaching its maximum response in the seeds. In accordance with our results, it was reported recently that, in Arabidopsis seeds, germination control by phytochromes is compartmentalized (Lee et al., Reference Lee, Piskurewicz, Tuecková, Carat, Chappuis, Strnad, Fankhauser and Lopez-Molina2012). This control is directed by the extra-embryonic tissues, mainly sensitive to phyB, where ABA is produced and released toward the embryo to inhibit phyA-regulated expansion in response to environmental cues. Even though we did not find evidence of a correlation between ABA levels and the VLFR-promoted seed germination (data not shown), the question remains whether any change in sensitivity to this hormone is involved in this response.
In conclusion, the WSA pretreatment notably changes the physiological state of the seeds, leading to a higher sensitivity to very low fluences of light in D. ferox seeds. These changes lead to a dormancy breakage that is likely to be occurring with differing timing in endosperm and embryo tissues, according to their response thresholds. These differences may also suggest that there is some kind of independence in the responses of EW and EGP. As a consequence, the weakening of the micropylar zone is the limiting factor for embryo expansion and radicle protusion in FR-promoted germination, being dependent on cell wall degrading enzyme activity, in particular β-mannosidase. It would be interesting to investigate how dormancy levels and the phyA-signalling pathway modulate these processes and what elements are involved in such regulation.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0960258513000263
Acknowledgements
We thank Dr Rodolfo Sánchez for helpful discussion of the data.
Financial support
This work was funded by Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad de Buenos Aires.
Conflicts of interest
None.