Introduction
Seeds stored in natural seed banks are subject to variable drought periods, which may last for months and generate a discontinuous hydration pattern; changes in the moisture content of the soil induce changes in the latency state of the seeds of different species (Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2010). Earlier research has demonstrated that some of the seeds that withstand long drought periods after one or several hydration events are able to germinate during a hydration period according to an apparent germination ‘experience’. Seeds from Stenocereus thurberi (Engelm.) Buxb., germinated 2–3 d earlier and had a 1.4- to two-fold shorter mean germination time after a 72 or 80 h hydration period followed by different dehydration times (1, 14, 70, 120 or 181 d). Seeds from Pachycereus pecten-aboriginum (Engelm. ex S.Watson) Britton & Rose, and Ferocactus peninsulae (A.A.Weber) Britton & Rose, showed similarly reduced germination times after single or multiple hydration–dehydration (HD) cycles. These data suggest the presence of a hydration memory in these seeds, defined as the ability of the seed to maintain the physiological changes associated with hydration, through discontinuous dehydration periods (Dubrovsky, Reference Dubrovsky1996).
The agricultural industry has developed a process known as ‘seed priming’ aiming at artificially inducing uniform, optimized germination. Successful germination of ‘primed’ seeds is dependent on many factors, such as priming method, oxygen availability and temperature (McDonald, Reference McDonald, Black and Bewley2000). Drought resistance mechanisms have been observed in response to seasonal environmental changes in other species (Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011), which elicit a similar physiological response and are thus known as ‘natural’ seed priming. The hydration memory or natural priming present in desert cacti, while physiologically very similar to both agricultural and natural priming, has evolved independently in response to particularly extreme environments; therefore, studying the expression profiles that underpin it will shed light on whether natural priming of phylogenetically distant species has the same genetic background. Hydration memory-capable cactus seeds have high drought tolerance after they have been subjected to HD cycles, and their germination percentages do not vary significantly, ultimately contributing to the survival of the species (Dubrovsky, Reference Dubrovsky1998).
Wild plant species have a largely unexplored diversity of mechanisms (Flood et al., Reference Flood, Harbinson and Aarts2011) and we think that they must be thoroughly understood, since they could potentially lead to very precise crop improvement solutions. Current efforts are being made towards engineering drought resistance into commercially interesting plants that do not possess it (Umezawa et al., Reference Umezawa, Fujita, Fujita, Yamaguchi-Shinozaki and Shinozaki2006; Cattivelli et al., Reference Cattivelli, Rizza, Badeck, Mazzucotelli, Mastrangelo, Francia, Marè, Tondelli and Stanca2008; Ashraf, Reference Ashraf2010). In this sense, we believe that the mechanism underlying the hydration memory process may have long-term biotechnological applications. However, after exhaustive bibliographical research, we did not find any reports concerning the molecular mechanisms involved in naturally occurring hydration memory. Conversely, seed priming of model organisms and crops has been thoroughly analysed both at the transcriptomic (Soeda et al., Reference Soeda, Konings, Vorst, van Houwelingen, Stoopen, Maliepaard, Kodde, Bino, Groot and van der Geest2005; Batlla and Benech-Arnold, Reference Batlla and Benech-Arnold2010) and proteomic (Dubrovsky, Reference Dubrovsky1996; Gallardo et al., Reference Gallardo, Job, Groot, Puype, Demol, Vandekerckhove and Job2001) levels, providing information that may or may not apply to naturally occurring seed hydration memory, since seed priming is a technological procedure and hydration memory is a natural stress response.
Since germination, from the imbibition of a dry seed to the emergence of the radicle, is a very complex and tightly regulated process, comprehensively reviewed by several authors, and fairly conserved across plant species (McDonald, Reference McDonald, Black and Bewley2000; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Holdsworth et al., Reference Holdsworth, Bentsink and Soppe2008; Nonogaki et al., Reference Nonogaki, Bassel and Bewley2010), we hypothesize that the hydration memory process is likely an effect of the regulation of known genes and pathways, rather than a separate pathway. The description of this regulation may ultimately serve to introduce or stimulate similar phenomena through manipulation of native plant regulation pathways in crops (Hussain and Peng, Reference Hussain and Peng2003; Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011).
Previously, we have successfully reproduced this phenomenon by germinating F. peninsulae seeds; we observed that the germination time was dramatically reduced after an HD cycle, as expected (Dubrovsky, Reference Dubrovsky1996, Reference Dubrovsky1998). As an initial approach to describing the molecular events that lead up to the enhanced germination after an HD cycle, we aimed to investigate whether there was differential expression of total proteins of seeds and seedlings subjected to the HD cycle. We have surveyed the total protein expression patterns of seeds subjected to HD and their germinating seedlings, through two-dimensional (2D) electrophoresis, and found differential expression levels of several proteins possibly involved in primary metabolism, ubiquitination and reserve protein availability regulation.
Materials and methods
Biological material and germination conditions
Ferocactus peninsulae var. townsendianus (Britton & Rose) N.P. Taylor fruits were collected in June 2012 in various sites located along the Mexican state of Baja California Sur, between 24°54′40″N and 22°54′32″N. Their seeds were extracted from the fruits, separated from the pulp, washed and air-dried with assistance from the Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México (FESI-UNAM) Seed Bank staff. Seeds were stored in dry conditions prior to the HD treatments. Seeds were germinated in standard plastic Petri dishes with a filter paper lining, 25 seeds per dish. Seeds were surface sterilized for 5 min in a solution containing commercial bleach (5.25% sodium hypochlorite) and 1 μl ml− 1 of 20% Triton X-100, and then placed on a filter paper-lined Petri dish moistened with 3 ml of distilled water. The Petri dishes were sealed and maintained in a Lumistell (Mexico City, Mexico) germination chamber under a 12-h day/night photoperiod, a 30°C constant temperature and 60–65% relative humidity for as long as necessary to observe germination; the filter paper moisture was constantly monitored and an additional 1 ml of distilled water was added when necessary to prevent it from drying. A seed was considered germinated when a protruding radicle was observed (Dubrovsky, Reference Dubrovsky1996; Flood et al., Reference Flood, Harbinson and Aarts2011).
Seed treatments
Seeds that were subject to a single HD cycle were incubated in germination conditions for 72 h, then blotted dry with a paper towel and air dried on a laboratory shelf (23–27°C, 50–60% relative humidity) and wrapped in a paper towel for 4, 15 or 70 d. Seeds that were subjected to multiple HD cycles were incubated in germination conditions for 24 h, then blotted dry with a paper towel, air dried on a laboratory shelf (23–27°C, 50–60% relative humidity) and wrapped in a paper towel for 4 d for each cycle. After the treatments, seeds were transferred back to germination conditions for as long as necessary to observe germination. The mean germination time (MGT) was calculated as
![]() $$\sum ( G _{ i } i )/\sum ( G _{ i }) $$
, where i is a germination day and G
i
is the number of seeds germinated at day I (Dubrovsky, Reference Dubrovsky1996; Umezawa et al., Reference Umezawa, Fujita, Fujita, Yamaguchi-Shinozaki and Shinozaki2006; Cattivelli et al., Reference Cattivelli, Rizza, Badeck, Mazzucotelli, Mastrangelo, Francia, Marè, Tondelli and Stanca2008; Ashraf, Reference Ashraf2010). The data were analysed using Student's t-test at P< 0.05.
$$\sum ( G _{ i } i )/\sum ( G _{ i }) $$
, where i is a germination day and G
i
is the number of seeds germinated at day I (Dubrovsky, Reference Dubrovsky1996; Umezawa et al., Reference Umezawa, Fujita, Fujita, Yamaguchi-Shinozaki and Shinozaki2006; Cattivelli et al., Reference Cattivelli, Rizza, Badeck, Mazzucotelli, Mastrangelo, Francia, Marè, Tondelli and Stanca2008; Ashraf, Reference Ashraf2010). The data were analysed using Student's t-test at P< 0.05.
Total RNA and protein were extracted from untreated seeds or from treated seeds after 1 HD cycle (72 h germination conditions, 4 d dry conditions). Two-day-old treated and untreated seedlings were collected, frozen in liquid nitrogen, ground and saved for RNA and protein extraction, until all the seedlings from each Petri dish were obtained.
Seed moisture content was determined in the dry, untreated seeds, 24-h hydrated seeds and dry, treated seeds, at the end of each dehydration period (4, 15 or 70 d) right before rehydration. The seeds were dried at 130°C for 1.5 h (International Seed Testing Association, 1985) and their moisture content was calculated by using the following formula: moisture content = (fresh mass − dry mass)/(fresh mass).
RNA and protein extraction
Both total RNA and protein were extracted using the AllPrep kit (Qiagen, Hilden, Germany) from the same sample: seeds and seedlings subjected, or not, to an HD cycle. Thirty-milligram samples of seeds or seedling tissue were frozen in liquid nitrogen and thoroughly ground, using a sterile mortar and pestle, homogenized by pipetting and processed according to the manufacturer's recommendations.
2D Electrophoresis and analysis
Isoelectric focusing was performed on an Ettan IPGphor 3 IEF System (GE Healthcare, Little Chalfont, Buckinghamshire, UK) following the manufacturer's recommendations. Briefly, a 7-cm Immobiline DryStrip Gel with a pH range 3–10 (GE Healthcare) was rehydration-loaded with 100 μg of total protein from each sample and run following a four-step protocol: step 1, 300 V for 200 Vh; step 2, a gradient to 1000 V for 300 Vh; step 3, a gradient to 5000 V for 4000 Vh; and a final step of 5000 V for 1250 Vh up to 5750 Vh with a limit of 50 μA/strip. The strips were equilibrated according to the manufacturer's protocol and subsequent 2D sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 12% 8 × 7.3 cm polyacrylamide gels, which were later stained with the SilverQuest silver staining kit (Invitrogen, Carlsbad, California, USA) and photographed with an AlphaImager gel documentation system (Alpha Innotech, now part of ProteinSimple, Santa Clara, California, USA). Image analysis was performed using the REDFIN Solo program (Ludesi AB, Lund, Sweden; www.ludesi.com). This software provides spot detection, segmentation and matching following a strict protocol. Protein spots are assigned a constant number in all gels that corresponds to a unique position. The integrated intensity of each spot is measured, background corrected and normalized according to the total protein loaded in each gel. Normalization removes systematic gel intensity differences originating from, for example, variations in staining, scanning time and protein loading, by mathematically minimizing the median expression difference between matched spots. The most evident and reproducible spots were partially characterized using the TagIdent tool available from the EsPASy Bioinformatics Resource Portal (www.expasy.org). For each analysed spot, the TagIdent output was a list of probable proteins within a 5% pI and molecular weight (MW) variation; the most likely protein was selected according to the tissue expression reported in the Uniprot database (www.uniprot.org).
Results
Hydration memory is a reproducible phenomenon
F. peninsulae seeds germinated after 8 d without additional treatment and in approximately 4 d after HD. Regardless of the extent or number of the HD cycles, the percentage of germinated seeds was substantially higher, reaching its highest value by days 4–5 while less than 20% of untreated seeds had germinated then (Fig. 1A); the final germination percentages remained constant around 75% both in treated and untreated seeds (data not shown). The MGT was thus significantly reduced after HD treatment (P< 0.05 Student's t-test, Fig. 1B) as previously observed in this species (Dubrovsky, Reference Dubrovsky1996). Based on these data, we concluded that in the present work we had successfully reproduced the hydration memory phenomenon. Seed water content was measured in dry and hydrated seeds to find out if hydration alone or HD treatments modified it. The water content showed a slight decrease once seeds were hydrated, but did not vary significantly in dehydrated seeds. No significant differences were found among treatments (see supplementary Fig. S1).

Figure 1 Effect of HD treatments on germination kinetics and mean germination time (MGT) of F. peninsulae seeds. (A) Germination curves of seeds subjected to 3, 15 and 70 d of dehydration (left) and 1, 2 or 3 HD cycles (right). (B) MGT was significantly reduced (P>0.05) after either different dehydration times (4, 15 and 70 d) (left), or 1–3 HD cycles (right).
Hydration memory-capable seeds maintain RNA and protein integrity through HD cycles
The RNA and protein patterns of both treated and untreated seeds and seedlings were analysed and compared to those of another cactus species Pachycereus ssp., which is not capable of surviving HD treatments (Fig. 2). In order to compare the RNA pattern (Fig. 2A), we extracted RNA from 25 seeds in each case, thus smaller F. peninsulae seeds yielded a lower amount RNA than those of Pachycereus ssp. The observed RNA patterns in F. peninsulae seeds that went through the HD cycle and those that had not were very similar (compare lanes 1 and 3 in Fig. 2A, left panel). Likewise, the RNA patterns from untreated and HD-treated seedlings remained unchanged (compare lanes 2 and 4 in Fig, 2A, left panel). On the other hand, Pachycereus ssp. seeds showed evident RNA degradation after an HD cycle (compare lanes 1 and 3 in Fig. 2A, right panel). RNA from HD-treated Pachycereus ssp. seedlings is not shown since HD-treated seeds failed to germinate.
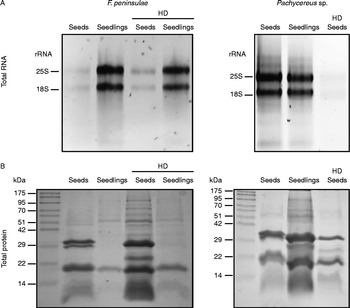
Figure 2 Total RNA (A) and total protein (B) patterns of untreated and HD-treated seeds of F. peninsulae and Pachycereus sp.
Regarding protein patterns, we observed the differential expression of several protein bands with apparent molecular weights ranging from 25 to 70 kDa, which were detectable only, or at much higher levels, in F. peninsulae seeds after the HD cycle. On the other hand, we observed a substantial reduction in total protein content in Pachycereus ssp. seeds after an HD cycle (Fig. 2B). HD-treated Pachycereus sp. seeds failed to germinate, so a protein pattern was unavailable in seedlings.
HD treatment possibly influences the expression of proteins participating in regulatory pathways
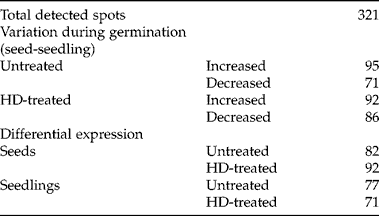
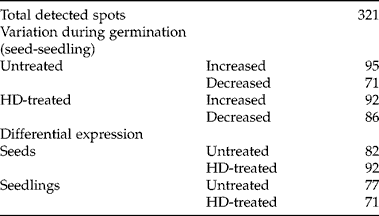
As an initial approach to describing the molecular events that lead up to the enhanced germination after an HD cycle, we aimed to investigate whether there was differential expression of proteins between seeds and seedlings subjected or not to the HD cycle. Two-dimensional electrophoresis assays revealed a total of 321 spots (Fig. 3). This protein pattern was reproducible using both Coomassie blue and silver staining (data not shown). Silver-stained gels were used for Ludesi REDFIN Solo analysis, which showed a variation of 20–30% of these spots during germination with or without treatment. The REDFIN Solo software can identify differences in spot intensity from one gel or group of gels to another. When comparing untreated seeds versus seedlings, we detected an increase in the intensity of 95 spots as an apparent consequence of germination (i.e. 95 spots were detected at higher levels in seedlings) and a decrease of 71 spots. In contrast, the analysis showed higher intensity of 92 spots and lower intensity of 86 with germination of HD-treated seeds. Next, we compared treated versus untreated seeds and seedlings independently. In seeds, we found 82 spots with higher intensity in untreated seeds and 92 in HD-treated ones; untreated seedlings showed 77 spots with higher intensity, and HD-treated ones only showed 71 (Table 1).

Figure 3 Two-dimensional protein patterns from F. peninsulae seeds and seedlings subjected, or not, to an HD cycle. (A) Untreated seeds, (B) untreated seedlings, (C) HD-treated seeds, (D) HD-treated seedlings.
Table 1 Detected protein spots on HD-treated or untreated germinating seeds, using REDFIN Solo software analysis
Thirty-seven spots, evident through Coomassie staining, were partially identified by homology using the pI and MW data. From these, 15 were common to HD-treated and untreated samples while 22 were differentially expressed. We found proteins involved in primary metabolism, storage, ubiquitination and germination regulation. Most of the common proteins (Table 2) play constitutive housekeeping roles, adding confidence to our identification method. From the differentially expressed proteins (Table 3) protease inhibitors and ubiquitin ligases stand out. Since proteinases play a fundamental metabolic role during the life cycle of plants, it has been proved that protease inhibitors are as important, because they regulate the former (Gomes et al., Reference Gomes, Oliva, Lopes and Salas2011). We found differential expression of at least one possible Bowman–Birk proteinase inhibitor (Table 3), which suggests its implication in proteinase regulation before germination, as it is present in untreated seeds; interestingly, it was also found in HD-treated seedlings, hinting at de novo synthesis as an interesting regulatory mechanism that deserves further assessment. Additionally, we detected a possible alpha-amylase inhibitor only in HD-treated seedlings, suggesting that the treatment induces de novo synthesis of metabolic regulators that control availability of stored energy sources, perhaps in preparation for the reinvigorated germination that takes place after discontinuous hydration periods. Two possible ubiquitination-related proteins were partially described: RING Finger E3 Ligase RHA2a and AtRbx1 (Table 3). The first one, detected in seedlings regardless of the treatment, has a described role in seed germination (Bu et al., Reference Bu, Li, Zhao, Jiang, Zhai, Zhang, Wu, Sun, Xie, Wang and Li2009); conversely, higher AtRbx1 mRNA expression has been observed in plant tissues containing actively dividing cells (Lechner, Reference Lechner2002), making it reasonable to speculate that it has a regulatory role in HD-treated F. peninsulae seeds, as enhanced germination has been observed in ubiquitin-overexpressing tobacco seeds under drought stress conditions (Guo et al., Reference Guo, Zhang, Gao, Xing, Li and Wang2008).
Table 2 Partially characterized protein spots common to all stages of F. peninsulae: untreated seeds (S), untreated seedlings (SG), HD-treated seeds (SHD) and HD-treated seedlings (SGHD)

Table 3 Partially characterized differential protein spots present in F. peninsulae untreated seeds (S), untreated seedlings (SG), HD-treated seeds (SHD) and HD-treated seedlings (SGHD)

Discussion
Ever since hydration memory was described (Dubrovsky, Reference Dubrovsky1996) very few studies have acknowledged its status as a natural phenomenon. We believe that hydration memory research has been largely neglected because it was overshadowed by extensive research on agricultural seed priming. Nevertheless, our experiments reproduced previous results that show that hydration memory-capable seeds are intrinsically resistant to repeated or extended drought periods, a condition not explored in primed commercially important seeds.
Hydration memory can be viewed from two complementary angles: from a wide angle it has been considered to be responsible for an increment in the vegetative growth of a variety of plant species throughout extensive areas such as the Sonoran desert (Salinas-Zavala et al., Reference Salinas-Zavala, Douglas and Diaz2002), whereas from a narrow angle, hydration memory entails tightly regulated metabolic processes that respond to environmental variables (Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011). The aim of this work was to provide insights on the molecular control of this process in a wild species.
Our findings suggest the presence of specific mechanisms of RNA and protein stability acting within seed hydration memory-capable seeds, as F. peninsulae mRNA and protein patterns remain virtually unchanged after HD treatment. A number of cis-acting elements that elicit selective messenger degradation have been described (Gutiérrez et al., Reference Gutiérrez, MacIntosh and Green1999); these elements, and possibly novel ones, are still to be described in desert cacti.
Regardless of the RNA or protein stability mechanism, we found that several proteins are expressed differentially in HD-treated as compared to untreated seeds. This suggests that post-HD germination may not be physiologically identical to regular germination, an assumption sustained by the observation of shorter germination times in HD-treated seeds. The total protein expression pattern is clearly different in untreated and HD-treated seeds and seedlings, which shows that HD-treated seeds have a metabolic signature characterized by higher expression of low molecular weight ( < 30 kDa) and low pI ( < 7) proteins. We have partially characterized in silico a number of proteins involved in storage, ubiquitination and germination regulation, which hint at the processes that this metabolic signature might comprise.
Previous studies on dormancy release have demonstrated differential expression of transcription regulation and chromatin remodelling genes at the mRNA level in dormant and after-ripened seeds, as well as the requirement of a balance of catabolism and synthesis of active gibberellic acids (GAs) to promote germination (Cadman et al., Reference Cadman, Toorop, Hilhorst and Finch-Savage2006). Moreover, it has been shown recently that such regulation may be linked to seasonal conditions (Footitt et al., Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011). Therefore, we hypothesize that discontinuous hydration might also have an effect on this regulation. Unpublished data from our lab hints at up-regulation of the GA synthesis pathways. However, seed germination is a very complex process and these findings suggest a regulatory network that remains to be analysed.
Seed germination responses to environmental cues through regulation of gene expression have only been explored by a few studies, such as that of Footitt et al. (Reference Footitt, Douterelo-Soler, Clay and Finch-Savage2011). The present study is a contribution to efforts being made towards understanding the relationship between protein expression and environmental conditions, particularly harsh ones, such as those found in arid environments. Plant species have adapted to these challenging conditions by developing specialized regulation mechanisms to withstand discontinuous conditions while competing for limited resources. Hydration memory is one such mechanism, acting at the germination and seedling establishment level; it provides the ability of accumulating higher biomass over a given period of time which is a clear advantage in such a competitive environment (Salinas-Zavala et al., Reference Salinas-Zavala, Douglas and Diaz2002).
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0960258513000317
Acknowledgements
We are grateful to UBIPRO-FESI-UNAM Seed Bank, for some of the seed material provided, and expert seed technical assistance.
Financial support
E.L.-U. received a post-doctoral grant from Direción General de Asuntos del Personal Académico – Universidad Nacional Autónoma de México (Academic Personnale General Direction – National Autonomous University of Mexico). This work was partially funded by Programa de Apoyoa a los Professors de Carrera (Careers Professors Support Program) 2011–2012.
Conflicts of interest
None.