Introduction
Seeds of most Prunus species are dormant at maturity and require prolonged cold or warm-plus-cold stratification to break dormancy (Crocker and Barton, Reference Crocker and Barton1931; Grisez, Reference Grisez and Schopmeyer1974; Chang and Werner, Reference Chang and Werner1984; Suszka et al., Reference Suszka, Muller and Bonnet-Masimbert1996). Removal of the seed covering layers shortens stratification time for dormancy break and improves germination of non-stratified seeds in peach (P. persica) (Tukey and Barrett, Reference Tukey and Barrett1936; Mehanna et al., Reference Mehanna, Martin and Nishijima1985), sweet cherry (P. avium) (Zielinski, Reference Zielinski1958) and wild plum (P. americana) (Giersbach and Crocker, Reference Giersbach and Crocker1932). An early study found that seed dormancy in Lovel peach was regulated by dormin, located primarily in the seed coat, and that the substance disappeared or was inactivated during cold stratification (Lipe and Crane, Reference Lipe and Crane1966). (Diaz and Martin Reference Diaz and Martin1972) identified dormin as abscisic acid (ABA) in Lovell peach seeds, and found that the amount of ABA in the seed coat was more than twice that in the embryo. Together, these results imply that dormancy in peach seeds is caused by mechanical and chemical inhibition associated with the seed covering layers.
The plant hormones abscisic acid (ABA) and gibberellins (GAs) are involved in seed dormancy and germination. Whereas ABA in embryo and/or seed coat appears to be important in the regulation of dormancy, GAs appear to promote germination once dormancy is lost (Bewley, Reference Bewley1997; Koornneef et al., Reference Koornneef, Bentsink and Hilhorst2002). ABA was implicated in regulation of embryo and testa-imposed dormancy in seeds of Acer (Pinfield et al., Reference Pinfield, Stutchbury and Bazaid1987, Reference Pinfield, Stutchbury, Bazaid and Gwarazimba1989). In seeds of Taxus mairei, reduction in ABA content after warm stratification was insufficient to break dormancy, and it was suggested that GAs might ultimately be involved in germination of the intact seed, because GA4+7 could replace the cold stratification requirement for germination (Chien et al., Reference Chien, Kuo-Huang and Lin1998). Cold stratification induced an increase of GA levels in embryos of European hazel (Corylus avellana), suggesting that GAs synthesized during cold treatment were responsible for dormancy break (Williams et al., Reference Williams, Bradbeer, Gaskin and MacMillan1974). Recently, Yamauchi et al. (Reference Yamauchi, Ogawa, Kuwahara, Hanada, Kamiya and Yamaguchi2004) demonstrated that a gene involved in GA biosynthesis in seeds of Arabidopsis thaliana was activated by cold stratification at 4°C. Increase in tissue sensitivity to GAs during cold stratification is another factor that may be involved in controlling seed germination (Derkx and Karssen, Reference Derkx and Karssen1993; Koornneef et al., Reference Koornneef, Bentsink and Hilhorst2002).
Prunus campanulata Maxim. (Rosaceae) is a common forest tree in the mountains of Taiwan, growing to 10 m in height. It produces red, campanulate flowers from January to March and dark red fruits from April to May, making it a valuable ornamental plant. This species is distributed in East Asia, from Japan (including Okinawa) and Taiwan to southern China. (Chien et al. Reference Chien, Chen and Yang2002b) showed that a combination of warm-plus-cold stratification broke dormancy and promoted full germination of this species. The purpose of the present study was to further characterize seed dormancy in P. campanulata. Thus, we determined the mechanical role of the endocarp and seed coat, and monitored changes in content of ABA and GAs in the endocarp, seed coat and embryo during dormancy break and germination in seeds of this species. Contents of these growth regulators were also measured in the endocarp, seed coat, embryo and pulp (exocarp and mesocarp) of immature and of freshly mature fruits. Since concentrations of ABA and GA are the result of ongoing synthesis, we used fluridone and paclobutrazol, inhibitors of ABA- and GA-synthesis, respectively, to examine the role of these hormones in the breaking and maintenance of dormancy.
Materials and methods
Plant materials
Fruits of P. campanulata were collected in the field when they were mature, i.e. dark red or dark purple in colour. More than 5000 mature fruits were collected from five trees at Alishan (23°32′N, 120°47′E), Chiayi County, central Taiwan at 2000 m elevation in early May 2002. Small amounts of green immature fruits were collected 3 weeks before maturity. Seeds were extracted by removing the pulp (exocarp+mesocarp) in water, and the clean sunken seeds (with endocarp) were used for subsequent treatments. Seeds of P. campanulata are 8–9 mm in length, and consist of a large embryo and a thin seed coat adhered to a small amount of endosperm. The seed is surrounded by a hard endocarp (Fig. 1). Thus, the word ‘seed’ in this paper refers to the true seed plus endocarp, which is the germination unit in Prunus. Moisture content was determined for both fresh and treated seeds using three replicates of 20 seeds each, and calculated on a fresh weight basis after oven drying for 17 h at 103°C (International Seed Testing Association, 1999).

Figure 1 Longitudinal section through a seed of Prunus campanulata. A small amount of endosperm adheres to the hypocotyl and radicle.
Effect of moist warm and moist cold stratification on germination
Freshly harvested seeds were placed on moist sphagnum in sealable polyethylene bags, and warm moist stratified for 4 or 6 weeks at 30/20°C (12/12 h) with 12 h of fluorescent light (80–100 μmol m− 2 s− 1) during the higher temperature period of the regime. They were then cold moist stratified at 4°C in the dark for 4, 8 or 12 weeks. A cold moist stratification treatment without a warm treatment was also carried out on fresh seeds in moist sphagnum for 4, 8 or 12 weeks, prior to testing them for germination. A treatment consisted of three replicates of 50 seeds each. Moisture content of the sphagnum was about 400% of its dry mass.
Effect of endocarp and seed coat on germination
True seeds (without endocarp, but seed coat retained) and isolated embryos (without endocarp and seed coat) from freshly harvested seeds were placed on moist sphagnum in sealable polyethylene bags and germinated at 30/20°C (12/12 h) with 12 h of fluorescent light during the high temperature period of the regime. A treatment consisted of three replicates of 50 seeds or embryos each. Germination was recorded three times a week, and germination percentages were calculated. Seeds (true seed plus endocarp) were used as a control.
Effect of GA3 on germination of intact seeds
Freshly harvested, mature seeds were treated with concentrations of 0 (water control), 1.3, 2.6 and 5.2 mM GA3 (potassium salt) (95% purity; Sigma, St. Louis, Missouri, USA) for 16 h at room temperature prior to germination. To enhance passage of GA3 solutions through the endocarp and seed coat, seeds with the various GA3 concentrations were placed in a vacuum container at a pressure of 30 cmHg (0.4 atmospheric pressure) for 16 or 72 h. Seeds with the endocarp removed were also treated with the above-described GA3 concentrations, as well as with 0.26 mM GA3 for 16 h. Each treatment consisted of 3 replicates of 50 seeds each. These treated seeds were then mixed with moist sphagnum for germination testing.
Effect of fluridone, paclobutrazol, GA3, GA4 and ABA on germination of true seeds
Fluridone {1-methyl-3-phenyl-5-[3-(trifluoromethyl)phenyl]-4-(1H)-pyridinone} (>97% purity) was obtained from Olchemim Ltd, Czech Republic; paclobutrazol [(2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1,2,4-triazolyl)-pentan-3-ol] (97% purity) from Wako Pure Chemical Industries, Ltd, Japan; ( ± )-ABA (99% purity) and GA4 ( ≥ 90% purity) from Sigma. Due to the inhibitory effect of the hard endocarp on germination, mature true seeds (without endocarp, but seed coat retained) were soaked for 24 h in double-distilled water (ddH2O), two concentrations (10 and 50 μM) of ABA, GA3, GA4, fluridone or paclobutrazol, and in combinations of these compounds. The solution-treated true seeds were then mixed with moist sphagnum and germinated at 30/20°C (12/12 h) with 12 h of fluorescent light during the higher temperature period of the regime. A treatment consisted of 3 replicates of 25 seeds each. Germination was recorded three times a week, and germination percentages were calculated. The seed lot used in this experiment was harvested in April 2006.
Germination tests
Unless otherwise stated, germination tests were carried out in a growth chamber for up to 12 weeks at an alternating temperature of 30/20°C (12/12 h) with 12 h of fluorescent light during the high temperature period of the regime, i.e. the same conditions as those used for warm stratification. Sphagnum served as the germination medium. Seeds were considered to have germinated when the radicle was at least 5 mm long. Germination was recorded weekly, and results were expressed as percent germination and as mean germination time (MGT) in days (Naylor, Reference Naylor1981). MGT = (Σn it i)/N, where n i is the number of seeds germinated in t i days from the beginning of the test, and N is the total number of germinated seeds at the end of the test. MGT is a measure of the rate of germination and of the sharpness of the germination peak.
Growth regulator analyses
The endocarp, seed coat (with adhering endosperm), embryo (cotyledons+axis) and pulp (exocarp+mesocarp) were separated individually from immature fruits, collected 3 weeks before maturity (green pulp), and from freshly mature fruits (dark red or dark purple). Further, the endocarp, seed coat and embryo were separated from seeds (endocarp+true seed) warm moist stratified only at 30/20°C for 6 weeks, from seeds cold moist stratified only at 4°C for 12 weeks, from seeds warm moist stratified at 30/20°C for 6 weeks and then cold moist stratified at 4°C for 8 weeks (non-dormant seeds) and from germinated seeds. In all cases, parts were separated from 50 fruits or seeds, lyophilized and weighed. Then, two replicates of each fruit/seed part were stored at − 80°C until they could be analysed for ABA and GAs. Endogenous ABA, GA1, GA3, GA4, GA7 and GA20 were determined by gas chromatography–mass spectrometry–selected ion monitoring (GC–MS–SIM).
Embryos, seed coats and pulp were ground separately in liquid N2 using a mortar and pestle, and the endocarps were finely pulverized using an ultracentrifugal mill (Retsch, Germany). Internal standards were added to each sample after grinding as follows: 100 ng of [2H6]ABA and 50 ng of [17,17-2H2]GAs (GA1, GA3, GA4, GA7 and GA20). Tissue extraction was carried out overnight at 4°C with 15 ml of 80% (v/v) methanol (MeOH), containing 0.4 mg of butylated hydroxytoluene (BHT) and 2 mg of ascorbate, and the residue was re-extracted in the same solvent for 2 h at 4°C. The MeOH extracts were combined and reduced to c. 1–2 ml of water residue using both prepurified N2 airflow and a SpeedVac (Savant Instruments, Massachusetts, USA).
The residue with internal standards was adjusted to pH 8.5 with 0.05 M potassium phosphate (K-Pi) buffer and passed through a polyvinylpolypyrrolidone (PVPP) column (approximately 5 g). The eluate was partitioned with ethyl acetate (EtOAc) (3 × 15 ml). The aqueous fraction was then adjusted to pH 3.0 with 0.5 M K-Pi buffer (pH 2.0) and partitioned with EtOAc (3 × 15 ml). The pooled EtOAc fractions were taken to dryness by the SpeedVac. The residue was dissolved in 0.05 M K-Pi buffer (pH 3.0) and loaded onto a preconditioned octadecylsilyl (ODS) silica column (approximately 3 g). The ODS-silica column was washed 3 times with ddH2O in 0.1% acetic acid (HOAc), and eluted with 80% aqueous MeOH containing 0.1% HOAc.
Following drying in vacuo using the SpeedVac, the sample was dissolved in 30% aqueous MeOH containing 0.1% HOAc and injected onto a Beckman System Gold high performance liquid chromatography (HPLC) system with LiChrosphere RP-18 column (250 × 4 mm internal diameter × 5 μm particle size, Merck, Germany) for further separation of ABA and GAs. Running conditions for HPLC were similar to those described by (Nakayama et al. Reference Nakayama, Koshioka, Matsui, Ohara, Mander, Leitch, Twitchin, Kraft-Klaunzer, Pharis and Yokota2001), as follows: solvent A was 100% MeOH and solvent B was 30% aqueous MeOH containing 0.1% HOAc; 0–2 min, 0% solvent A; 2–42 min, 0–100% solvent A; 42–52 min, 100% solvent A; flow rate, 0.6 ml min− 1; fractionation, at 1 min intervals. Retention times of ABA and the GAs in HPLC: 17–20 min, GA1 and GA3; 26–28 min, GA20 and ABA; 29–30 min, GA7; and 34–35 min, GA4.
GC–MS–SIM analysis
The HPLC fractions were dried using a SpeedVac and derivatized by adding ethereal diazomethane, then dried with N2. GAs and ABA were further trimethysilylated with 50 μl pyridine plus 100 μl of N, N′-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) (Macherey-Nagel, USA) at 90°C for 30 min. The derivatized samples were analysed using a HP 6890 GC and 5973 MSD with a DB-1 capillary column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness, J&W Scientific, USA). Operating conditions of GC–MS were similar to those described by (Nakayama et al. Reference Nakayama, Koshioka, Matsui, Ohara, Mander, Leitch, Twitchin, Kraft-Klaunzer, Pharis and Yokota2001). The carrier gas was helium at a flow rate of 1 ml min− 1, and the interface temperature of the ion source was 250°C. The MS source temperature was 200°C, and the electron energy was 70 eV. The split/splitless injector was used in the splitless mode at 250°C. The oven temperature was programmed to begin at 60°C for 2 min, then raised to 210°C at a rate of 30°C min− 1 and to 280°C at a rate of 2°C min− 1, where it remained for 5 min. The sample was dissolved in 10 μl of hexane, and 1 μl of it was injected. For ABA, GA1, GA3, GA4, GA7 and GA20, the m/z ratios of 190/194, 506/508, 504/506, 284/286, 222/224 and 418/420, respectively, were used for quantification.
Statistical analysis
Radicle protrusion data were transformed to percentages based on number of treated seeds. Concentrations of GAs and ABA were calculated from two replicates and expressed as the mean ± SD. Germination percentages (mean ± SE) were calculated, and means were compared by analysis of variance (ANOVA) and by a least significant difference (LSD) test at the 5% level of significance (SAS Institute, 2004).
Results
Effect of moist warm and moist cold stratification on germination
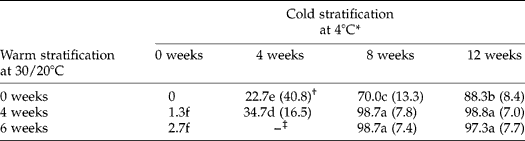
Fresh seeds from mature fruits germinated to < 3% during incubation at 30/20°C for 16 weeks. However, stratification of seeds at 30/20°C and then at 4°C, or only at 4°C, increased germination (Table 1). Thus, for example, seeds stratified at 4°C for 12 weeks germinated to 88%, while those stratified at 30/20°C for 4 or 6 weeks, followed by 4°C, for 8 weeks germinated to 99%. Mean germination time decreased (i.e. rate increased) after warm and/or cold stratification. Differences in germination of seeds in response to stratification also were linked to fruit maturity at harvest. Thus, the germination percentage of seed lots of fruits harvested before they were mature was < 50% of that of mature seeds treated at 4°C for 12 weeks (data not shown).
Table 1 Effect of cold and of warm-plus-cold stratification on percent germination and mean germination time of Prunus campanulata seeds at 30/20°C
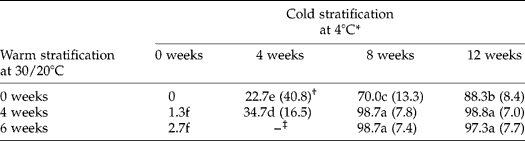
* Means (n = 3) with the same letter do not differ significantly (LSD, P = 0.05).
† Values in parentheses are mean germination time (MGT) in days.
‡ –, Not tested.
Effect of endocarp and seed coat on germination
Seeds from freshly harvested fruits were dormant and, thus, failed to germinate during incubation for 21 d or longer (Fig. 2). Removal of the endocarp, however, resulted in a gradual increase in germination percentage; 25% of the seeds germinated in 21 d. Isolated embryos from fresh seeds germinated completely within 14 d. Seeds with endocarp only removed, and treated with 0.26 mM GA3, germinated to 90% after 21 d (Fig. 2).

Figure 2 Germination percentages of Prunus campanulata seeds with both endocarp and seed coat removed (open circles), endocarp only removed (closed circles), endocarp removed and then treated with 0.26 mM GA3 (closed triangles) and intact seeds (closed squares). Each point represents the mean of 3 replicates of 50 seeds. Vertical bars indicate SE. Points with a different letter are significantly different at that incubation time (LSD, P = 0.05).
Effect of GA3 on germination of intact seeds
Germination of freshly mature seeds increased only slightly (to 7%) in 5.2 mM GA3 (Table 2). Soaking seeds in a GA3 solution in a vacuum chamber at 30 cmHg increased germination somewhat, e.g. to 18% and 31%, after 16 and 72 h of vacuum treatment with 5.2 mM GA3. However, >60% of the remaining seeds remained ungerminated and MGT was high.
Table 2 Effect of GA3 treatment on percent germination of non-stratified Prunus campanulata seeds

* Fresh intact seeds were soaked with GA3 and then incubated at 30/20°C for germination.
† Means (n = 3) with the same letter do not differ significantly (LSD, P = 0.05).
‡ Values in parentheses represent the mean germination time (MGT) in days.
Effect of fluridone, paclobutrazol, GA3, GA4 and ABA on germination of true seeds
Intact seeds did not germinate, and true seeds (without endocarp, but with seed coat retained) gradually germinated to 21% in 27 d (Table 3). However, fluridone stimulated germination of true seeds. Thus, seeds treated with 10 and 50 μM fluridone germinated to 45 and 91%, respectively, in 13 d and to 73 and 95%, respectively, in 27 d. Addition of 10 μM ABA or 10 μM paclobutrazol prevented seed germination completely. A combined fluridone and ABA treatment increased seed germination, and the effectiveness was similar to that of fluridone alone. Paclobutrazol plus GA3 and paclobutrazol alone maintained seed dormancy. However, the inhibitory effect of 10 μM paclobutrazol was partially reversed by 10 and 50 μM GA4, to 23 and 39% germination, respectively, in 27 d (Table 3). When exogenous fluridone and paclobutrazol were applied together to Prunus true seeds, no germination occurred.
Table 3 Effect of ABA, GA3, GA4, fluridone and paclobutrazol on germination percentages (means±SE) of Prunus campanulata seeds after 6, 13, 20 and 27 d at 30/20°C

*The endocarp was removed from fresh seed but the seed coat was retained. Means (n = 3) with the same letter in a column do not differ significantly (LSD, P = 0.05).
Abscisic acid content of various tissues in dormant and moist stratified seeds
ABA contents of P. campanulata differed among the embryo, seed coat (including attached endosperm), endocarp and pulp (only from fresh fruit) (Fig. 3). ABA content was 6660 pg per endocarp, 2850 pg per seed coat and 1520 pg per embryo in fresh seeds. The combined ABA contents of seed coat and endocarp was up to 6.2-fold of that in the embryo (Fig. 3). Total ABA content of warm and warm-plus-cold moist stratified seeds was approximately 6- to 12-fold lower than that of fresh seeds. Cold stratification for 12 weeks also reduced ABA to 343 and 506 pg per seed in the embryo and seed coat, respectively, but ABA in the endocarp remained high (4897 pg per seed), compared to that in fresh seeds. ABA content was 665 pg per endocarp, 183 pg per seed coat and 416 pg per embryo in germinating seeds. In seeds harvested before maturity, ABA was 25,631 pg per endocarp, 7824 pg per seed coat and 6514 pg per embryo, and total ABA level in the embryo and coverings (seed coat and endocarp) of fruits harvested 3 weeks before maturity was approximately 3.6 times higher than the level in those layers of freshly mature fruits. ABA in the pulp was 35,760 pg per seed pulp in mature fruits and 41,680 pg per pulp of fruits at 3 weeks before maturity.

Figure 3 Abscisic acid content of the various seed parts from fresh or from moist stratified Prunus campanulata seeds. Results are presented as means ± SD from 2 replicates of 50 seeds each.
Gibberellin contents of various tissues in dormant and moist stratified seeds
The biologically active GA1, GA3, GA4, GA7 and the precursor GA20 were detected in the embryo, seed coat (including attached endosperm) and endocarp of fresh and stratified seeds (Fig. 4). There were marked differences in GA1 and GA4 contents between dormant fresh seeds and moist stratified seeds. The GA1 content of embryos of fresh seeds was relatively high. A high content of GA4, 173 pg per embryo, was measured in warm-plus-cold stratified seeds and 148 pg and 156 pg per embryo in cold stratified and germinating seeds, respectively. Total amounts of (active) GAs (GA1+GA3+GA4+GA7) in pg per embryo was 289 in fresh embryos, 140 in warm stratified embryos, 264 in warm-plus-cold stratified embryos, 280 in cold stratified embryos and 293 in germinating embryos. There were high GA4 amounts in the embryo (229 pg) and seed coat (504 pg) and of GA1 in the endocarp (360 pg) of seeds harvested 3 weeks before maturity.

Figure 4 Gibberellic acid (GA) contents of the various seed parts from fresh and moist stratified Prunus campanulata seeds. Results are presented as means ± SD from 2 replicates of 50 seeds each.
Discussion
Freshly harvested seeds of P. campanulata germinated to low percentages. Warm followed by cold moist stratification completely released dormancy and promoted germination. Cold moist stratification alone also stimulated a high percentage of the seeds to germinate (Table 1). Moist cold stratification has been widely used for breaking dormancy and promoting the maximum percentage and rate of germination (Schopmeyer, Reference Schopmeyer1974; International Seed Testing Association, 1999). The beneficial effects of cold stratification on tree seed germination have been reported by (Wang Reference Wang1987) and Wang and Berjak (Reference Wang and Berjak2000), among others. Since the embryo of P. campanulata is fully developed at seed maturity (Fig. 1), the seed coat (true seed coat+endocarp) is water permeable and cold stratification breaks dormancy, Prunus seeds are classified as physiologically dormant (Nikolaeva, Reference Nikolaeva and Khan1977; Baskin and Baskin, Reference Baskin and Baskin2004).
Exogenous application of GA3 or of GA4+7 has been reported to be effective in breaking dormancy and in substituting for a cold stratification requirement in many seeds (Powell, Reference Powell and Davies1987; Nicolás et al., Reference Nicolás, Nicolás and Rodríguez1996; Chien et al., Reference Chien, Kuo-Huang and Lin1998, Reference Chien, Chen and Chang2002a; Hidayati et al., Reference Hidayati, Baskin and Baskin2000; Chen et al., Reference Chen, Li, Han, Wang and Chien2005). Results of our study show that the longer the GA3 treatment, the higher the seed germination percentage. However, GA3 was not very effective in enhancing germination, even when vacuum infiltration was used to facilitate hormone uptake. It is more likely that the physical constraint imposed by the thick endocarp (1.03 mm thickness) was responsible for blocking entrance of GAs into the seed. Furthermore, the high concentration of ABA in the endocarp could have inhibited radicle protrusion.
ABA is involved in the maintenance of primary dormancy in tree seeds. Its concentration declines in seeds or in embryo tissues during cold or warm stratification (Pinfield et al., Reference Pinfield, Stutchbury, Bazaid and Gwarazimba1989; Chien et al., Reference Chien, Kuo-Huang and Lin1998, Reference Chien, Kuo-Huang, Shen, Zhang, Chen, Yang and Pharis2004; Chen et al., Reference Chen, Li, Han, Wang and Chien2005). In Fagus sylvatica, genetic analysis has identified the crucial role of ABA in the regulation of dormancy and of GAs in seed germination (Lorenzo et al., Reference Lorenzo, Nicolás, Nicolás and Rodríguez2002; González-García et al., Reference González-García, Rodríguez, Nicolás, Rodríguez, Nicolás and Lorenzo2003; Mortensen et al., Reference Mortensen, Rodríguez, Nicolás, Eriksen and Nicolás2004). When ABA concentration is high in both endocarp and seed coat of freshly mature seeds of P. campanulata (Fig. 3), seed dormancy is at its greatest depth. Removal of both endocarp and seed coat resulted in germination of the embryo (Fig. 2). Furthermore, warm-plus-cold stratification decreased the ABA concentration of the endocarp, seed coat and embryo, and simultaneously released seed dormancy. A low ABA content was detected in seeds that were warm stratified only, but the seeds failed to germinate. The most likely explanation for this phenomenon is that the concentration of GAs in warm stratified embryos was low compared to the GA concentrations of cold stratified and of germinating seeds (Fig. 4), and the low levels of GAs did not promote germination. On the other hand, GA4 in warm-plus-cold stratified seeds increased significantly in the embryo, resulting in 99% germination. In large-scale expression studies of GA-responsive genes during Arabidopsis seed germination, an increase in the synthesis of active GA4 was the principal contributor to increased GA levels after imbibition of seeds at 4°C (Ogawa et al., Reference Ogawa, Hanada, Yamauchi, Kuwahara, Kamiya and Yamaguchi2003).
Studies on woody plants indicated that the GA content of seeds increases during cold stratification. For example, cold stratification induced an increase in GA3 and in GA7 in peach (P. persica) seeds (Mathur et al., Reference Mathur, Couvillon, Vines and Hendershott1971), in GA1 in Corylus avellana embryos (Williams et al., Reference Williams, Bradbeer, Gaskin and MacMillan1974), in GA4+7 in apple seeds (Halińska and Lewak, Reference Halińska and Lewak1987) and in GA1, GA3 and GA4 in P. buergeriana seeds (Chen et al., Reference Chen, Li, Han, Wang and Chien2005). In the present study, GA4 contents of embryos from cold stratified seeds were up to three times that of embryos from fresh or warm stratified seeds, and this coincided with a high germination percentage of cold stratified seeds. The ABA content of the endocarp of 12-week cold stratified seeds was as high as that of freshly mature seeds (but it was low in the seed coat and embryo); however, 88% of the seeds germinated after cold stratification. The high content of endocarp ABA may be caused by a reduced rate of ABA catabolism or reduced rate of leakage from the endocarp at the cold temperature. Indeed, we found 2.86, 2.54 and 2.62 ng of ABA per g dry weight of sphagnum in the sphagnum medium of fresh, cold and warm-plus-cold stratified seeds, respectively (LSD, P>0.05). We speculate that an antagonistic interaction between ABA and GAs, especially the increased GA4 content, allowed the seed to germinate.
ABA concentrations in pulp, endocarp, seed coat and embryo of fruits harvested 3 weeks before maturity were high, but these levels declined to some extent in mature seeds (Fig. 3). ABA may play a role in preventing seeds from germinating during the later stages of seed maturation, although contents of GA4 in the embryo and seed coat, and GA1 and GA3 in the endocarp, were high in seeds harvested 3 weeks before maturity. Lower levels of GAs in freshly mature seeds and the presence of ABA help to maintain dormancy (Berry and Bewley, Reference Berry and Bewley1992; Bewley, Reference Bewley1997).
Fluridone decreases concentrations of endogenous ABA in seeds and can increase seed germination (Xu and Bewley, Reference Xu and Bewley1995; Bianco et al., Reference Bianco, Garello and Le Page-Degivry1997; Le Page-Degivry et al., Reference Le Page-Degivry, Garello and Barthe1997). Fluridone very effectively enhanced germination of true seeds (without endocarp) of P. campanulata (Table 3). Surprisingly, ABA did not counteract the effect of the fluridone, and dormancy was not induced, even though ABA alone inhibited germination strongly. ABA and GAs share the initial steps of the terpenoid pathway for their biosynthesis in plants (Crozier et al., Reference Crozier, Kamiya, Bishop, Yokota, Buchanan, Gruissem and Jones2000). Thus, we speculate that the inhibition of ABA biosynthesis by fluridone might not only have decreased ABA content, but also increased GA content, thereby resulting in germination; exogenous ABA plus GA3 gave a similar result (see Table 3).
Treatment of fresh Prunus seeds with paclobutrazol had little effect on germination, and the small amount of inhibition was reversed by GA4, but not by GA3. Thus, we suggest that the effect of paclobutrazol resulted from the inhibition of GA biosynthesis, and GA4 might be the major hormone to initiate seed germination. Furthermore, combinations of fluridone and paclobutrazol maintained seed dormancy, which suggests strongly that GAs are required for seed germination when ABA biosynthesis is blocked and ABA content declines.
Our study provides strong evidence that the endocarp and seed coat play a role in seed dormancy in P. campanulata. Further, they suggest that both endogenous ABA and GAs may be involved in dormancy-break and germination. The seed-covering layers contain high amounts of ABA, which is decreased by warm-plus-cold stratification. Maximum germination percentages occurred when the seeds were warm stratified and then cold stratified. GA content was highest after dormancy was broken. We suggest that: (1) the decrease in ABA content in the covering layers signifies the removal of the chemical barrier to radicle growth; and (2) the increase in GA4 concentration in the embryo after cold stratification increases the growth potential of embryo to the point where it can overcome the mechanical resistance of the seed coat and endocarp. It is not known if the mechanical resistance of the seed covering layers weakens during dormancy break, but endocarps of warm-plus-cold stratified seeds were much easier to cut and separate than those of non-stratified seeds. Decreased ABA content in the seed coat and endocarp, and increased GA in embryo, might be widespread in Prunus species. Several early studies have shown that these seed covers play an important role in dormancy in this genus, since embryos isolated from the seeds germinate readily (Giersbach and Crocker, Reference Giersbach and Crocker1932; Tukey and Barrett, Reference Tukey and Barrett1936; Zielinski, Reference Zielinski1958; Mehanna et al., Reference Mehanna, Martin and Nishijima1985).
Acknowledgements
The authors express grateful thanks to Professors Jerry M. Baskin and Carol C. Baskin, Department of Biology, University of Kentucky, and Ben S.P. Wang, Petawawa Research Forest, Canadian Forest Service, Canada, for comments on the manuscript. We thank Professor Richard P. Pharis, Canada, for the stable isotope-labelled abscisic acid and Professor Lewis N. Mander, Australia, for the stable isotope-labelled gibberellins. This research was supported financially by the National Science Council, Taiwan (NSC 93-2313-B-054-010).