Introduction
The oaks (Quercus spp.) are described as the most ecologically and economically important tree species in North America (Bonner and Karrfalt, Reference Bonner and Vozzo2008; Cavender-Bares, Reference Cavender-Bares2016). Approximately 58 Quercus species are native to the United States and 50 of these grow in the eastern United States (Stein et al., Reference Stein, Binion and Acciavatti2003). The Quercus species of the eastern United States are represented by two groups commonly referred to as the red oaks and the white oaks. Within the Quercus subgenus, the red oaks are placed in section Lobatae, and the white oaks are in section Quercus. Phylogenetically, these sections represent two major American oak clades that are of northern temperate origin and diverged ca. mid-Eocene (Hipp et al., Reference Hipp, Manos, González-Rodríguez, Hahn, Kaproth, McVay, Avalos and Cavender-Bares2018). Thereafter, sympatric parallel diversification of Lobatae and Quercus occurred as species moved southward during the Oligocene to latter Miocene, and ecological diversification proceeded within clades (Hipp et al., Reference Hipp, Manos, González-Rodríguez, Hahn, Kaproth, McVay, Avalos and Cavender-Bares2018). Indeed, in the eastern United States, representatives of both sections are common canopy components in mesophytic to xeric upland forests (Abrams, Reference Allen and Farmer1992), and in bottomland forests that often are subject to periodic inundation (Hodges, Reference Hodges and Barrett1994; King and Keeland, Reference King and Keeland1999; Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001).
To date, we have only a broad understanding of acorn germination dynamics for a relatively few eastern United States Quercus species, and the majority of past studies have focused on those of section Lobatae. For example, Bonner and Vozzo (Reference Bonner and Karrfalt1987) reported that the length of cold stratification required to ‘enhance acorn germination’ varied among nine Quercus species of Lobatae. Peterson (Reference McGee1983) found that increased lengths of cold stratification reduced the time to maximum germination in Quercus nigra L. acorns, and Hopper et al. (Reference Hopper, Smith and Parrish1985) quantified this same trend in acorns of Quercus rubra L. We can infer from these studies that physiological dormancy (PD) is present in acorns of many species of section Lobatae; however, it is unclear whether different levels of PD are present.
Seeds may possess non-deep, intermediate or deep PD (Baskin and Baskin Reference Baskin and Baskin2004, Reference Baskin and Baskin2014). The level of PD is identified by seed dormancy break and germination response to the length of stratification (warm and/or cold) and to treatment with gibberellic acid (GA3). In other words, seeds with deep PD require longer periods of stratification (>12 weeks) for dormancy break than those with non-deep PD (≤12 weeks). Those with intermediate PD require 8–12 weeks of stratification. Additionally, GA3 promotes germination in seeds with non-deep PD, may or may not promote germination in those with intermediate PD and would not be effective in seeds with deep PD (Baskin and Baskin, Reference Baskin and Baskin2014).
Quercus pagoda grows in bottomland hardwood forests of the eastern United States and often in association with other Quercus species such as Q. laurifolia Michx., Q. nigra and Quercus phellos L. (Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001). Acorns of Q. pagoda Raf. (Section Lobatae) require at least 12 weeks of cold stratification for dormancy break and possess non-deep PD (Hawkins, Reference Hawkins2019a). However, past research (e.g. Peterson, Reference McGee1983; Hopper et al., Reference Hopper, Smith and Parrish1985; Bonner and Vozzo, Reference Bonner and Karrfalt1987) suggests that different levels of PD may be present among species of section Lobatae, and thus, acorn dormancy break requirements of Q. pagoda may differ from that of other sympatric Quercus species. The primary objective of this research was to identify dormancy break and germination requirements in acorns of Q. nigra and Q. phellos. This component of the research will determine the level of PD in acorns of these two species and allow for comparison of dormancy break and germination requirements among three bottomland Quercus species. This will add to our limited knowledge of the ecology of acorn germination dynamics within a bottomland forest habitat and begin to elucidate phylogenetic associations of dormancy types or level of PD among species of section Lobatae.
The second objective of this study was to determine if varying acorn treatments, such as length of cold stratification and/or post-stratification temperature in a controlled environment, are required for interspecific germination synchrony in acorns of Q. nigra and Q. phellos. In the last 70 years, a significant decline in Quercus spp. abundance in the eastern United States forests has resulted from a culmination of timbering practices, e.g. high grading (McGee, Reference Peterson1972), fungal pathogens (Bruhn et al., Reference Bruhn, Wetteroff, Mihail, Kabrick and Pickens2008; Kelley et al., Reference Kelley, Fierke and Stephen2009), insect damage (Stephen et al., Reference Stephen, Salisbury and Oliveria2001; Starkey et al., Reference Starkey, Oliveria, Mangini, Mielke and Spetich2004; Haavik et al., Reference Haavik, Jones, Galligan, Guldin and Stephen2012) and changes in fire disturbance patterns (Guyette et al., Reference Guyette, Muzika and Dey2002; Arthur et al., Reference Arthur, Alexander, Dey, Schweitzer and Loftis2012). In turn, much attention has focused on mitigating Quercus spp. decline by employing artificial regeneration practices in Quercus depleted forests and on former agricultural sites (Stanturf et al., Reference Stanturf, Gardiner, Hamel, Devall, Leininger and Warren2000; Dey et al., Reference Dey, Jacobs, McNabb, Miller, Baldwin and Foster2008). The success of these regeneration efforts may be enhanced by synchronizing acorn germination to produce even-aged Quercus seedlings (Dey et al., Reference Dey, Jacobs, McNabb, Miller, Baldwin and Foster2008).
Materials and methods
The species
Q. nigra grows along the Coastal Plain from southern New Jersey and Delaware; south to Florida; west to eastern Texas; north to Missouri and east to Virginia (Vozzo, Reference Vozzo, Burns and Honkala1990). This species grows on a variety of sites ranging from poorly drained bottomlands to ridges and high flats (Vozzo, Reference Vozzo, Burns and Honkala1990; Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001). Mature trees are moderately flood-tolerant (Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001), and acorns have been shown to retain viability up to 30 days during winter or spring submergence (Guo et al., Reference Guo, Shelton and Lockhart1998). However, the effects of submergence on Q. nigra acorn viability have not been tested beyond 30 days.
Q. phellos ranges from New York; west to Missouri; south to Texas, east to Florida and north to Delaware (Schlaegel, Reference Schlaegel, Burns and Honkala1990). The species grows on a variety of soil types in bottomland forests, but most often is found on high flats and loamy ridges of recent alluvium (Schlaegel, Reference Schlaegel, Burns and Honkala1990; Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001). Mature Q. phellos trees are moderately flood-tolerant (Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001), and acorns of this species have been reported to retain viability for up to 84 days of winter submergence (Hawkins, Reference Hawkins2019b).
Sampling and conditions
In November 2012, mature acorns were harvested directly from randomly selected Q. phellos trees growing in Starkville, Mississippi (33.4504°N, 88.8184°W) and from Q. nigra trees growing in Amory, Mississippi (33.9843°N, 88.4881°W). Acorns were immediately taken to the laboratory and remained in covered 19-L buckets at ambient temperature (~24°C) for 16 h before receiving experimental treatments.
Five incubators were set at 12 h/12 h diurnal alternating temperature regimes that approximate seasonal temperatures within the range of the two Quercus species. One of five incubators was set at 5/1°C (winter) and used for cold stratification. Temperature regimes for the remaining four incubators were 15/6°C (early spring/late fall), 20/10°C (late spring/early fall), 25/15°C (early/late summer) and 30/20°C (summer). All incubators were set to provide 12 h of light (50 μmol m−2 s−1) during the high-temperature period and 12 h of uninterrupted darkness during the low-temperature period.
Effects of cold stratification on acorn dormancy break
Acorns were placed on sand moistened with distilled water in 16 cm × 16 cm × 5 cm clear plastic dishes. Plastic lids were placed on the dishes to retard moisture loss. Three replicates of 50 acorns per dish for each species were used in each treatment. Dishes were placed in a completely randomized design in each of the treatments. Acorns received 0 (control), 6, 12 or 18 weeks of cold stratification at 5/1°C. Acorns used as controls (0-week cold stratification) were placed directly in the test temperatures (15/6, 20/10, 25/15 and 30/20°C). Following each cold stratification treatment, acorns received an incubation time of 12 weeks in each of the test temperature regimes. Germination was recorded at 2-week intervals. Emergence of the radicle (at least 1 mm) was the criterion for germination. At the conclusion of the incubation period, acorns that did not germinate were assessed for viability. Acorns containing firm, white embryos were scored as viable, and those with soft, brown to grey embryos were scored as non-viable.
Effects of gibberellic acid (GA3) on acorn dormancy break
To determine whether gibberellic acid (GA3) substitutes for cold stratification, three replicates of 50 acorns for each species were placed in plastic containers (described in the cold stratification experiment) containing sand moistened with either distilled water (control), or a solution of 10, 100 or 1000 mg l−1 GA3. Acorns were incubated at 25/15°C for 12 weeks. This incubation temperature was used because it is too high to be effective for cold stratification (Stokes, Reference Stokes and Ruhland1965). Germination was recorded at 2-week intervals. At the conclusion of the incubation period, acorns that did not germinate were assessed for viability as described previously in the effects of cold stratification experiment.
Statistical analyses
Means and standard errors for germination proportions were calculated based on the number of viable acorns. Non-viable acorns in each replicate were ≤6%. For each Quercus species, a factorial repeated measures analysis of variance (ANOVA), based on the binomial distribution, was used to test for fixed effects and interaction of (1) length of cold stratification, length of incubation and incubation temperature in the germination experiment and (2) solution concentration and length of incubation in the GA3 experiment. Multiple comparison was used to compare germination proportions among incubation temperatures at a given incubation time. Tukey HSD was used for multiple comparison to control Type I error rates. The SAS procedure GLIMMIX was used to carry out all analyses (SAS Institute Inc., 2007). Following analyses, all proportions were converted to percentages for presentation.
Results
For purposes of clarity later in the Discussion section, results focus on germination response at the 4-weeks incubation time for identification of acorn dormancy break requirements and dormancy type (sensu Baskin and Baskin, Reference Baskin and Baskin2014), and at the 12-weeks incubation time to further assess germination responses to main effects and/or their interactions over time.
Effects of cold stratification on acorn dormancy break
Germination in Q. nigra acorns was affected by all variables (length of stratification, incubation temperature and incubation time) and their interactions (Table 1). In the control (0-week cold stratification), mean cumulative germination percentages at 4 weeks of incubation were ≤7 ± 3%, and ranged from 1 ± 1% (15/6°C) to 58 ± 9% (30/20°C) at 12 weeks of incubation (Fig. 1A). With 6 weeks of cold stratification, followed by 4 weeks of incubation, mean germination was higher for acorns incubated in the 30/20°C temperature regime (48 ± 4%; d.f. = 3, F = 96.81, P < 0.0001) than those at the lower incubation temperatures (≤10 ± 1%) (Fig. 1B). Thereafter, during incubation times of 4–12 weeks, mean germination percentages for acorns receiving 6 weeks cold stratification and incubation temperatures of 25/15 and 30/20°C increased to 90 ± 3 and 100 ± 0%, respectively (Fig. 1B). During this same incubation time range, mean germination percentages for acorns incubated at 20/10 and 15/6°C increased to 45 ± 11 and 8 ± 3%, respectively (Fig. 1B).
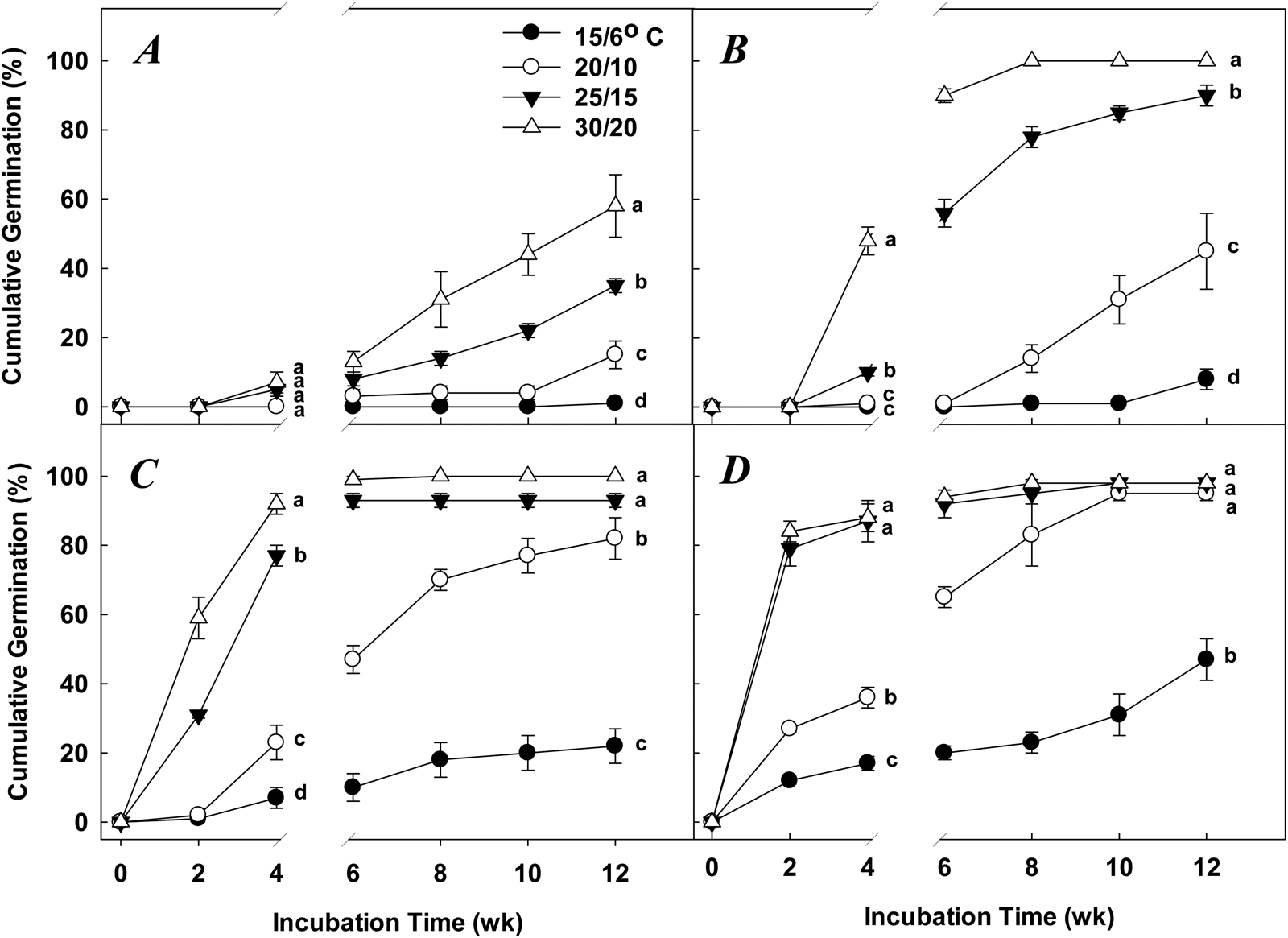
Fig. 1. Mean (±SE) cumulative germination percentages for Q. nigra acorns receiving (A) 0 week (control), (B) 6 weeks, (C) 12 weeks or (D) 18 weeks of cold stratification at 5/1°C and incubated for 12 weeks in four alternating temperature regimes. Means at 4 or 12 weeks incubation with dissimilar letters are significantly different (Tukey's HSD, P = 0.05).
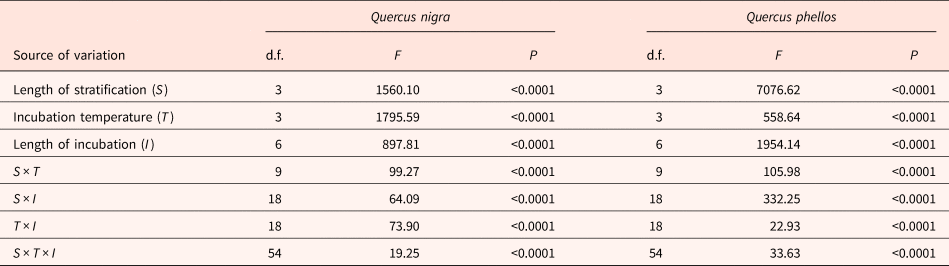
Table 1. Results of ANOVAs showing the effects and interactions of length of cold stratification (0, 6, 12 and 18 weeks), length of incubation (2, 4, 6, 8, 10 and 12 weeks) and incubation temperature (15/6, 20/10, 25/15 and 30/20°C) on germination of Q. nigra and Q. phellos acorns
Q. nigra acorns receiving 12 weeks of cold stratification and 4 weeks of incubation germinated to a higher percentage than those in the lower incubation temperatures (d.f. = 3, F = 136.71, P < 0.0001) (Fig. 1C). However, with 12 weeks of incubation, there was no difference in mean cumulative germination percentages for acorn in the 25/15 and 30/20°C temperature regimes (Fig. 1C), and these percentages were significantly higher (d.f. = 3, F = 73.57, P < 0.0001) than those for acorns in the two lower incubation temperatures (Fig. 1C). There was no difference in mean cumulative germination percentages for acorns receiving 18 weeks cold stratification and incubated for 4 weeks at 25/15 or 30/20°C (Fig. 1D). At 12 weeks incubation, there was no difference in mean cumulative germination percentages among acorns in the 20/10, 25/15, and 30/20°C incubation temperatures (Fig. 1D). Mean cumulative germination percentages for acorns incubated at 15/6°C did not exceed 47 ± 6%, regardless of incubation time (Fig. 1D).
Q. phellos acorn germination was affected by length of cold stratification, length of incubation, incubation temperature and the interaction of these variables (Table 1). In the absence of cold stratification, acorns did not germinate at an incubation time of 4 weeks, regardless of incubation temperature, and cumulative germination was ≤29 ± 1% among incubation temperatures with 12 weeks of incubation (Fig. 2A). With 6 weeks of cold stratification and 4 weeks of incubation, cumulative germination for Q. phellos acorns was highest (43 ± 1%) in the 30/20°C incubation temperature (d.f. = 3, F = 289.75, P < 0.0001) (Fig. 2B). Over the course of an additional 8 weeks of incubation, cumulative germination percentages (76 ± 2 to 87 ± 3%) at 20/10, 25/15 and 30/20°C were greater (d.f. = 3, F = 197.63, P < 0.0001) than those at 15/6°C (Fig. 2B).
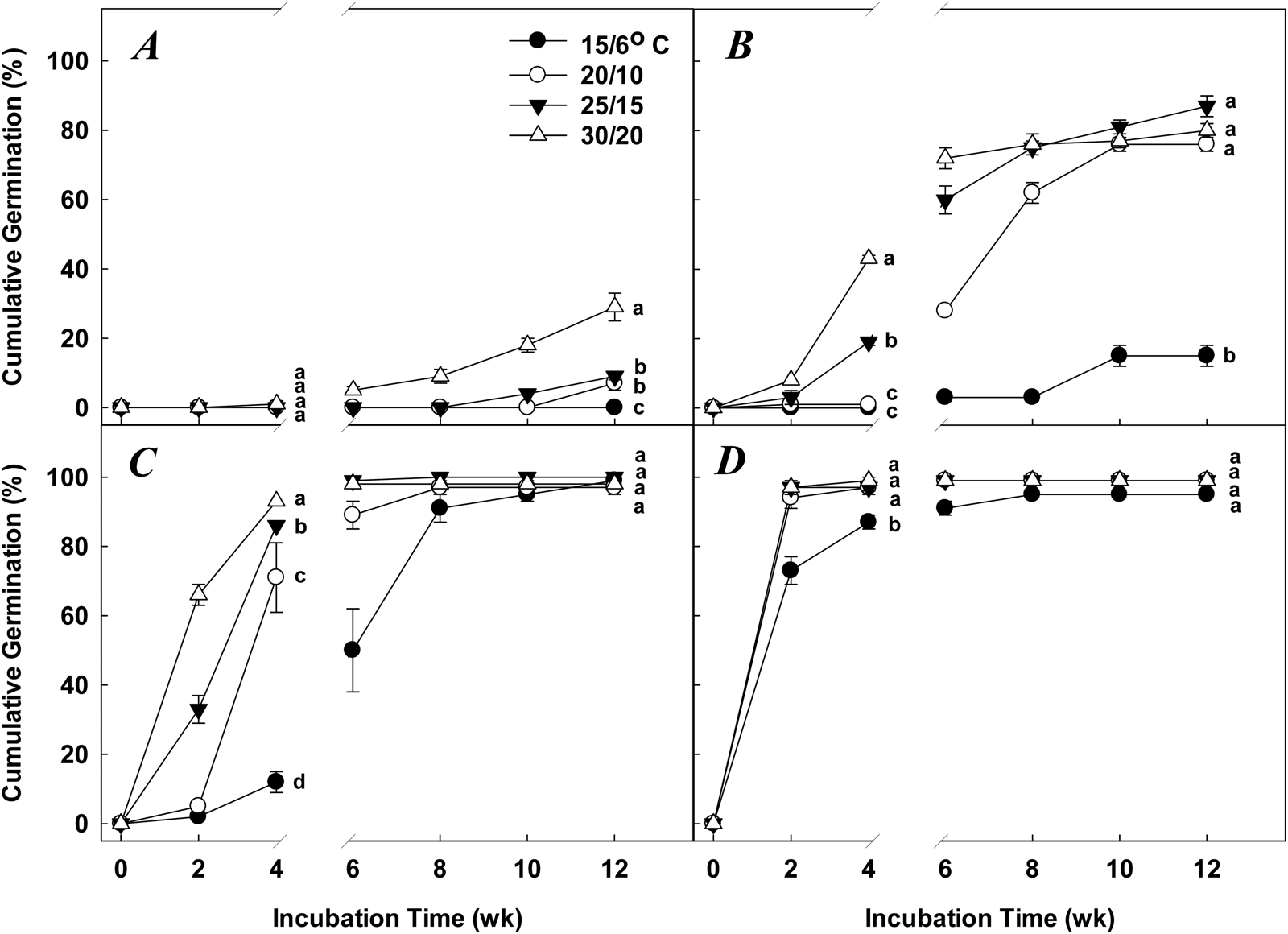
Fig. 2. Mean (±SE) cumulative germination percentages for Q. phellos acorns receiving (A) 0 week (control), (B) 6 weeks, (C) 12 weeks or (D) 18 weeks of cold stratification at 5/1°C and incubated for 12 weeks in four alternating temperature regimes. Means at 4 or 12 weeks incubation with dissimilar letters are significantly different (Tukey's HSD, P = 0.05).
Germination percentages ranged from 12 ± 3 to 93 ± 0% for Q. phellos acorns receiving 12 weeks of cold stratification and 4 weeks of incubation (Fig. 2C). However, with 12 weeks of incubation, cumulative germination percentages (≥97 ± 2%) were not significantly different among incubation temperatures (Fig. 2C). Cumulative germination percentages ≥87 ± 2% among all incubation temperatures were achieved with 18 weeks cold stratification and 4 weeks of incubation, and there was no difference in cumulative germination percentages at 12 weeks of incubation (Fig. 2D).
Effects of gibberellic acid (GA3) on acorn dormancy break
Length of incubation (d.f. = 6, F = 38.76, P < 0.0001), and the interaction GA3 concentration and length of incubation (d.f. = 18, F = 3.09, P = 0.0007), had significant effects on cumulative germination percentages in Q. nigra acorns. Germination percentages (0–5 ± 3%) among the control (distilled water) and GA3 solutions were low and not significantly different at 4 weeks of incubation (Fig. 3). However, at 6 weeks incubation, acorn cumulative germination percentages in the 100 and 1000 mg l−1 GA3 solutions were greater than those of the control and 10 mg l−1 GA3 solution. At 8 weeks incubation, acorn cumulative germination percentages were greater in the three GA3 solutions than that of the control (Fig. 3). At the 12 weeks incubation time, there was no difference in acorn cumulative germination percentages among treatments and the control (Fig. 3).

Fig. 3. Mean (±SE) cumulative germination percentages for Q. nigra (solid line) and Q. phellos (broken line) acorns receiving 0-week cold stratification and incubated for 12 weeks at 25/15°C in distilled water (control), or a solution of 10, 100 or 1000 mg l−1 of gibberellic acid (GA3). Means at 12 weeks incubation with dissimilar letters are significantly different (Tukey's HSD, P = 0.05).
The overall ANOVA showed that GA3 concentration (d.f. = 3, F = 0.01, P = 0.9993), length of incubation (d.f. = 6, F = 0.28, P = 0.9463) or the interaction of these variables (d.f. = 18, F = 0.24, P = 0.9991) had no effect on germination of Q. phellos acorns. Over the course of 12 weeks of incubation, acorn cumulative germination percentages among GA3 concentrations (and control) remained ≤9 ± 1% (Fig. 3).
Discussion
The process of seed dormancy break is characterized by changes in germination percentages over a range of post-stratification temperatures in response to a treatment such as cold or warm stratification. When dormancy break occurs, seed germination response to a specific treatment is observed within 2–4 weeks of seed placement in a range of incubation temperatures (Baskin and Baskin, Reference Baskin and Baskin2004, Reference Baskin and Baskin2014). This incubation time is used to identify seed dormancy break in response to a treatment since changes in germination percentages beyond this time may reflect the interaction of treatment and incubation time (Baskin and Baskin, Reference Baskin and Baskin2014). In light of this, dormancy type for acorns of Q. nigra and Q. phellos was determined by acorn cumulative germination percentages at 4 weeks of incubation.
With 12 or 18 weeks of cold stratification and 4 weeks of incubation, cumulative germination percentages for Q. nigra acorns were ≥77% but only in incubation temperatures of 25/15 and 30/20°C. However, with 18 weeks of cold stratification and 10 or 12 weeks of incubation, cumulative germination percentages for acorns incubated at 20/10, 25/15 and 30/20°C were not significantly different (≥95 ± 2%). This suggests that acorns of Q. nigra may require >18 weeks of cold stratification to germinate to higher percentages over a wider range of temperatures (<20/10°C) and achieve dormancy break. On the other hand, dormancy break in Q. phellos acorns occurred with 18 weeks of cold stratification. Cumulative germination percentages for acorns of this species were ≥87% at 4 weeks of incubation in all test temperature regimes. Gibberellic acid was not an effective substitute for cold stratification in either species, as germination percentages were ≤5 ± 3% for both species at the 4 weeks incubation time. Collectively, these data indicate that acorns of Q. nigra and Q. phellos possess deep PD (sensu Baskin and Baskin, Reference Baskin and Baskin2004, Reference Baskin and Baskin2014).
In the Lobatae clade, Q. nigra and Q. phellos are closely related species, appearing on separate, but adjacent branches stemming from a node with an estimated divergence in the mid-Miocene (Hipp et al., Reference Hipp, Manos, González-Rodríguez, Hahn, Kaproth, McVay, Avalos and Cavender-Bares2018). Q. nigra is a sister taxon to Q. arkansana Sarg., and Q. phellos is a sister taxon to Q. elliottii Wilbur (syn. Q. pumila Walter) (Hipp et al., Reference Hipp, Manos, González-Rodríguez, Hahn, Kaproth, McVay, Avalos and Cavender-Bares2018). Both Q. arkansana and Q. elliottii are upland forest species and, thus, occupy a different ecological niche than Q. nigra and Q. phellos. Dormancy type for Q. elliottii is not known; however, cumulative germination percentages in Q. arkansana are significantly greater for acorns receiving 90–120 days of cold stratification (Wirges and Yeiser, Reference Wirges and Yeisiker1984). This suggests that acorns of Q. arkansana may possess deep PD, and the level of PD may be the same as that of the sister taxon, Q. nigra. Summarily, deep PD is present in two closely related Quercus species of similar habitat (Q. nigra and Q. phellos), and potentially the same is closely related Quercus species of dissimilar habitats (Q. phellos and Q. arkansana).
It should be noted that Q. pagoda, a bottomland species that often grows with Q. nigra and Q. phellos, appears on a separate, less diverse branch of the Lobatae clade than Q. nigra and Q. phellos, and one that diverged from the latter two species branch ca. mid-Miocene (Hipp et al., Reference Hipp, Manos, González-Rodríguez, Hahn, Kaproth, McVay, Avalos and Cavender-Bares2018). Q. pagoda is sister taxon to Q. ilicifolia, which grows on dry, sandy barrens, or rocky hillsides. Acorns of Q. pagoda possess non-deep PD (Hawkins, Reference Hawkins2019a), and those of Q. ilicifolia possess an unknown level of PD, and possibly some level of epicotyl dormancy (Allen and Farmer, Reference Abrams1977). Although, epicotyl dormancy is not expressed in Q. pagoda acorns, the time between germination and epicotyl emergence increases with decreasing post-germination temperature, but this is unrelated to the length of cold stratification received (Hawkins, Reference Hawkins2019a). This suggests similar (PD), although not identical, dormancy types between sister taxa (Q. pagoda and Q. ilicifolia) that grow in dissimilar habitats, and different levels of PD in less closely related species (Q. pagoda vs Q. nigra and Q. phellos) that grow together in mixed stands in the eastern United States bottomland forests.
Differences in dormancy type or level of PD in congeners with respect to species relatedness or habitat are not an atypical occurrence in temperate deciduous forests (Li et al., Reference Li, Baskin and Baskin1999; Hawkins et al., Reference Hawkins, Skojac, Schiff and Leininger2010a). However, recognition of dormancy break and germination requirements in Quercus species within and among habitats is highly relevant to large-scale oak research and artificial regeneration efforts. In both instances, comparative morphometric measurements are sometimes used to assess seedling response to abiotic variables (Anderson and Pezeshki, Reference Anderson and Pezeshki2000) and/or identify traits that enhance the success of seedling establishment of outplanted nursery generated seedlings (Dey et al., Reference Dey, Jacobs, McNabb, Miller, Baldwin and Foster2008). Comparative measurements of an even-aged seedling cohort, particularly within the first months following seedling emergence, ensures that morphological and/or physiological differences among seedlings are not age-related. Intraspecifically, germination synchrony and thus an even-aged seedling cohort may be achieved by ensuring dormancy break in acorns of the Quercus species of interest, followed by post-germination temperatures appropriate for epicotyl emergence (Hawkins, Reference Hawkins2019a). However, if dormancy break requirements differ between acorns of one or more species, it may be possible to subject acorns of the Quercus species to the same length of cold stratification, but then adjust the post-stratification temperature to achieve interspecific germination synchrony. For example, in this study, 18 weeks of cold stratification was sufficient for dormancy break in acorns of Q. phellos, but not for those of Q. nigra. However, with 18 weeks of cold stratification, 84 ± 3% of Q. nigra acorns and 97 ± 2% of Q. phellos acorns germinated within 2 weeks in an incubation temperature of 30/20°C (i.e. synchronous germination).
The interaction of variables (length of cold stratification, post-stratification temperature and time) that contribute to acorn dormancy loss make it difficult to predict the influence of different levels of PD on germination synchronicity, and subsequent seedling emergence among Q. nigra and Q. phellos acorns in a bottomland habitat. At the time of dispersal, acorns begin receiving some level of stratification. Although autumn dispersal of Q. nigra and Q. phellos acorns is relatively synchronous (Cypert and Webster, Reference Cypert and Webster1948; Hawkins, pers. observation), time elapsed from dispersal to spring temperatures that satisfy germination temperature requirements will vary with year and latitude. Similarly, the length of time that acorns receive temperatures effective for cold stratification (0–10°C; Stokes, Reference Stokes and Ruhland1965) may vary annually within and among latitudes throughout the species’ ranges.
An additional abiotic influence on acorn germination dynamics of bottomland Quercus species is submergence from flooding which may vary annually in timing and duration (Hawkins et al., Reference Hawkins, Baskin and Baskin2010b). For example, 63 days of winter submergence (water temperature range, 4–13°C) was an effective substitute for cold stratification and, thus, sufficient for dormancy break in Q. pagoda acorns (Hawkins, Reference Hawkins2019b). In contrast, dormancy break was not achieved in Q. phellos acorns with 63 or 84 days of winter submergence (Hawkins, Reference Hawkins2019b). Guo et al. (Reference Guo, Shelton and Lockhart1998) reported that germination percentages for Q. nigra acorns receiving up to 30 days of winter submergence were not significantly greater than those receiving 0-day winter submergence. Additionally, submergence duration may vary within acorn cohorts due to the heterogeneous topography associated bottomland hardwood forests in the eastern United States (Gardiner, Reference Gardiner, Hodges, Fristoe and Connor2001; Gardiner et al., Reference Gardiner2004).
Conclusions
PD is the most prevalent dormancy type in seeds of woody species in the canopy and subcanopy of the eastern United States temperate deciduous forests (Baskin and Baskin, Reference Baskin and Baskin2014). Among species of the Lobatae section, acorn dormancy break and germination requirements may differ between species that possess the same level of PD, and Quercus species of the same habitat may possess different levels of PD. In this study, acorns of the closely related Q. nigra and Q. phellos possessed the same level of PD, and a different level of PD than that of a more distantly related Quercus species. However, an accurate description of biogeographical or phylogenetic associations of acorn dormancy type (or level of PD) among species of Lobatae will require ecologically relevant studies of more representatives of this section. Additionally, information gained from these studies may provide methods for refining artificial regeneration practices aimed at restoration of Quercus spp. in forests of the eastern United States.
Acknowledgements
The author thanks Rory Thornton for help with data collection, Dr. Charles Sabatia for direction in statistical analyses and Dr. Jeffrey Walck and Dr. Siti Hidayati for review of an earlier draft of this manuscript.







