Introduction
Since the beginning of husbandry and breeding, humans have been manipulating plants by artificial selection, and have produced many modifications in a wide variety of plant species. The changes that differentiate domesticates from their wild ancestors are known as domestication syndromes (Fuller, Reference Fuller2007). Increases in fruit size or reductions on seed dispersal mechanisms are examples of some commonly modified traits (Bai and Lindhout, Reference Bai and Lindhout2007; Fuller and Allaby, Reference Fuller, Allaby and Østergaard2009). Another important feature associated with domestication is the reduction or even complete loss of seed dormancy (De Wet and Harlan, Reference De Wet and Harlan1975; Hillman and Davies, Reference Hillman and Davies1990; Smith, Reference Smith, Zeder, Emshwiller, Bradley and Smith2006; Jones, Reference Jones, Fairbairn and Weiss2009). Dormancy is defined as the failure to germinate under favourable ambient conditions (Bewley, Reference Bewley1997). It has been well established that morphological and physiological mechanisms determine the germination capacity of a particular seed (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). Isolated embryos may present an incapacity to grow that may be the consequence of an underdeveloped state or the presence of chemical inhibitors (i.e. abscisic acid, ABA). In other cases, the embryo has the capacity to germinate, but germination is blocked or retarded by the coat. The presence of inhibitors and mechanical restrictors in the tissue surrounding the embryo brings about limitations to water or gas diffusion and are characteristics of the embryo that impede its growth (Bewley, Reference Bewley1997; Steinbrecher and Leubner-Metzger, Reference Steinbrecher and Leubner-Metzger2017).
Five Cucurbita species (C. pepo L.; C. moschata Duch, C. maxima Duch., C. argyrosperma Huber and C. ficifolia Bouché) have been domesticated in the Americas. In the case of Cucurbita maxima subspecies maxima Duch. ex Lam, domestication was conducted from its wild ancestor C. maxima subsp. andreana (Nauding) Filov, which is still occasionally observed as ruderal in different areas of South America (Whitaker and Bemis, Reference Whitaker and Bemis1964; Nee, Reference Nee1990; Decker-Walters and Walters, Reference Decker-Walters, Walters, Kiple and Ornelas2000; Sanjur et al., Reference Sanjur, Piperno, Andres and Sel-Beaver2002). Compared with other domesticated cucurbits, C. maxima is the species that can be grown at the widest altitudinal range and, together with C. ficifolia, has the highest tolerance to low temperatures (Lira Saade, Reference Lira Saade1995).
Cucurbit seeds that are frequently recovered from archaeological surveys display a wide range of variations in the size and in the histological characteristics of their seed coats depending on the age of the site (Lema et al., Reference Lema, Capparelli and Pochettino2008; Martínez et al., Reference Martínez, Perez, Lema and López Anido2015). These variations may be the result of human manipulation. Seeds of the wild, C. maxima subsp. andreana, are prevented from germinating several months after dispersal; in contrast, the domesticated Cucurbita maxima subsp. maxima seeds do not display restrictions to germination immediately after being released from the fruit (F. López Anido, unpublished results). Thus an association between morphological changes with a typical physiological process linked to domestication (i.e. loss of seed dormancy) may help to reconstruct the processes involved in plant domestication (Hillman and Davies, Reference Hillman and Davies1990).
The experiments reported here were designed to unravel the physiological mechanisms establishing seed dormancy in Cucurbita maxima that were lost during domestication.
Materials and methods
Plant material
Experiments were conducted at the Instituto de Fisiología Vegetal (INFIVE), Universidad Nacional de La Plata, La Plata, Argentina. The plant material was generated ad hoc under controlled conditions at the Campo Experimental Villarino, Universidad Nacional de Rosario, Zavalla, in the province of Santa Fe, Argentina (33° 01′ S; 60° 53′ W). It has a temperate climate, loamy soil and average annual rainfall of 950 mm.
The experiments were performed with the two subspecies of Cucurbita maxima, namely: C. maxima subsp. maxima Duch. ex Lam (domesticated) and C. maxima subsp. andreana (Naudin) Filov (wild ancestor). The domesticated accession (coded 93 in this study), is the cultivar ‘Redonda Verde’, from Feltrin Seed Company Brazil, and belongs to the Zapallito cultivar group. Two wild accessions were used: accession MAX 81 (coded 130) from the IPK Genebank, Gatersleben, Germany (collected in the province of Entre Ríos, Argentina); and accession G 29254 (coded 160) from the Northeast Regional PI Station Geneva, USA (collected in the province of Córdoba, Argentina). Their hybrids (F1) were coded: 93 × 130, 93 × 160, 130 × 93 and 160 × 93, the first accession being the parental mother. The experiments were performed during the first six months from seed collection as after this time seeds start to lose dormancy.
Germination experiments
The experiments were repeated at least three times and ten seeds of each genotype were used for each biological replicate. Seeds or naked embryos were laid on wet filter papers imbibed with 5 ml of water inside Petri dishes that were covered with foil and incubated at 16, 22 and 28°C. The germination tests lasted 10 days. Germination was scored when the radicle protruded more than 2‒3 mm out of the seed coat. Naked embryos were considered as germinated when they had elongated between 2 and 3 mm.
ABA and gibberellins (GA) treatments
Naked embryos of genotypes 93, 130, 93 × 130, 130 × 93 were used to verify the effect of ABA on germination. They were incubated in the presence of 5 ml 0, 0.1, 1 and 10 µM ABA (mixed isomers, Sigma) at 16, 22 and 28°C for 10 days.
Seeds of wild (accession 130) and domesticated genotypes or naked embryos were imbibed at 28°C in Petri dishes containing two filter papers moistened with 5 ml of distilled water or 100 µM GA3 (Sigma) or 300 µM paclobutrazol (PAC, PhytoTechnology Laboratories).
ABA assay
Seeds from the 93 (domesticated) and 130 (wild) accessions were imbibed at 28°C in triplicate in distilled water as described above. Seeds were sampled at 0, 12, 24, 48 and 72 h after imbibition, embryos were dissected, frozen in liquid N2 and stored at ‒80°C until processing. Seed coats dissected at 0 h were also frozen and stored as described for embryos. Each sample was then lyophilized, powdered, weighed and stored at ‒30°C until assayed for ABA content.
Distilled water was added to each powdered sample (100:1 v/w) and left overnight on a shaker at 4°C. After centrifugation at 7000 g for 4 min, 50 µl of the supernatant was used to assay ABA with a radioimmunoassay as described by Steinbach et al. (Reference Steinbach, Benech-Arnold, Kristof, Sánchez and Marcucci Poltri1995) using the mono-clonal antibody AFR MAC 252 (Quarrie et al., Reference Quarrie, Whitford, Appleford, Wang, Cook, Henson and Loveys1988), and tritiated ABA (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). The average and standard error of three biological replicates, each measured twice, was reported.
Statistical analysis
Normality of the data was tested with Omnibus Normality of Residuals (P ≥ 0.05) and homogeneity of variance with the modified Levene equal variance test (P ≥ 0.05). Those data that were not suitable for parametric tests were analysed with a Kruskal‒Wallis one-way ANOVA with Dunn's post hoc test (P ≤ 0.05). Those data suitable for parametric analysis were analysed using Tukey's comparisons test.
Results
Germination of seeds and embryos from domesticated and wild subspecies and their reciprocal hybrids at different incubation temperatures
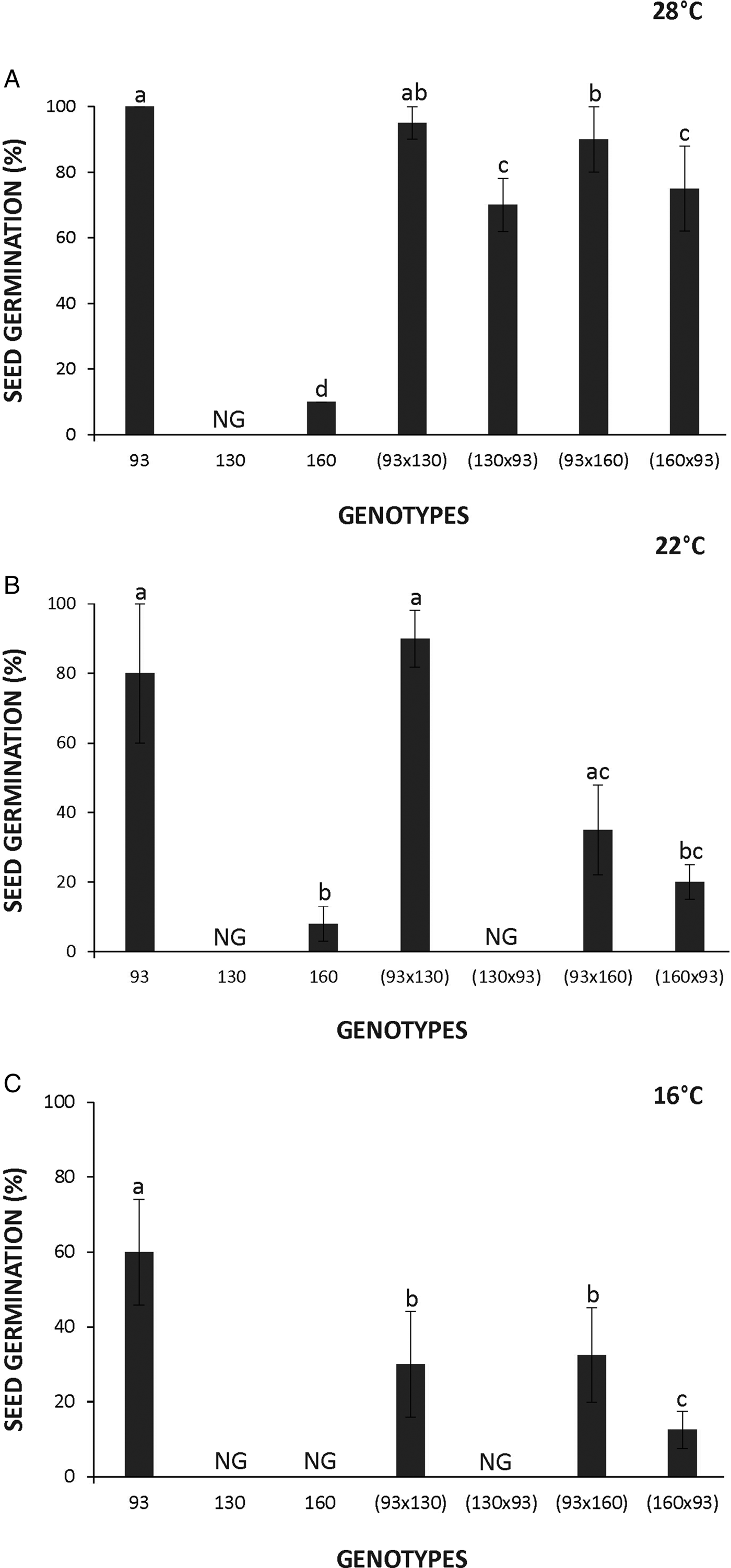
The seeds of the domesticated Cucurbita rapidly germinated, attaining 100% of germination after 48 h of imbibition at 28°C (Fig. 1A). Furthermore, germinated seeds of the domesticated accession were observed inside the fruit (Fig. 2). In contrast, seeds of the wild genotypes displayed dormancy evidenced by the complete lack of germination in the 130 accession and only 7% germination in the 160 accession after 9 days of imbibition at 28°C. It is noteworthy that seeds of the wild genotypes did not germinate even during the following months after being harvested and stored under dry conditions. Seed germination of 160 and 130 accessions was achieved at around 6 and 12 months after storage, respectively. Hybrid seeds obtained from the domesticated genotype used as the mother plant showed almost 100% germination during the first 2 days after imbibition. However, hybrid seeds produced from the reciprocal cross using a wild mother plant displayed low germination percentages (Fig. 1A‒C). Seed dormancy expression was exacerbated when imbibition was performed at lower temperatures (i.e. 22 and 16°C) particularly in the wild accessions (130 and 160) and the hybrid produced using 160 or the domesticated genotype 93 as mother plant (Fig. 1B and C).
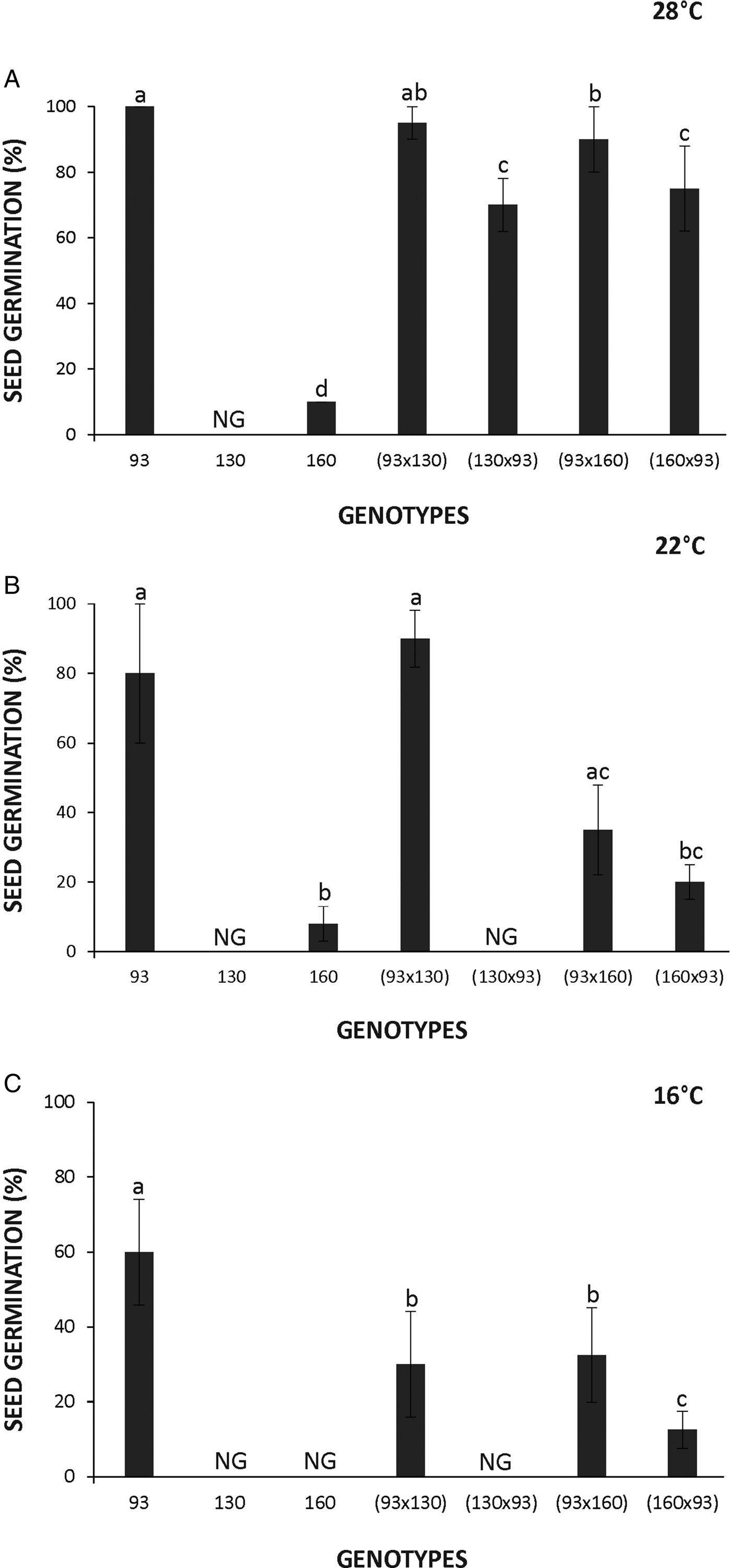
Figure 1. Seed germination of Cucurbita maxima subsp. maxima (93, domesticated) and C. maxima subsp. Andreana (130 and 160, wild ancestor) and their hybrids at the indicated temperatures. The first accession indicates the parental mother used for each cross. ‘NG’ indicates no germination. The data were obtained from at least three independent experiments using three biological replicates of 10 seeds. Results are expressed as means ± SEM. Different letters indicate significant differences (Kruskal–Wallis one-way ANOVA with Dunn's post hoc test, P ≤ 0.05).

Figure 2. Photographs showing the capacity of seeds of Cucurbita maxima subsp. maxima Duch. ex Lam (domesticated genotype) to germinate inside the fruit.
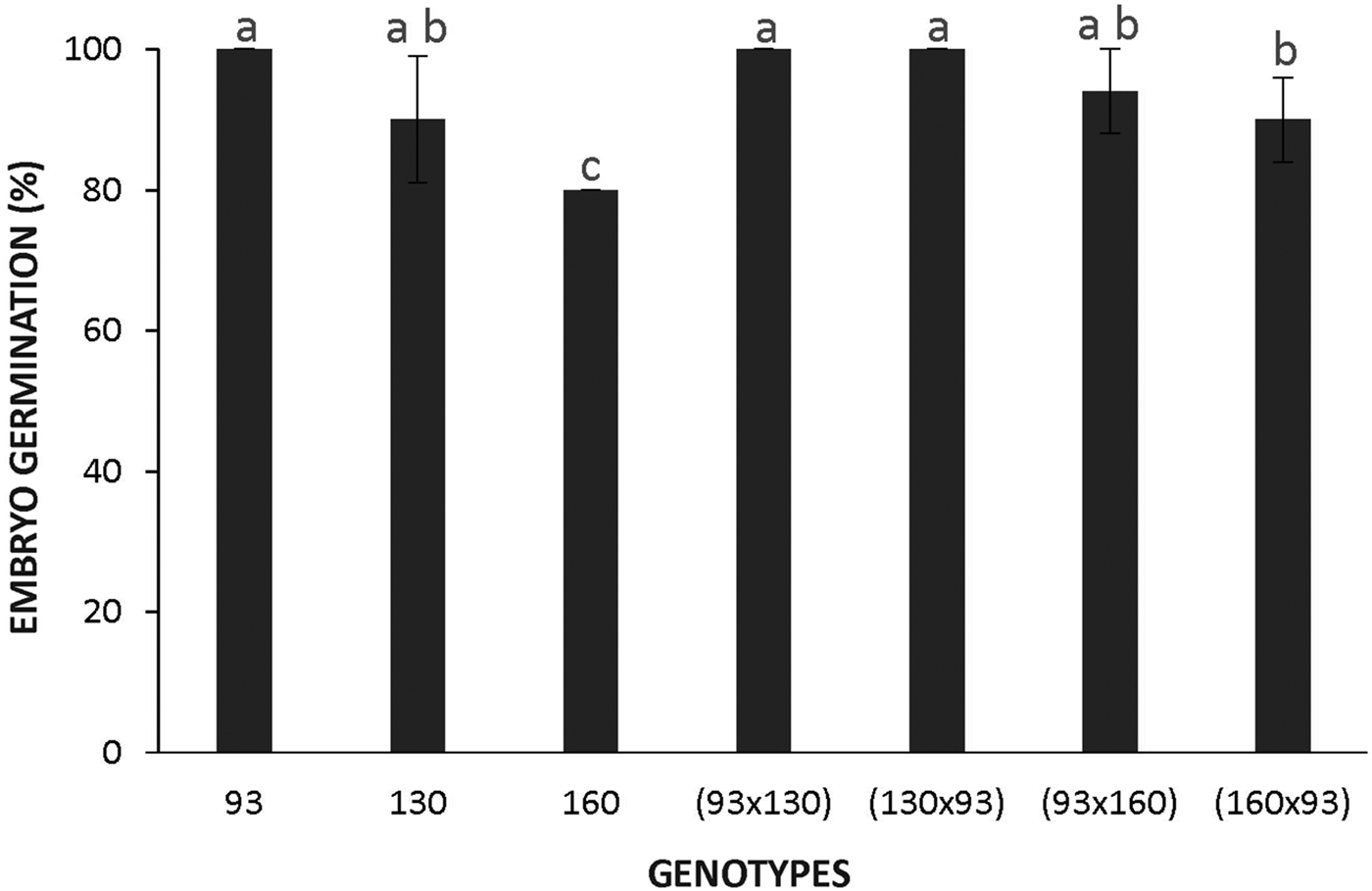
In contrast, naked embryos from all genotypes incubated at 28°C reached 80‒100% germination (Fig. 3), demonstrating that an almost null germination observed in seeds from accessions 160 and 130, and low germination in the crosses 130 × 93 and 160 × 93, can be attributed to seed coat imposed dormancy. Figure 4 shows that the highest temperature does not affect germination of the domesticated or wild genotypes, whereas the low temperature reduced the germination of the wild embryos to around 40%. This decreased germination at the low temperature in the wild subspecies suggests that dormancy is also dependent on the embryo.
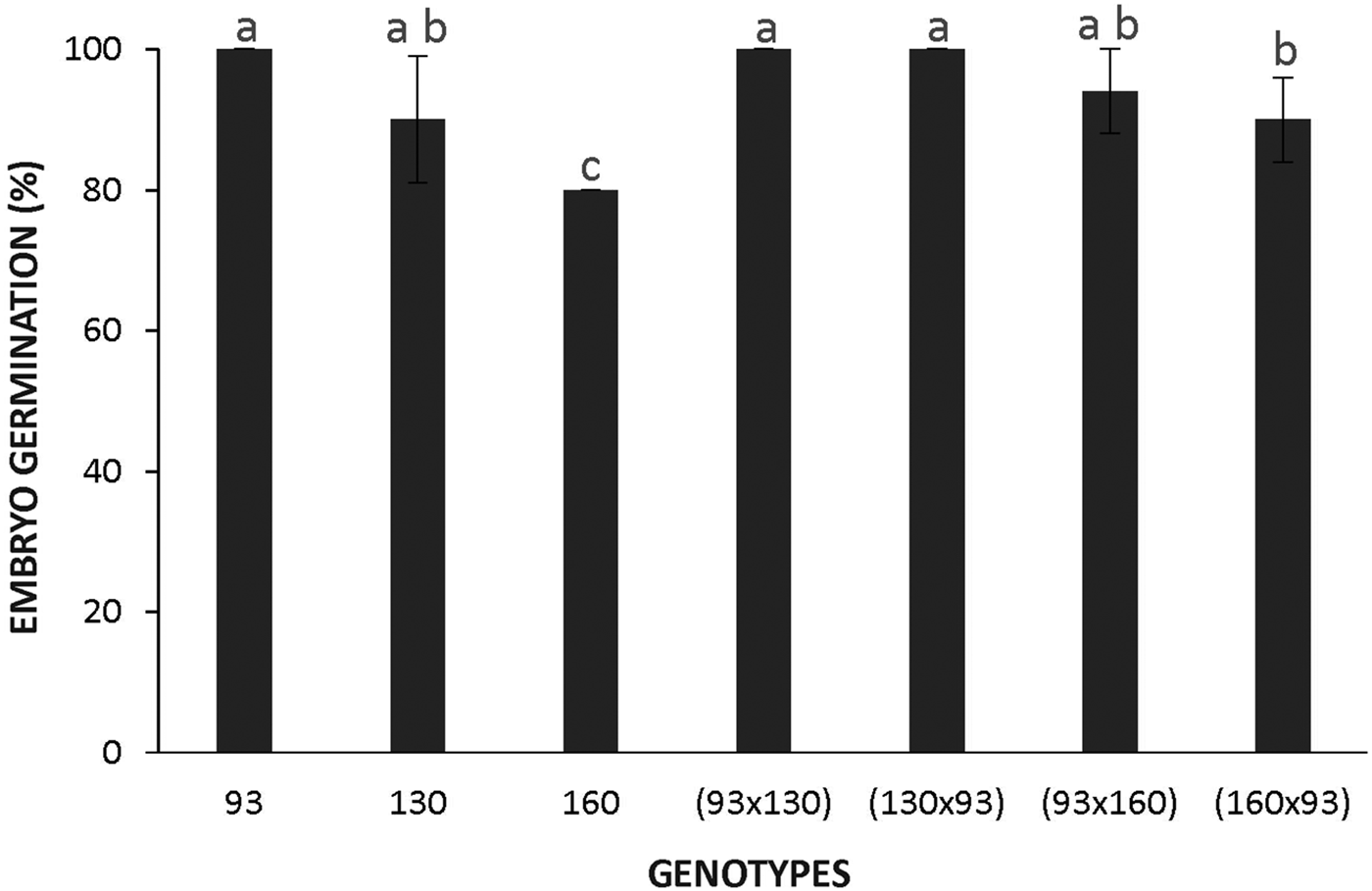
Figure 3. Germination of naked embryos of Cucurbita maxima subsp. maxima (93, domesticated) and C. maxima subsp. andreana (130, wild ancestor) and their hybrids at 28°C. The first accession indicates the parental mother used for each cross. The data were obtained from at least three independent experiments using three biological replicates of 10 embryos. Results are expressed as means ± SEM. Different letters indicate significant differences (Kruskal–Wallis one-way ANOVA with Dunn's post hoc test, P ≤ 0.05).

Figure 4. Effects of ABA on embryo germination of Cucurbita maxima subsp. maxima (93, domesticated) and C. maxima subsp. andreana (130, wild ancestor) and their hybrids at (A) 28°C, (B) 22°C and (C) 16°C. The first accession indicates the parental mother used for each cross. The data were obtained from at least three independent experiments using three biological replicates of 10 embryos. Results are expressed as means ± SEM. Different letters indicate significant differences (Kruskal–Wallis one-way ANOVA with Dunn's post hoc test, P ≤ 0.05). NG, no germination.
Seed coat permeability to water
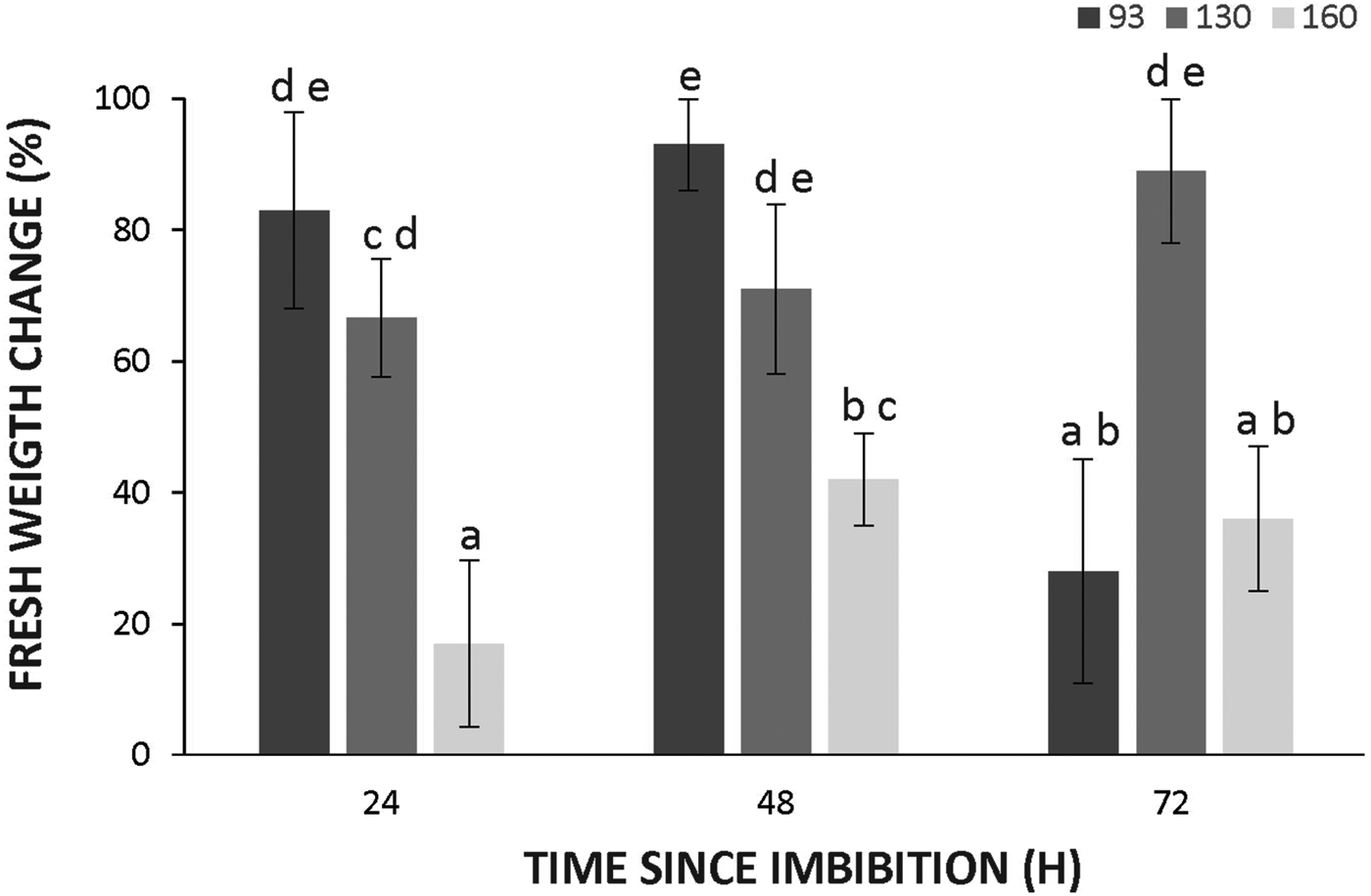
To assess whether differences in dormancy were due to differences in seed coat permeability to water, the fresh weight increment of the seeds was measured during the first 3 days of imbibition. The increment in fresh weight of seeds was significantly lower in wild genotypes at 24 and 48 h (Fig. 5), suggesting a decreased imbibition rate. However, water effectively reached the embryos as demonstrated by safranin uptake by intact seeds (supplementary figure S1) at this time, indicating that teguments did not restrict water uptake.
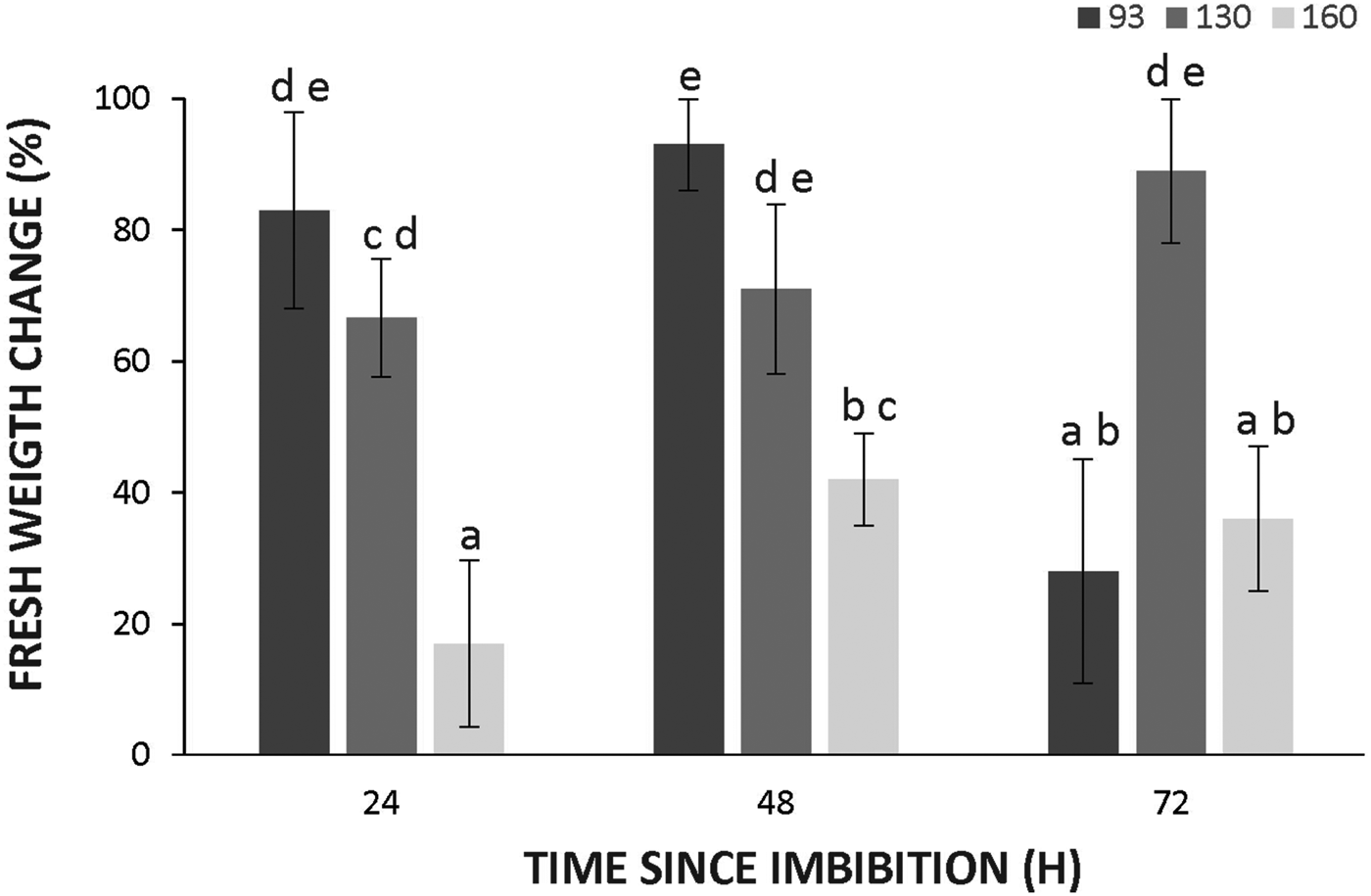
Figure 5. Changes in water uptake of seed of Cucurbita maxima subsp. maxima (93, domesticated) and C. maxima subsp. andreana (130 and 160, wild ancestors) after 24, 48 and 72 h of imbibition at 28°C. The increments were calculated as: [(FW24, 48 or 72 h – FW before imbibition )/FW before imbibition ] × 100. The data were obtained from at least three independent experiments using three biological replicates of 10 seeds. Results are expressed as means ± SEM. Different letters indicate significant differences (two-way ANOVA, P ≤ 0.05).
Responses of Cucurbita embryos to ABA and temperature
The effect of ABA on germination was assayed using naked embryos of the domesticated (93) and the wild accessions (130) and their hybrids under different temperatures (Fig. 4A‒C). At 28°C and 0.1 µM ABA, only wild type embryos showed a partial inhibition of germination. The embryos of the hybrid genotype produced using the maternal wild type displayed partial germination blockage at 1.0 and 10.0 µM ABA (Fig. 4A). In addition, the embryos of the domesticated and those of the hybrid generated using the maternal domesticated genotype presented partial germination only at the highest hormone concentration. The inhibition of germination increased with the concentration of ABA (Fig. 4A‒C) and was more pronounced for all genotypes at the lower temperatures (i.e. 22 and 16°C, Fig. 4B and C).
ABA content
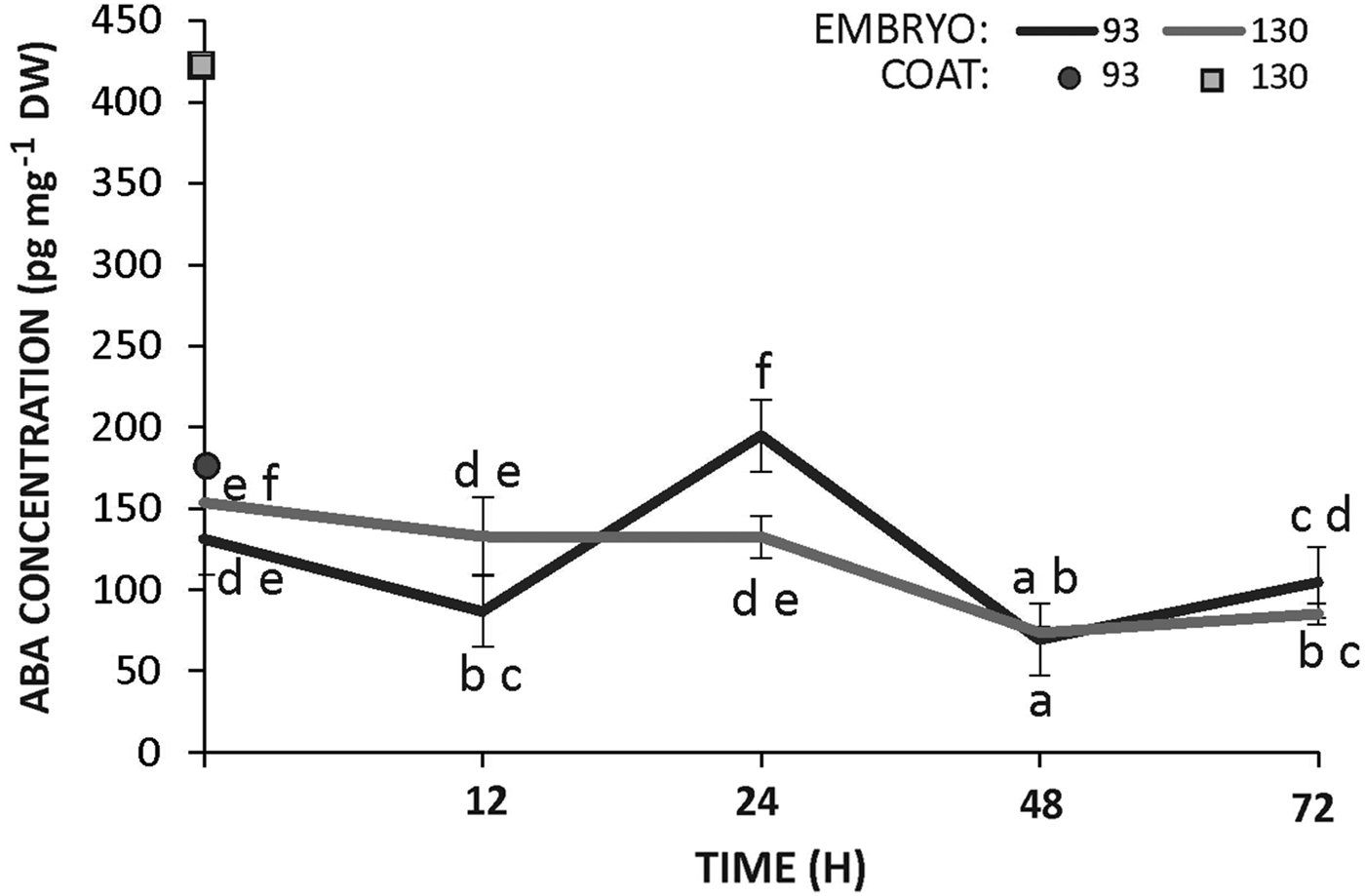
The concentration of ABA was measured in the seed coats (prior to imbibition) and in the embryos (prior to and during incubation) of accessions 93 and 130. Seed coats from the wild accession (130) had an ABA content that was threefold higher than that observed in the teguments of the domesticated seeds (Fig. 6). In contrast, the concentration of ABA was similar in the embryos of both genotypes throughout imbibition, showing almost no change or a slight decreasing trend with the progress of incubation (Fig. 6).
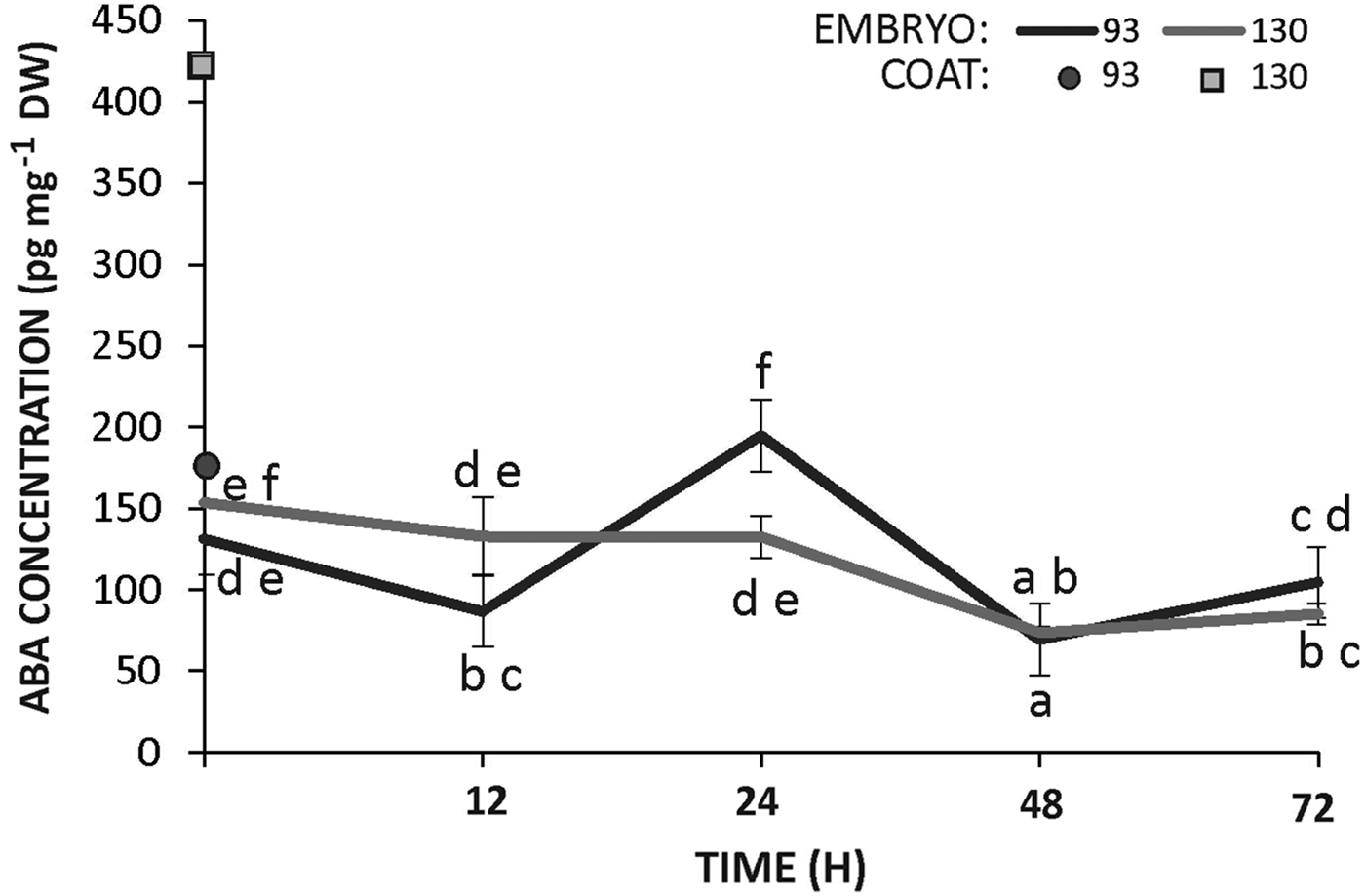
Figure 6. Concentration of ABA in the seed coat and embryo of Cucurbita maxima subsp. maxima (93, domesticated) and C. maxima subsp. andreana (130, wild ancestor) during seed germination at 28°C. The data were obtained from at least three independent experiments using three biological replicates and 10 seeds each. Results are expressed as means ± SEM. Different letters indicate significant differences (two-way ANOVA, P ≤ 0.05).
The effect of GA on seed germination
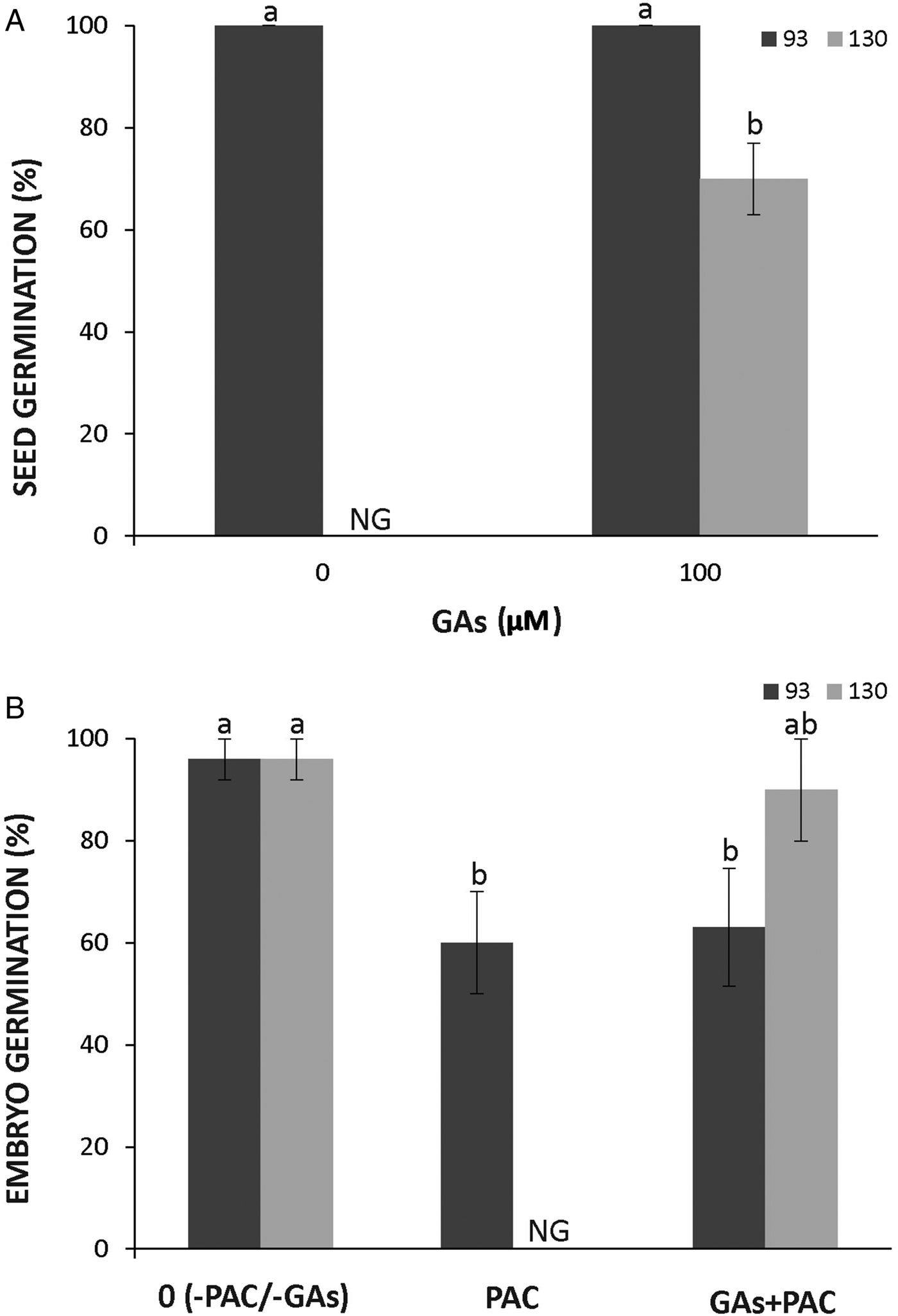
The seeds of the domesticated subspecies showed full germination after GA treatment; however, GA partially removed dormancy in wild seeds imbibed at 28°C as about 60% of them germinated (Fig. 7A). To assess whether GA synthesis might trigger germination, naked embryos were imbibed in 300 µM PAC. The treatment with PAC completely blocked the germination of wild embryos but only partially (ca 50%) blocked that of the domesticated ones (Fig. 7B). The effect of PAC was reverted by the application of exogenous GA in wild embryos but not in domesticated ones (Fig. 7B).
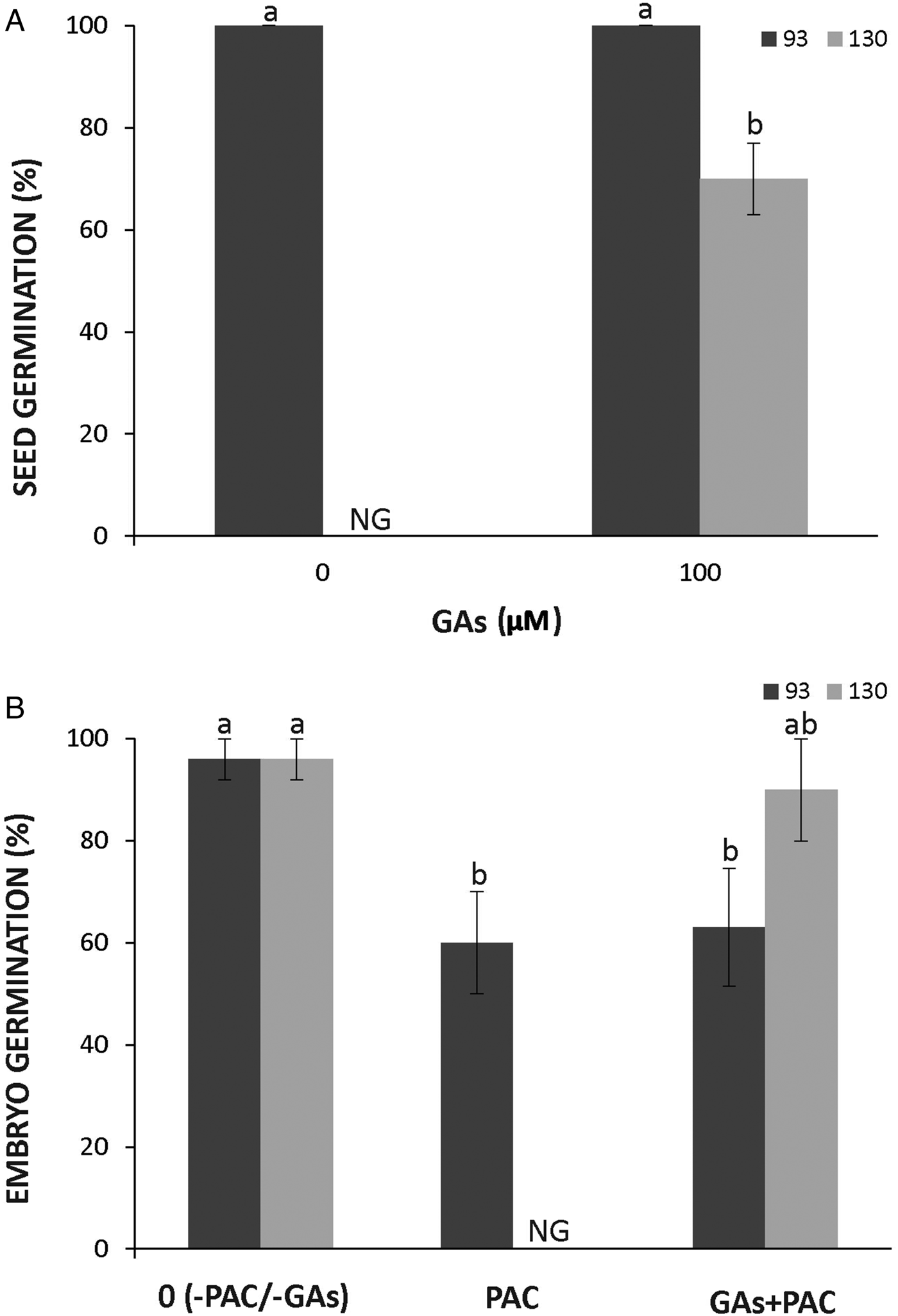
Figure 7. Effect of GA in the germination of seeds (A) and embryos (B) of Cucurbita maxima subsp. maxima (93, domesticated) and C. maxima subsp. andreana (130, wild ancestor) at 28°C. Seeds were treated with 100 µM GA and embryos with 100 µM GA and 300 µM PAC (inhibitor of GA synthesis). The data were obtained from at least three independent experiments using three biological replicates of 10 seeds or embryos. Results are expressed as means ± SEM. Different letters indicate significant differences (Kruskal–Wallis one-way ANOVA with Dunn's post hoc test, P ≤ 0.05). NG, no germination.
Discussion
Domestication leads to morphological and physiological modifications in edible plant organs. Experiments using seeds of wild and domesticated Cucurbita subspecies and their hybrids allow for the study of those changes taking place as a consequence of human manipulation. This work presents evidence showing changes in coat ABA concentration and ABA embryo sensitivity behind the control of seed dormancy in this species.
Role of seed coat in dormancy
The seeds of the wild Cucurbita subspecies displayed dormancy in contrast to those from the domesticated genotypes (Fig. 1). The lack of restriction for germination of embryos of all genotypes indicates that dormancy is imposed in part by the seed teguments (Fig. 3). Seed coats from the wild subspecies have some properties that allow them to impose dormancy with an intensity that is much higher than that of the domesticated subspecies. Hybrids obtained using the domesticated subspecies as the mother plant showed almost no dormancy. The observation that this coat-imposed dormancy had different intensities depending on the direction of the reciprocal cross points out the maternal origin of this feature. In muskmelon, the seed coat impedes the embryo from growing during imbibition by preventing its expansion and seed water uptake (reviewed in Welbaum, Reference Welbaum1999). However, in this study, wild-type embryos did imbibe, albeit at a lower rate (Fig. 5), indicating that mechanisms other than seed coat impermeability are involved in the maintenance of dormancy.
ABA sensitivity and temperature effect
ABA is well recognized as the main hormone inhibiting seed germination by imposing and maintaining dormancy in many species (Nambara et al., Reference Nambara, Okamoto, Tatematsu, Yano, Seo and Kamiya2010). The sensitivity to ABA also determines a block to the completion of germination (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). In seeds of barley and those of many other species, dormancy is released by a decrease both in the content and responsiveness of ABA by the embryo (Benech-Arnold et al., Reference Benech-Arnold, Giallorenzi, Frank and Rodriguez1999, Reference Benech-Arnold, Gualano, Leymarie, Côme and Corbineau2006; Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006). In this study, ABA supplementation reduced the capacity of the naked embryos to germinate, but this effect was more pronounced in the wild genotype (Fig. 4). Furthermore, embryos of the hybrid genotypes showed an intermediate response to ABA, indicating that differences in the intensity of seed coat-imposed dormancy between genotypes might be mediated in part by differences in the embryo sensitivity to ABA.
Both genotypes displayed temperature-dependent dormancy as it was expressed more intensely at 16°C (Fig. 4). This was accompanied by an increase in embryo responsiveness to ABA. The lowest ABA concentration treatment had no effect on the domesticated genotype but decreased the germination of wild embryos by ca 40 and 95% at 28 and 16°C, respectively. In the absence of ABA, naked embryos of the domesticated subspecies germinated under all temperatures but those of the wild genotype exhibited reduced germination percentages, particularly at 16°C. Overall, these results suggest that embryonic sensitivity to ABA have been reduced during domestication. The mechanism through which this reduction took place remains to be elucidated but an alteration in the functionality or availability of one or more proteins involved in the ABA signalling cascade is quite likely (Finkelstein et al., Reference Finkelstein, Gampala and Rock2002).
The concentration of ABA in wild and domesticated seeds
Besides ABA sensitivity, synthesis can take place during seed imbibition, thereby maintaining dormancy (Bewley, Reference Bewley1997; Gubler et al., Reference Gubler, Millar and Jacobsen2005). While ABA sensitivity was different between domesticated and wild embryos, ABA synthesis would represent another factor determining differences in dormancy. Indeed, during development, immature muskmelon seeds germinate when both the ABA concentration and ABA sensitivity decrease (Welbaum, Reference Welbaum1999). Here, ABA levels in the embryos were similar in both genotypes during the first 72 h of imbibition (Fig. 6). However, the ABA content in the dry seed coats of the wild genotype was threefold higher than that of seeds from the domesticated genotype. This difference must have been instrumental for the determination of the different dormancy between subspecies, a hypothesis that is supported by the fact that hybrid seeds produced on a wild ancestor (responsible for the genotype of the seed coat) had a deeper dormancy than seeds from its reciprocal cross. Decrease in ABA content of tissues surrounding the embryos might allow for their germination as observed in some species such as pine or rose (Feurtado et al., Reference Feurtado, Ambrose, Cutler, Ross, Abrams and Kermode2004; Pipino et al., Reference Pipino, Leus, Scariot and Van Labeke2013 and references therein). We did not carry out experiments to elucidate the nature of this differential ABA accumulation in the seed coats of wild and domesticated subspecies.
In contrast to ABA, an increase in GA content in the embryo promotes germination of many species (Bethke et al., Reference Bethke, Libourel, Aoyama, Chung, Still and Jones2007; Diaz-Vivancos et al., Reference Diaz-Vivancos, Barba-Espín and Hernández2013). The ABA/GA balance controls seed dormancy (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006; Piskurewicz et al., Reference Piskurewicz, Jikumaru, Kinoshita, Nambara, Kamiya and Lopez-Molina2008). In this study, dormancy of the wild genotype was partially released by the treatment with exogenous GA (Fig. 7A). In addition, the supplementation of GA reversed the dormancy in PAC-treated wild embryos further suggesting a coat-imposed germination blockage mediated by ABA (Fig. 7B). A complete inhibition of embryo germination by PAC treatment suggests that newly synthesized GA is needed for the germination of wild embryos (Fig. 7B). In contrast, the partial inhibition observed in the domesticated genotype indicates that the level of GA was high enough to allow their growth and that this physiological trait is affected by domestication. However, hybrid embryos and seeds were not analysed and dose‒response curves for GA and PAC must be performed in future experiments to demonstrate the role of GA synthesis in the dormancy of cucurbit seeds. In addition, the concentration of GA must be measured to unravel its participation in dormancy release in the domesticated subspecies.
Conclusions
At present, studies on domestication of C. maxima characterized the seminal cover at the anatomical level (Lema et al., Reference Lema, Capparelli and Pochettino2008). Our results suggest that domestication led to a reduction in both the concentration of ABA present in the seed coat, and in the sensitivity of the embryos to this hormone that, eventually, resulted in an attenuated dormancy in the seeds from the domesticated Cucurbita subspecies. This attenuated dormancy is further supported by the capacity of domesticated seeds to germinate under a wide range of temperatures.
Supplementary material
Supplementary Figure S1. Microscope observations of water uptake by Cucurbit seeds using staining with safranin (50% w/v) of sclereid cells of the coat. Scale bar scale, 10 µm.
A, coat sections of accession 130 after 10 min of incubation in water.
B, coat sections of accession 93 after 10 min of incubation in water.
C, coat sections of accession 130 after 5 h of incubation in water.
D, coat sections of accession 93 after 5 h of incubation in water.
E, coat sections of accession 130 after 5 h min of incubation in safranin.
F, coat sections of accession 130 after 5 h min of incubation in safranin.
G, coat sections of accession 130 after 24 h min of incubation in safranin.
H, coat sections of accession 93 after 24 h min of incubation in safranin.
I, coat sections of accession 130 after 48 h min of incubation in safranin.
J, coat sections of accession 93 after 48 h min of incubation in safranin.
To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258517000320
Acknowledgements
The authors wish to thank Dr Diego D. Fanello and Ing Ana Moretti for their assistance with the statistical analyses. L.V., A.C., F.L.A., R.B.A. and C.G.B. are researchers at CONICET. M.A.B. was a fellow student of Universidad Nacional de La Plata. This study was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (AC, PIP 0459), Agencia Nacional de Promoción Científica y Tecnológica (AC, PICT 2013-2539) and Universidad Nacional de La Plata (AC, N734).
Financial support
This study was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 0459), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2013-2539) and Universidad Nacional de La Plata (N734).