Introduction
Orchid seeds are small and simple, with average lengths of 500‒900 µm, the smallest merely being 100 µm long (Barthlott et al., Reference Barthlott, Große-Veldmann and Korotkova2014). Orchid seedlings grow as parasites on fungi, in this way compensating for a very limited provision of maternal seed reserves. In natural environments, mycorrhizal fungi are required for successful germination and establishment of seedlings (e.g. Rasmussen and Rasmussen, Reference Rasmussen and Rasmussen2014). At maturity an orchid seed contains an undifferentiated globular embryo within the thin-layered and usually transparent testa. There is no endosperm, and it is doubtful if double fertilization even takes place in Orchidaceae (Kodahl et al., Reference Kodahl, Johansen and Rasmussen2015).
Orchids occupy a broad range of habitats, and seed germination niches obviously vary accordingly. Our knowledge of functional and evolutionary variation in orchid seeds is largely based on external structural features such as testa cell morphology, the size and length/breadth of seeds, and air space/embryo ratios (e.g. Clements and Molvray, Reference Clements, Molvray, Pridgeon, Cribb, Chase and Rasmussen1999; Tsutsumi et al., Reference Tsutsumi, Yukawa, Lee, Lee and Kato2007; Verma et al., Reference Verma, Kranti Thakur, Sembi and Vij2012; Barthlott et al., Reference Barthlott, Große-Veldmann and Korotkova2014). We still have surprisingly little understanding of the functional and ecological consequences of this variation in outer structure. Few studies (such as Rauh et al., Reference Rauh, Barthlott and Ehler1975; Barthlott and Ziegler, Reference Barthlott and Ziegler1980; Prutsch et al., Reference Prutsch, Schardt and Schill2000) attempt to link structure and function.
With respect to seed coat chemistry and its functional importance, the information is even more limited. Most information is based on histochemistry where processed or fresh plant material is stained with a pigment that interacts at molecular scale with components of cells and cell walls (e.g. Fowler and Greenspan, Reference Fowler and Greenspan1985; Ruzin, Reference Ruzin1999). An advantage of light microscopy staining techniques is the sub-cellular and spatially precise characterization. However, the specificity of stains cannot be taken for granted, and there is a risk of misidentifying a component, or not identifying each component in a mixture of similarly staining components. Some stains used to locate lipids in plant tissues will also react with lignin and other phenolic substances (Brundrett et al., Reference Brundrett, Kendrick and Peterson1991), and thus the traditionally acknowledged lipid component in the orchid seed testa has recently been brought into question (Barsberg et al., Reference Barsberg, Rasmussen and Kodahl2013 and references therein).
In terrestrial orchids, cell walls of testa have been reported to contain lignin, suberin, polyphenols and cuticular materials, all contributing to the hydrophobic nature of mature seeds (Carlson, Reference Carlson1940; Harvais, Reference Harvais1980; Yeung et al., Reference Yeung, Zee and Ye1996; Lee et al., Reference Lee, Lee, Yeung and Chung2005). Strong hydrophobicity enhances flotation of Disa seeds, enabling them to move along streams (Kurzweil, Reference Kurzweil1993). The heavily lignified seed testa in Vanilla and Cyrtosia (Nishimura and Yukawa, Reference Nishimura and Yukawa2010; Yang and Lee, Reference Yang and Lee2014) is thought to make them resistant to gut juices and thus be an adaptation to animal dispersal (Rodolphe et al., Reference Rodolphe, Severine, Michel, Pascale, Grillo and Venora2011; Suetsugu et al., Reference Suetsugu, Kawakita and Makoto Kato2015). As the testa of most orchid seeds at maturity only consists of a few cell layers (in C. formosanum only two; Lee et al., Reference Lee, Lee, Yeung and Chung2005), the lignification of cell walls could also be a necessary reinforcement, preventing collapse during periodic desiccation, during wind dispersal and after settling on an exposed surface.
Contrary to the uncertain specificity for stain techniques, vibrational spectroscopy is in principle a direct link between molecular structures and the resulting vibrational spectrum, i.e. band positions and their intensities. This link is most transparent for infrared (IR) and Raman spectroscopy, as IR and Raman spectra are dominated by fundamental transitions as opposed to near-IR spectroscopy, where spectra are dominated by overtone and combination transitions. Both IR and Raman spectroscopy can be combined with microscopy without the need for chemical fixation or staining. Fourier transform infrared (FT-IR) spectroscopy has a long record of analysis of plant materials, especially wood polymer constituents, such as cellulose, hemicelluloses and lignin (Hergert, Reference Hergert, Sarkanen and Ludwig1971; Faix, Reference Faix1991; Kačuráková et al., Reference Kačuráková, Capek, Sasinková, Wellner and Ebringerová2000). Previously we have shown that extremely small samples of orchid seeds can be analysed by the Attenuated Total Reflectance (ATR) set-up, i.e. ATR-FT-IR spectroscopy (Harrick, Reference Harrick1967), and we provided basic chemical information with a good signal-to-noise ratio on fresh and intact plant material (Barsberg et al., Reference Barsberg, Rasmussen and Kodahl2013).
An exceptional version of lignin, C-lignin, was recently discovered by nuclear magnetic resonance (NMR) spectroscopy in seed coats of certain species belonging to Orchidaceae and Cactaceae (Chen et al., Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012, Reference Chen, Tobimatsu, Jackson, Nakashima and Ralph2013). While the widespread ‘classical’ G/S lignin is mainly derived from coniferyl (G) and sinapyl (S) alcohol, C-lignin is entirely synthesized from caffeyl alcohol, which polymerizes through only one type of linkage involving the Cα and Cβ positions of one precursor and the catechol hydroxyls of the other. The resulting benzodioxane linkage adopts either cis or trans configuration (each equivalent with a pair of the R/R, S/S, S/R and R/S configurations), but in practice natural C-lignin was found to be dominated by the trans configuration (Chen et al., Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012).
Among orchids, C-lignin was identified in three species of Vanilla, but could not be detected in an unspecified Phalaenopsis (Chen et al., Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012). It was not found in association with G/S-lignin in the seeds, while G/S-lignin might occur in other organs of the same plants. However, both lignins were found together in the seed coats of several species of Euphorbiaceae and Cleomaceae, and for a single species (Cleome hassleriana) C-lignin appeared after G/S-lignin deposition within the same testa layers (Tobimatsu et al., Reference Tobimatsu, Chen, Nakashima, Escamilla-Treviño, Jackson, Dixon and Ralph2013). In a study of Cactaceae, the distribution in evolutionary clades suggested that synthesis of C-lignin is readily switched on and off (Chen et al., Reference Chen, Tobimatsu, Jackson, Nakashima and Ralph2013). C-lignin thus appears to be a selective advantage in seed survival or germination strategy of certain plant species. Its precise function in seeds, however, is unknown, as are the consequences of the preferred trans configuration. Recent theoretical work has, based on simplified assumptions, hypothesized the consequences of C-lignin structure and stereochemistry for its polymer conformation (Berstis et al., Reference Berstis, Elder, Crowley and Beckham2016).
In the present work, we again demonstrate the utility of ATR-FT-IR spectroscopy for seed analysis, using three orchid species as models, and compare the chemical compositions of the surface layer in developing seeds. As the field decays within a few micrometres from the ATR crystal surface (onto which the outer seed coat surface is placed) this method effectively analyses the outer periclinal cell wall of testa, i.e. the cell walls of the outermost cell layer (Harrick, Reference Harrick1967). We were able to obtain a reference sample of pure C-lignin (see Acknowledgements), so that we could demonstrate its FT-IR bands. This enabled us to discriminate C-lignin and G/S-lignin vibrational signatures, and thus to obtain hitherto unprecedented detailed information on seed coat chemical composition with respect to these lignins. Key questions we addressed were: (1) can the FT-IR spectrum of C-lignin be distinguished from G/S lignin?; (2) comparing three different orchid species, how does chemical composition of the testa change from fertilization to maturity?; (3) what is the role of C-lignin for seed coat properties?; and (4) can specific differences in testa composition (including C-lignin) be related to species, habitats and germination strategies?
Materials and methods
Plant material and study species
Our study organisms were Neuwiedia veratrifolia Blume (here after referred to as Neuwiedia), Cypripedium formosanum Hayata (hereafter Cypripedium) and Phalaenopsis aphrodite subsp. formosana Christenson (hereafter Phalaenopsis); these were chosen to represent three subfamilies of Orchidaceae and a variety of habitats (Fig. 1). Neuwiedia and Cypripedium each represent a basal branch in the phylogenetic tree of Orchidaceae, while Phalaenopsis belongs in one of the advanced groups (Chase et al., Reference Chase, Cameron, Barrett, Freudenstein, Dixon, Kell, Barrett and Cribb2003). Plants of Neuwiedia and Phalaenopsis were maintained in the greenhouse of the National Museum of Natural Science, Taichung, Taiwan, while Cypripedium formosanum was found on Hehuan Mountain [voucher specimens Yung-I Lee 201232, 201017 and 201402, respectively, kept in the herbarium at Taiwan National Museum of Natural Science (TNM)]. All seeds were produced by self-pollination, and after harvest were freeze-dried and stored at −20°C.

Figure 1. The three study organisms in their habitats. (A) Neuwiedia veratrifolia growing in tropical forest in leaf litter; (B) Cypripedium formosanum on forest floor of warm temperate forest on the Hehuan Mountain; (C) Phalaenopsis aphrodite growing on a tree branch in tropical forest.
The three orchid species differed greatly in mature seed size and shape, the seeds of Phalaenopsis being much smaller than the others (Fig. 2). The colour of the testa was light brown in Phalaenopsis and Cypripedium, but pale in Neuwiedia except for the strongly pigmented central part which was due to the inner seed coat adjacent to the embryo (which in our preparation was removed together with the embryo and thus not analysed).

Figure 2. Representative seeds of the three orchid species studied: Neuwiedia veratrifolia (arrow), Cypripedium formosanum (arrowhead), and Phalaenopsis aphrodite subsp. formosana (double arrowhead). Scale bar, 1 mm.
Neuwiedia is a rain forest terrestrial growing in deep leaf litter and shade under stable tropical conditions of high temperatures and year-round high humidity. It blooms in midsummer and seeds mature at the end of the year (Y.-I. Lee, personal observation). As decomposition is fast under these circumstances, we hypothesize that Neuwiedia seeds survive only a short time after dispersal and rapidly come into growth. Cypripedium is a terrestrial living in mountainous regions in warm temperate to subtropical temperatures that are seasonally fluctuating, i.e. dryer and cooler in winter. In culture it requires a period of cool temperatures to come into flower (Lee, Reference Lee2003); in nature it flowers in April and seeds mature in November. It seems likely that the seeds have a resting time after dispersal, during which they receive a period of chilling, even occasional snow coverage (Lee et al., Reference Lee, Chung, Yeung and Lee2015). Seeds of both terrestrial orchids may need to survive a passage through the digestive tract of some soil invertebrates, and are prone to attacks by micro-organisms. In contrast, Phalaenopsis is an epiphyte in tropical forests where temperatures are warm year-round (20‒30°C) and precipitation is reduced in summer. It flowers March‒April and the seeds mature in October. As seedlings with rhizoids and hyphal connections are probably more capable of staying attached to tree bark than the ungerminated seed, it would seem likely that the seeds germinate immediately after dispersal, under the warm and humid winter conditions. The Phalaenopsis seed is thus thought to largely avoid biotic threats, but desiccation could be a problem, both as dispersed seed lodged on a bark surface and as a young seedling.
Lignin reference materials
A sample of high-purity C-lignin with no detectable G/S-lignin (extracted from vanilla seed coats; see Chen et al., Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012) was kindly donated by John Ralph, Department of Biochemistry, University of Wisconsin, Madison, USA. High-purity G- and G/S-lignins were prepared from gymnosperm and angiosperm debarked wood ground into particle sizes less than 0.5 mm and 2.0 mm, respectively, the two wood types being Picea abies (L.) Karsten (Norway Spruce) and Betula pubescens Ehrh. (Downy Birch). Wood particles (0.50 g) were suspended in a solution of 9 g acetic acid:1 g demineralized water within 50 ml falcon tubes at 90°C for 4 h under mild agitation, and the solution was then passed through a pore 4 filter (nominal maximum pore size 10‒16 µm). Ten parts (w + w) deionized water was added to insolubilize lignin, and the solution was then centrifuged for 30 min to precipitate lignin. The liquid was carefully removed and the lignin rinsed three times with deionized water (3 × 50 ml). After the third rinse, the tubes that contained a thin surface layer of lignin were dried in vacuum. The lignin samples were then extracted at ca 22°C for ca 1 min with toluene (20 ml added). The samples were then centrifuged for 30 min, the toluene carefully decanted and the samples dried.
FT-IR spectroscopy
Using a stereomicroscope, embryos and inner seed coat (carapax) were removed with preparation needles and scalpel blades from 10‒12 seeds at each developmental stage. Without any chemical treatment or mounting liquid, the empty seed coats were spread on the table of the Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, Massachusetts, USA) equipped with a monolithic diamond single reflection ATR unit (PIKE Technologies, Madison, Wisconsin, USA) according to Barsberg et al. (Reference Barsberg, Rasmussen and Kodahl2013). The rigid structure and tapering ends of the seed allowed only analysis of the outer seed coat surface. A good contact between sample and ATR-crystal surface was ensured by the pressure device of the unit (maximum 30,000 psi). A typical maximum of 10 seeds (testas) were brought into contact with the ca 2 × 2 mm2 crystal surface, the actual contact area probably being much smaller than the projected area of the seeds. The temperature of the crystal surface was set at T = 30°C. For background, 200 scans were acquired and each sample spectrum was obtained from 100 scans at a resolution of 4 cm−1. The software atmospheric correction procedure was used to correct for superposed H2O and CO2 bands caused by slightly changing atmospheric conditions. All spectra were first baseline corrected by subtraction of the A-value at wavenumber position of 1900 cm−1 (i.e. a simple linear ‘flat’ line), then normalized by the integration (sum of A-values) across the 1000–1100 cm−1 interval within which all spectra attain the maximal absorbance. These normalized AN spectra are presented in arbitrary units, and are reported as the average of four to six spectra obtained for different seeds of the same species and developmental stage.
The FT-IR spectra showed high reproducibility and little variation within the same type of seed samples (see Supporting information), and characteristic bands clearly showed the chemical differences between developmental stages and between species. Some molecular components showed several characteristic bands. We focus on those bands that exhibited least overlap and could be reliably interpreted; thus we exclude the hydroxyl stretch region (ca 3100–3600 cm−1) and the region chosen for normalization (1000–1100 cm−1), where most molecular components have strongest absorption caused mainly by C-O stretching vibrations.
The observations are supported by difference spectra ΔAN = AN (j) – AN (i), which show positive and negative changes between two adjacent developmental stages i and j. Difference spectra ΔAN can reveal details not immediately seen when comparing the absolute AN spectra. Interpretation of difference spectra requires some caution as the spectra are influenced both by optical, mechanical and sample effects such as chemical (compositional) heterogeneity of sample surface layers. Thus the concern may arise that such effects are magnified in difference spectra (Harrick, Reference Harrick1967). In the supporting information we present an exemplary study of these effects for the Neuwiedia seed samples in their three developmental stages. We assess the magnitude of possible artefacts, and conclude that the difference spectra presented in the following, and the bands they reveal, can indeed be assigned the developing chemistry of the seed surface layers.
Results
Lignin references
All lignins showed typical ν(Ar) ring stretching vibrations at ca 1600 and 1510 cm−1, as well as τ(Ar-H) out of plane torsional vibrations positioned within the 800–900 cm−1 interval (Fig. 3; Larsen and Barsberg, Reference Larsen and Barsberg2010). The pure C-lignin FT-IR band structure clearly differed from wood lignins (representing G- and G/S-lignins) across the whole spectrum, but most significantly in the intervals 700‒900 cm−1 and 1400‒1650 cm−1 (Fig. 3); the 1400‒1500 cm−1 interval only contained one distinct band placed at 1440 cm−1 about midway between the two bands observed for wood lignins, and the τ(Ar-H) bands were exceptionally strong relative to the ca 1510 cm−1 band. At least three such C-lignin bands can be discerned, i.e. at 869 (medium strength), 813 (strong) and 782 cm−1 (weak shoulder). Furthermore, there was no strong band from carbonyl structures corresponding to the band at ca 1730 cm−1 for wood lignins. The centre-band position of the typical ca 1510 cm−1 lignin band is for both wood lignins at 1509 cm−1, whereas for C-lignin it is slightly displaced at 1504 cm−1. In the spectral interval ca 900‒1400 cm−1 that typically can be dominated by strong bands of other cell wall polymers, e.g. cellulose and hemicelluloses, C-lignin bands are observed at 1165, 1205 (weak), 1270 (very strong) and 1370 cm−1 (weak).

Figure 3. ATR-FT-IR spectra of extracted G/S-lignin and C-lignin (displaced vertically for clarity, absorbance A in arbitrary units).
The wood lignin spectra contained lipid impurity bands (not shown) that were removed by toluene extraction of the samples. This also contributes to identification of lignin bands as they are nearly insensitive to the extraction. G-lignin of spruce and G/S-lignin of birch gave ν(Ar) bands observed at 1592 and 1594 cm−1, respectively, but these bands are relatively broad, and both consist of at least two adjacent components. In the characteristic τ(Ar-H) interval, bands are observed at 855, 818 and 733 cm−1 (G-lignin) and 873, 820 and 727 cm−1 (G/S-lignin). For C-lignin, no band is observed near the ca 730 cm−1 position. The observed G- and G/S-lignin bands are consistent with previous studies (Hergert, Reference Hergert, Sarkanen and Ludwig1971; Vázquez et al., Reference Vázquez, Antorrena, González and Freire1997; Constant et al., Reference Constant, Wienk and Frissen2016).
Mature seed coats
At maturity, our model species showed qualitative as well as quantitative differences in seed surface chemistry (Fig. 4A). Compared with Neuwiedia and Cypripedium, Phalaenopsis showed a distinct lignin band at ca 1510 cm−1, which is weaker in Cypripedium and absent in Neuwiedia. Two lignin bands at ca 825 and 860 cm−1 were distinct and of significant intensity in Neuwiedia, less so in Cypripedium, and in Phalaenopsis only a relatively weak band at ca 830 cm−1 appeared. By comparison with the spectra of lignin references (Fig. 3), and with the more detailed information obtained from spectra for different developmental stages (see next section), these bands can be assigned C-lignin and G/S-lignin, respectively. This indicates that C-lignin was present in Neuwiedia and Cypripedium (825 and 860 cm−1 bands) but not in Phalaenopsis for which only the G/S-lignin band at 830 cm−1 was detected. All three species shared a cellulose band at 665 cm−1, but only Phalaenopsis showed the distinct (but weak) cellulose band at 897 cm−1. These bands are highly specific for cellulose (Barsberg, Reference Barsberg2010). The lipid bands at 2853, 2922 and 3009 cm−1 were most pronounced in Cypripedium and Phalaenopsis, but much less so in Neuwiedia (Fig. 4B; see also Table 1 for assignment of bands).

Figure 4. ATR-FT-IR spectra for mature seeds of three different orchid species. (A) Absolute spectra; (B) detail of the range 2800‒3050 cm‒1. Phal, Phalaenopsis aprodite (blue in B); Neuw, Neuwiedia veratrifolia (red in B); Cyp, Cypripedium formasonum (black in B).
Table 1. Characteristic lipid and lignin band positions of seed testa in mature state
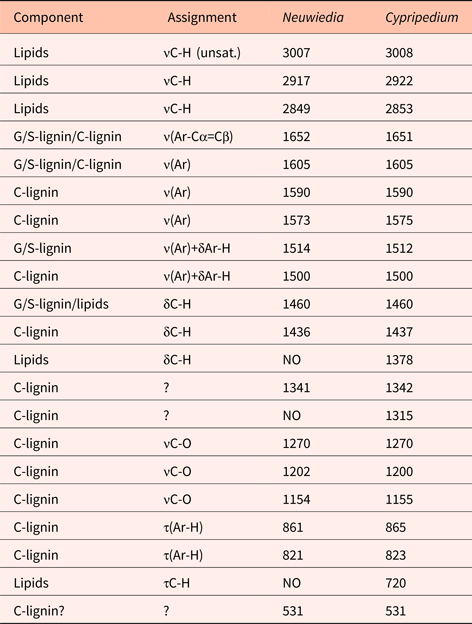
The component type, vibration type and position (in cm‒1) as observed for Neuwiedia and Cypripedium are listed. We do not list band positions for Phalaenopsis, as its seed coats contain no C-lignin, and all other bands are in practically same location as Cypripedium. The symbols ν, δ and τ denote vibrational modes dominated by bond stretching, bending or torsion, respectively. For assignments, see Hergert, Reference Hergert, Sarkanen and Ludwig1971 (G/S-lignin), and Guillén and Cabo, Reference Guillén and Cabo1997 (lipids).
Seed coat development
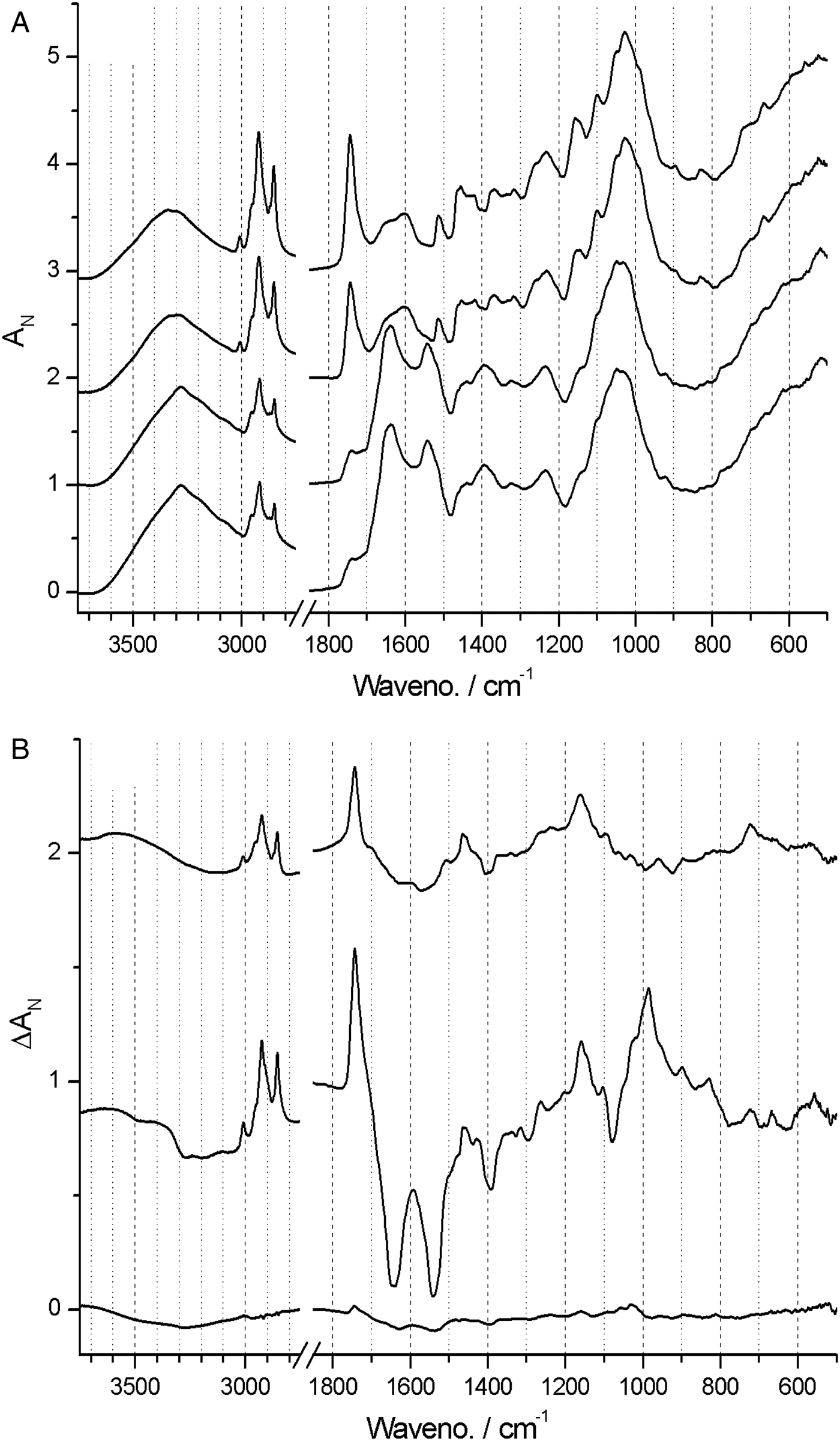
In Phalaenopsis, lignin deposition was observed only in the near-mature and mature stages of testa development (Fig. 5A) corresponding well with G/S-lignin, with main component at ca 1513 cm−1 as well as the τ(Ar-H) band at ca 830 cm−1. Cellulose deposition coincided with lignin deposition and is observed particularly at 665 and 897 cm−1 but also at ca 990 and 1155 cm−1. There was a steady build-up of lipids (bands at 3009, 2922, 2853, 1743 and 720 cm−1) also in the late stages (Guillén and Cabo, Reference Guillén and Cabo1997).
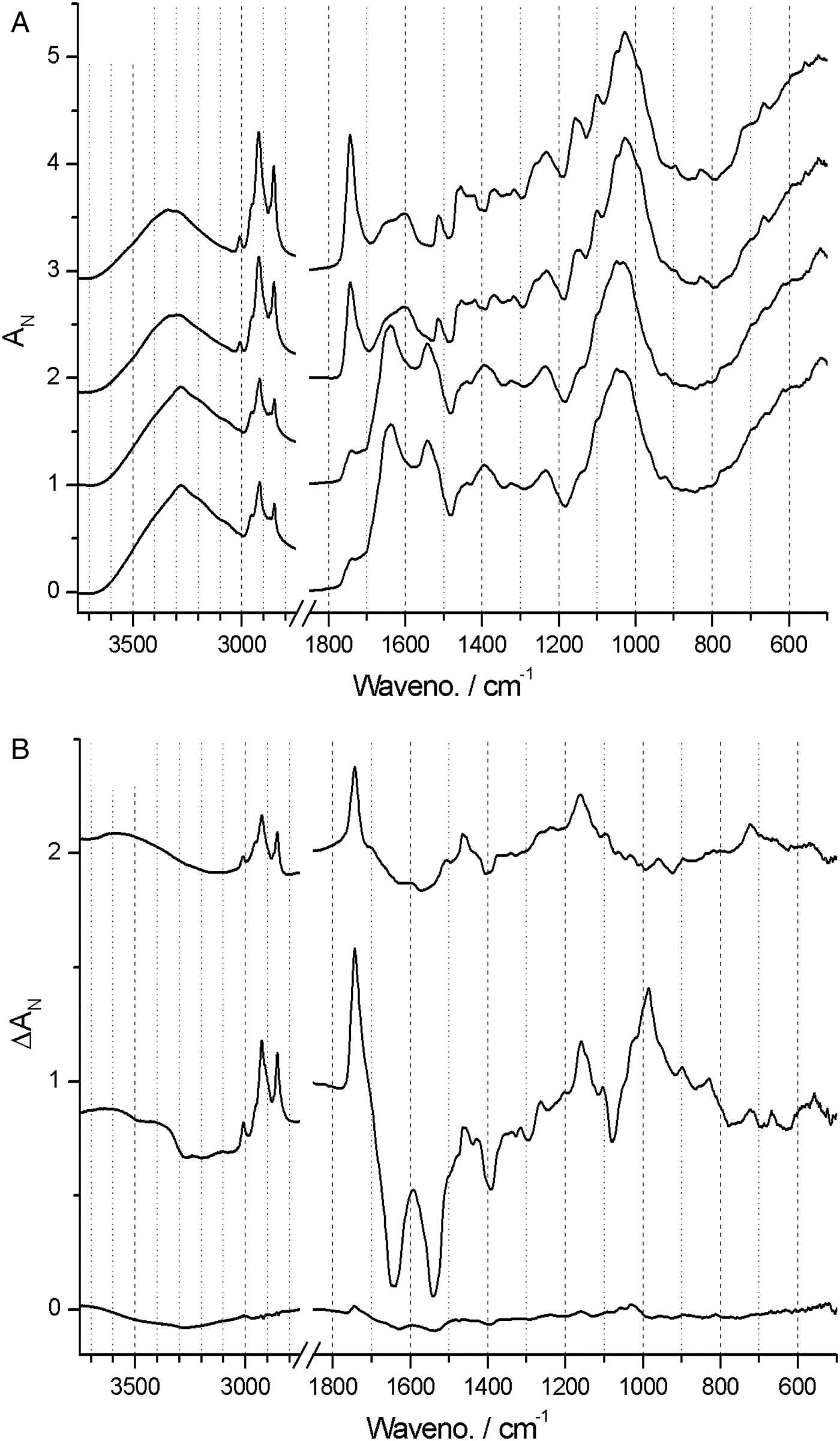
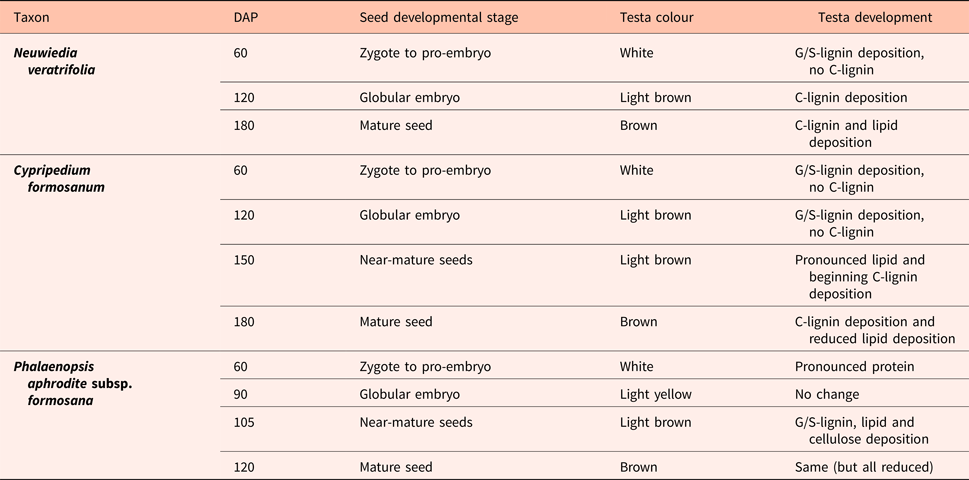
Figure 5. (A) Absolute (normalized) FT-IR spectra of young (bottom) to mature (top) stages of Phalaenopsis aphrodite seed coats at days after pollination (DAP) = 60 (young), 90, 105 and 120 (mature). (B) Difference ΔA spectra showing developmental change from one state to next state, i.e. 90‒60, 105‒90 and 120‒105 DAP.
Difference ΔAN spectra – which show the incremental spectral changes from one developmental stage to the next – allow for a more detailed account of surface chemistry changes and band assignments. In Phalaenopsis there was little surface chemistry development at early seed development, as indicated by the nearly flat first ΔAN spectrum (Fig. 5B). The remaining two ΔAN spectra enhance the changes noted above for the absolute spectra. The strong protein bands (amide I and II at 1640 and 1540 cm−1) disappeared from young to near-mature stage. Negative ΔAN of these bands is evident, and this may to some degree conceal the growth of G/S-lignin bands at ca 1600 and 1510 cm−1. The build-up of lipids and cellulose is evident. The change from near-mature to mature stage is relatively small, and is dominated by accumulation of lipids and lignin. A detailed analysis was carried out between 1480 and 1560 cm−1, i.e. the ‘valleys’ on each side of the G/S-lignin band. By simulating band shape as a sum of two Lorenzian bands and a linear baseline, the band at ca 1510 cm−1 was found to consist of two components, located at 1507 and 1517 cm−1, thus showing some heterogeneity of the G/S-lignin. The seeds of Phalaenopsis thus matured by the concomitant build-up of lipids, and of typical lignocellulosic material with G/S-lignin.
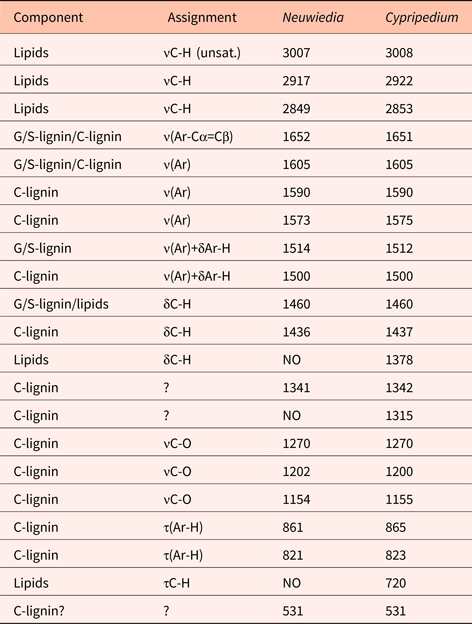
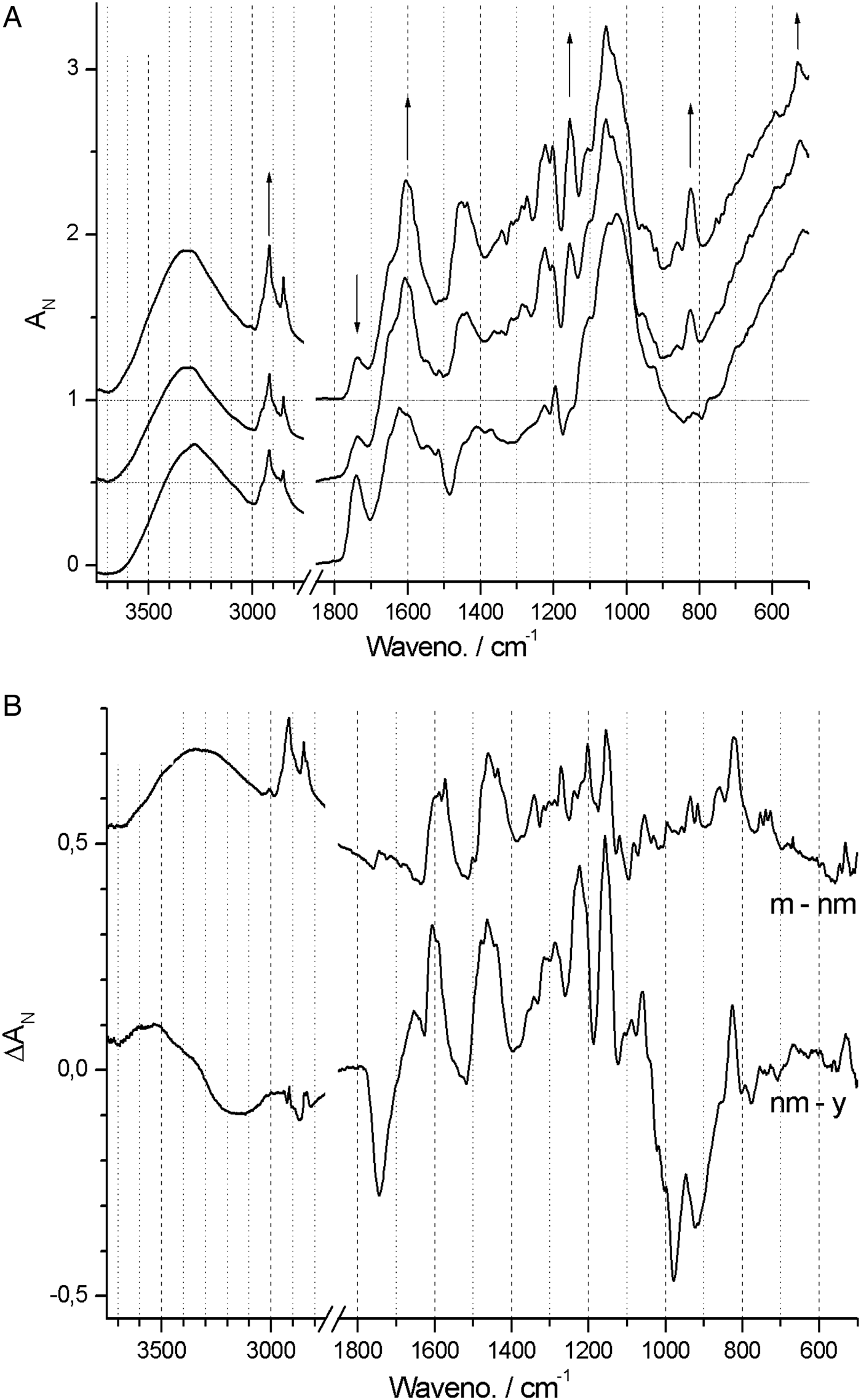
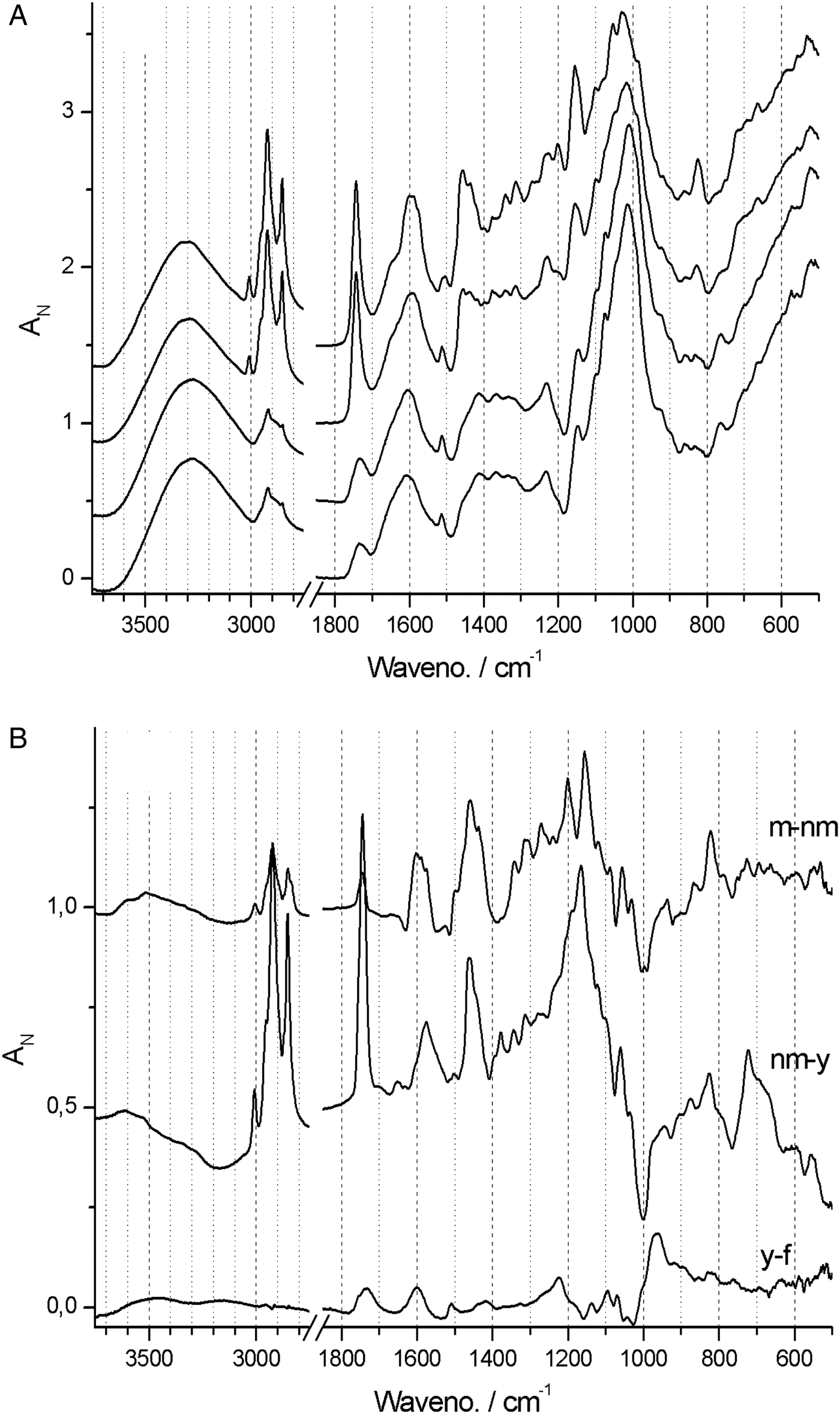
In contrast, spectra from both Neuwiedia (see Fig. 6A) and Cypripedium (see Fig. 7A) indicated lignified testa already in the earliest seed stage development with a distinct G/S-lignin band at 1514 and 1512 cm−1, respectively, but with no further accumulation of this form of lignin during seed maturation (Figs 6B and 7B). These two species differed most markedly from Phalaenopsis by their development of an additional aromatic polymer component which, by comparison with the lignin references (Fig. 3), can be assigned to C-lignin. The C-lignin component is synthesized during the later stages, and is clearly distinguished by its ν(Ar) band with down-shifted position at 1500 cm−1 and by the two observed τ(Ar-H) bands at 821 and 861 cm−1 (Neuwiedia; Fig. 6) and 823 and 865 cm−1 (Cypripedium; Fig. 7), the most intense (ca 820 cm−1 component) of which is much stronger than the 1500 cm−1 band. For Cypripedium this additional 1500 cm−1 band is observed as an apparent broadening of the 1512 cm−1 band at the mature stage. This broadening is in fact the superposition of two independent bands, i.e. the 1512 cm−1 G/S-lignin band and the 1500 cm−1 C-lignin band. The ΔAN spectra reveal the growth of the 1500 cm−1 band (not fully separable from the broad band at ca 1460 cm−1) together with the τ(Ar-H) bands, and with bands at 1155 and 1200 cm−1 (Fig. 7B). These ΔAN spectra also expose a complex build-up of the broad ca 1600 cm−1 band in terms of three discernible components at 1605, 1590 and 1575 cm−1, where the 1575 and 1590 cm−1 components also appear associated with C-lignin, cf. Table 1.
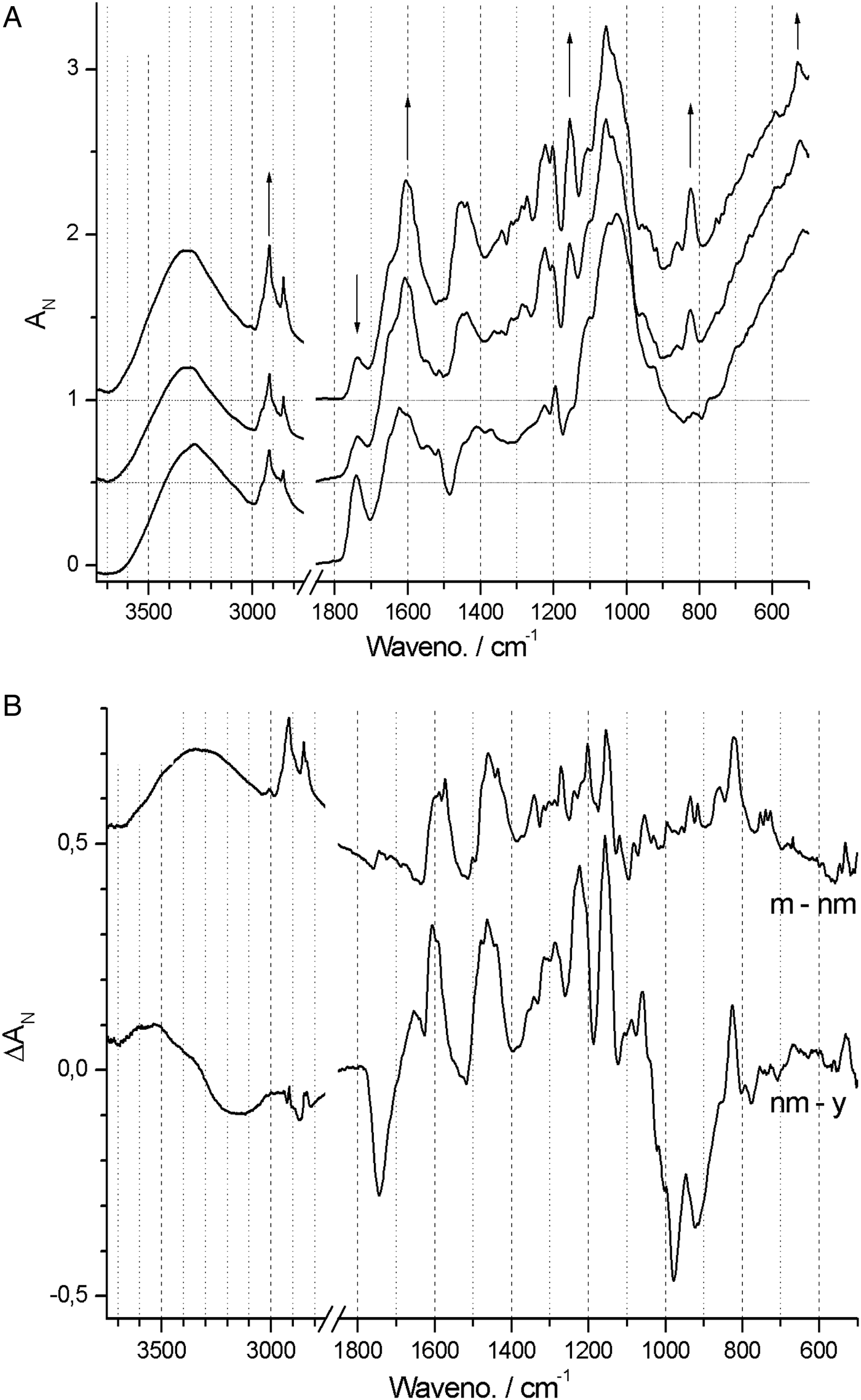
Figure 6. (A) Absolute (normalized) FT-IR spectra of Neuwiedia veratrifolia seed coats at three stages: 60, 120 and 180 days after pollination (DAP). Positions and directions of some major changes in the spectrum (mostly associated C-lignin) are marked by arrows, the latter when seeds are mature. (B) Difference ΔA spectra: 120‒60 and 180‒120 DAP.

Figure 7. (A) Absolute (normalized) FT-IR spectra of Cypripedium formosanum seed coats at 60, 120, 150 and 180 days after pollination (DAP). (B) Difference ΔA spectra: 120‒60, 150‒120 and 180‒150 DAP.
None of the 897 and 665 cm−1 cellulose bands could be detected at any stage in Neuwiedia, and only the latter could be clearly detected in near-mature and mature stages of Cypripedium, suggesting a late cellulose synthesis. In Cypripedium there was pronounced development of lipid components from young to near-mature stage (3008, 2922, 2853, 1743 and 720 cm−1) then levelling off towards the mature state. Neuwiedia showed initially a relatively modest lipid component with additional small build-up from near-mature to mature state with bands at 3007, 2917 and 2849 cm−1. The 720 cm−1 band, observed for both Cypripedium and Phalaenopsis, could not be clearly discerned. For Neuwiedia a significant decrease of the ca 1743 cm−1 band was observed from the young to near-mature state during which the lipid component showed no growth (Fig. 6B). For Cypripedium the same developmental period showed the significant growth of the lipid ca 1743 cm−1 band (Fig. 7B).
The lipid component of Cypripedium appears similar to that of Phalaenopsis (both with lipid bands at 3008, 2922 and 2853 cm−1), and the ΔAN spectra show that the lipids are deposited mainly during young to near-mature development, The different band positions for Neuwiedia (2917 and 2849 cm−1, weak 3008 cm−1 band) and the clear lack of the 1743 cm−1 band suggest that it generates a different lipid, or that the 2917 and 2849 cm−1 bands may be associated with C-lignin. The ΔAN spectra reveal a relatively broad 723 cm−1 lipid band for Cypripedium and Phalaenopsis that appears as a single component. For Neuwiedia no band is observed at that position; instead three resolved narrow components are observed at 727, 739 and 753 cm−1. As the C-lignin has no corresponding bands these three components are tentatively assigned the lipid component.
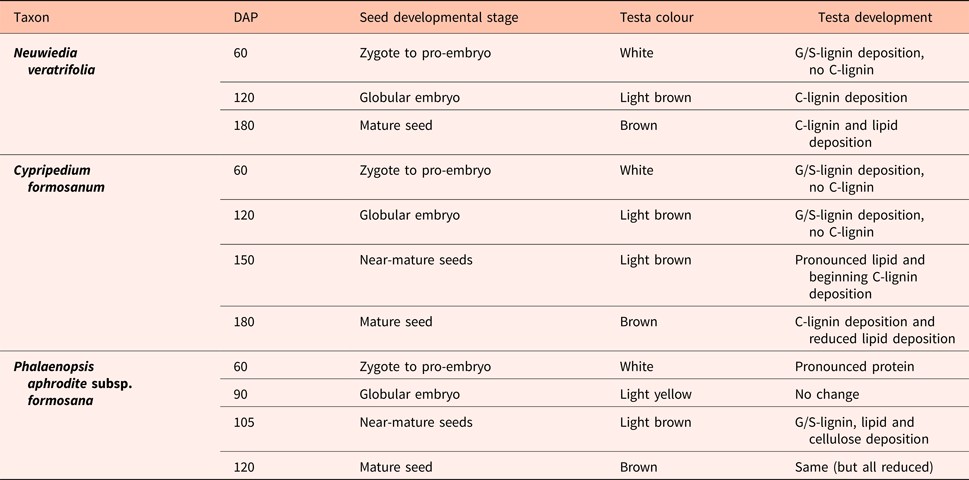
In conclusion, C-lignin deposition is initiated at the young state in Neuwiedia, and an additional lipid deposition at the near-mature state, i.e. there is a time lag between the onset of their deposition. For Cypripedium this time lag is reversed with significant deposition of a different lipid type that precedes C-lignin deposition towards the mature state. These qualitative differences are summarized in Table 2.
Table 2. Major events in seed testa chemical development as observed by FT-IR spectroscopy (Figs 5–7)
DAP, days after pollination.
Discussion
We have provided the assignment of C-lignin FT-IR bands and have found its spectrum to differ from that of G/S-lignin. It should, however, be noted that no ‘pure’ isolated G/S-lignin exists (and strictly speaking no fully representative spectrum) except for chemical features shared by various lignin preparations, e.g. its aromatic nature and ring substitution pattern. Once extracted and isolated from the cell wall the lignin isolate is inevitably chemically changed relative to its native state. Its chemical state depends on the kind of plant (species, location, etc.) and type and intensity of delignification, e.g. solvent, catalyst, temperature, etc. (Dence and Lin, Reference Dence and Lin1992; Hergert, Reference Hergert, Young and Akhtar1998). It is thus the main traits of the C-lignin versus G/S-lignin spectrum that differ as we have described. These traits have their origin in the basic structural differences that result from the different types of lignin precursor units, i.e. caffeyl alcohol versus coniferyl and sinapyl alcohol.
Thus the answer to our first key question (see end of Introduction) is affirmative. The C-lignin FT-IR spectrum is worthy of future more detailed studies. In principle, three different C-lignin sub-structures should contribute to the vibrational bands: the catechol end group, the 3,4/Cα,Cβ-ether bonded units (within the polymer), and the cinnamyl alcohol end group that contains a Cα=Cβ double bond. The relative proportion of these sub-structures, which depends on the degree of polymerization, is likely to control the relative intensity of τ(Ar-H) bands and other bands so that in situ C-lignin might not be fully represented by the extracted C-lignin reference.
Our ATR-FT-IR analysis provides a credible and detailed account of the developing chemistry of the seed coat surface, and thus enables us to answer the second key question. We chose the three model species to represent different subfamilies and expected to find many general features common to the whole family. It was surprising that the species differed so markedly in seed coat chemistry, both in mature composition and in the timing of deposition of different molecular components. The ontogenetic results strongly suggest that deposition order, and relative amounts of lipids, G/S-lignin and C-lignin, determine fundamental properties of the surface layers that are significant for seed biology. Differing seed surface chemistries could differentiate dormancy patterns, substrate attachment, stress tolerance and longevity of orchid seeds, all of which could participate as drivers in speciation. At this stage, however, the functional importance of this diversity is unclear.
These few examples give a first glimpse of what could be a tremendous seed diversity beyond morphology. The chemical build-up of the seed coat might also have implications for practical propagation. Rather harsh pre-treatments are routinely used for encouraging germination in vitro, presumably because inhibiting substances in this way are extracted, i.e. chemical scarification (e.g. Thompson et al., Reference Thompson, Edwards and Van Staden2001; Zeng et al., Reference Zeng, Zhang, Teixeira da Silva, Wu, Zhang and Duan2014). The required duration of pre-treatment may depend on the quality and quantity of chemicals deposited in the testa of species and seed provenance in question.
Our third key question concerned the role of C-lignin in seeds. Based on previous reports (Carlson, Reference Carlson1940; Chen et al., 2011; Barsberg et al., Reference Barsberg, Rasmussen and Kodahl2013) and our present work it appears that G/S-lignin is a common feature in testa of all orchid seeds, whereas C-lignin is not consistently present (Table 3). The seed coats of Cypripedium calceolus and Dactylorhiza incarnata showed characteristic G/S-lignin bands (Barsberg et al., Reference Barsberg, Rasmussen and Kodahl2013), but they did not show the strong bands at ca 825 and 860 cm−1 that we now can assign to C-lignin. A previous work found both lignins in seed coats of other angiosperm families: Cactaceae (Chen et al., Reference Chen, Tobimatsu, Jackson, Nakashima and Ralph2013), Euphorbiaceae and Cleomaceae (Tobimatsu et al., Reference Tobimatsu, Chen, Nakashima, Escamilla-Treviño, Jackson, Dixon and Ralph2013), although without consistency, i.e. several species in both families did not contain C-lignin in their seed coats.
Table 3. Seed testa C-lignin occurrence and germination strategies of orchid species
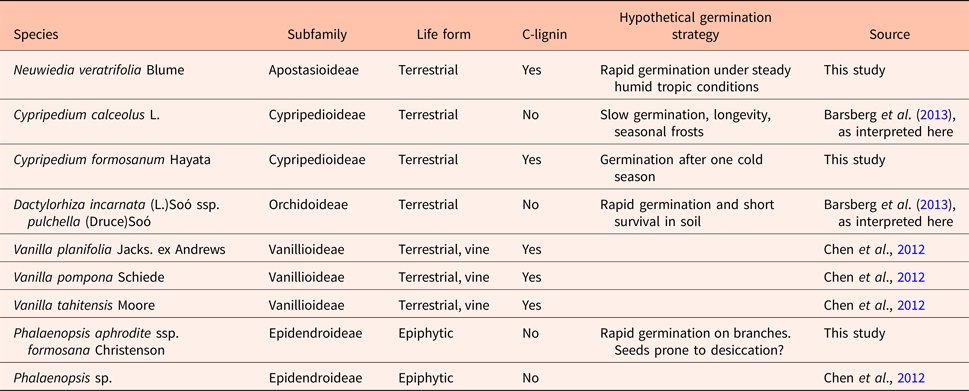
Data on C-lignin presence in Cypripedium calceolus and Dactylorhiza majalis were obtained by revisiting FT-IR spectra previously published (Barsberg et al., Reference Barsberg, Rasmussen and Kodahl2013). Germination strategy indicated here should be treated with reservation, as there is little experimental evidence to germination in situ (however, see Rasmussen and Pedersen, Reference Rasmussen and Pedersen2011 for C. calceolus). In the case of Dactylorhiza incarnata, we rely on Sletvold et al. (Reference Sletvold, Øien and Moen2010), for a study of a related species with no lasting seed bank. Most other information in that column is thus based on observation in nature and qualified assumptions (see section on study species in Materials and methods, and Discussion).
There does not seem to be a close connection between seed coat pigmentation and C-lignin (cf. Fig. 2 with the fact that Phalaenopsis did not develop any). The dark seed coat of Vanilla seeds known to contain C-lignin corresponds to the light brown membranous testas in our study species of which two contained C-lignin (Fig. 2). The degree of pigmentation could be regulated by C-lignin concentration, or thickness of a possible C-lignin layer, but also other substances such as tannins, fatty acids, etc. could contribute differently between C-lignin-containing species. The dark mid-portion of the seeds of Neuwiedia and Cypripedium is a pigmentation of the inner integument (carapax) that was not analysed here.
The preferred single-type (β-O-4) and dominant all-trans linkage suggest spatially linear or possibly helical conformations of C-lignin oligomers or polymers (Berstis et al., Reference Berstis, Elder, Crowley and Beckham2016). G/S-lignin polymerization results in no regular repeating structure, but in a statistical occurrence of several different linkage types with no (strongly) preferred linkage stereochemistry. The occurrence of G-units also enables branching of G/S-lignin through the so-called ‘dibenzodioxocin’ linkages that are composed of three instead of two units. G/S-lignin is thus disordered and has no detectable optical activity.
These significant differences of C-lignin versus G/S-lignin structure suggest different functional roles for seed coat properties (Tobimatsu et al., Reference Tobimatsu, Chen, Nakashima, Escamilla-Treviño, Jackson, Dixon and Ralph2013). The seeds of Phalaenopsis are much smaller than those of terrestrial orchids, have less hydrophobicity and the G/S-lignin is primarily deposited in radial walls of the testa (Lee et al., Reference Lee, Yeung, Lee and Chung2008). This suggests that G/S-lignin can also be present as a strengthening of the testa without adding significantly to hydrophobicity of the seed. In general, however, in mature orchid seeds, both C-lignin, G/S-lignin and lipids could contribute to the hydrophobic nature.
If the suggested linear structure of C-lignin holds true, then cooperative inter-molecular interactions (dispersion and/or hydrogen bonding) may enable dense parallel packing of C-lignin chains to form a hydrophobic impermeable seed coat barrier. A slight thermodynamic preference for the trans- over the cis-linkage has been predicted (Berstis et al., Reference Berstis, Elder, Crowley and Beckham2016), and a dominant 96–97% occurrence of the trans-linkage is observed (Chen et al., Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012). The implications of this may be that a mixed trans/cis stereochemistry could reduce structural order and worsen the barrier properties of C-lignin. We may thus speculate that the simple build-up of the C-lignin polymer is part of nature's recipe towards optimizing seed coat properties. C-lignin supramolecular organization may furthermore allow for a large contact surface of strong interaction with long carbon chains of lipid structures.
The biosynthetic pathways to C-lignin or G/S-lignin are very similar. Indeed, C-lignin precursor caffeyl alcohol leads to the G-lignin precursor coniferyl alcohol by a single methylation step of a catechyl hydroxyl group. In contrast to previous orchid seed coat studies (Chen et al., Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012) we found both types of lignin to be synthesized in the same seed coat sequentially, apparently by a switch in enzyme activity during seed maturation. This appears to be a general trait across very diverse plant species not just limited to orchids (Tobimatsu, Reference Tobimatsu, Chen, Nakashima, Escamilla-Treviño, Jackson, Dixon and Ralph2013). In general it is hard to tell whether all testa cells contain the whole synthetic machinery, or if production of certain cell wall components is allocated to particular cell types within the testa. However, considering the high surface specificity of the ATR-FT-IR set-up (see Introduction), the C-lignin and G/S-lignin deposition that we found must occur in the outer integument in the outer periclinal cell walls. Thus the temporal switch from G/S-lignin to C-lignin synthesis entails no spatial switch.
Our final question arises in the broader context of whether we can link the chemistry and presumed functions of the two types of lignin to species, habitats and germination strategies?
Although the molecular structure of C-lignin strongly suggests a functional difference relative to G/S-lignin, it is too early to speculate about what the implications are for germination biology of the seed in question. Looking at the assumed germination strategies that we outlined in introduction of our study species, we find C-lignin both in species with presumed rapid germination (Neuwiedia) and with germination that is probably delayed by a chilling period (Cypripedium formosanum; Table 3). We also find lack of C-lignin in similar situations (Dactylorhiza and Phalaenopsis versus Cypripedium calceolus). The occurrence of C-lignin is not linked to the tropics, cf. Neuwiedia and Vanilla-species versus Phalaenopsis (all tropical but only the two former producing C-lignin), or to the terrestrial life form (terrestrial species C. calceolus and Dactylorhiza incarnata lacking C-lignin, but C. formosanum developing it). The phylogenetic trends are also unclear. Among the about 100 Vanilla species, the three studied by Chen et al. (Reference Chen, Tobimatsu, Havkin-Frenkeld, Dixona and Ralph2012) are closely related. Indeed, V. tahitensis is considered a hybrid with V. planifolia as one parent, and V. pompona belongs in the same subgenus, all of which are Neotropical species (Cameron, Reference Cameron, Havkin-Frenkel and Belanger2011). As they share the C-lignin development, it could be a common derived character in that clade.
The smooth seed surface of Vanilla species (Cameron and Chase, Reference Cameron and Chase1998) differs markedly from most orchid seeds, such as those studied here, and we find that among orchids, the presence of C-lignin is not associated exclusively to Vanilla seeds. The fact that the two Cypripedium species that have been looked at so far differ in C-lignin development, suggests that this feature is not conservative, a conclusion also drawn for other plant families (Chen et al., Reference Chen, Tobimatsu, Jackson, Nakashima and Ralph2013; Tobimatsu et al., Reference Tobimatsu, Chen, Nakashima, Escamilla-Treviño, Jackson, Dixon and Ralph2013). As we found that it arises near the termination of seed development, C-lignin could simply be lost by a shortening of the time spent in the ovary, i.e. the stage of its production is not reached before the seed is released. As long as the functional effects of C-lignin are unclear, and cannot be confidently related to habitats and germination strategies, positive or negative selection cannot be inferred.
As for the C-lignin, it appears that no unequivocal answer can be given for the functional role of lipids. We use the term ‘lipids’ in a broad sense since the characteristic bands we observe at ca 2850 and 2920 cm−1 can generally be expected for all components containing long > C10 saturated carbon chains, e.g. suberin and/or cuticular components as well. The 1743 cm−1 band (ester carbonyl) suggests a triglyceride lipid type for both Phalaenopsis and Cypripedium of this study, but of these two only Cypripedium seed coats developed C-lignin. Previously published spectra of C. calceolus and D. incarnata suggested a lack of lipids (fig. 4b in Barsberg et al., Reference Barsberg, Rasmussen and Kodahl2013), but their weak presence may have been overlooked. These lipids do not have a strong band at 1743 cm−1, as for Phalaenopsis and Cypripedium (respectively Figs 5 and 7) in the present study, but do appear comparable to the lipids of Neuwiedia seed coats (Fig. 6). Thus species within the same genus, Cypripedium calceolus and C. formosanum, but not very closely related (Fatihah et al., Reference Fatihah, Fay and Maxted2011; Li et al., Reference Li, Liu, Salazar, Bernhardt, Perner, Yukawa, Jin, Chung and Luo2011), with no known difference in germination strategy, differed in respect to C-lignin content (only C. formosanum produced C-lignin; Table 3) as well as in the amount of seed coat lipids. We know that C. calceolus may survive many years in the ground (Rasmussen and Pedersen, Reference Rasmussen and Pedersen2011) so it seems that neither C-lignin nor heavy lipid deposits are necessary requirements for seed longevity.
Conclusions
We have conducted a combined FT-IR spectroscopic study of orchid seed coat ontogenesis with a special focus on C-lignin, G/S-lignin and lipids for seed coat chemistry and properties. In order to distinguish these components we have provided and assigned the characteristic FT-IR bands of C-lignin for the first time.
ATR-FT-IR spectroscopy can discriminate C-lignin from G/S-lignin and can thus monitor their deposition in orchid seed coats in days after pollination. The method is specific for the outermost seed coat layer. We discriminated and detected other molecular components including various lipids in the seed coats, and demonstrated a surprising variability in seed coat chemistry among orchid species. The previously observed preference of C-lignin for formation of its trans linkage form, and its predicted near-linear extended polymer conformations suggest its arrangement into dense hydrophobic films or barriers, fundamentally different from the expected more complex amorphous polymers of G/S-lignin. Such arrangement of C-lignin might also be optimal for interactions with lipid components to improve overall barrier properties and resistance of the seed coat.
We found, however, no obvious correlation between differential lignin and lipid contents in seed coats, and assumed germination niches or habitats of the studied species. This conclusion remains the same when we included a few other species from existing studies, and it may hold true also for the seed coats of the other plant families studied recently. To obtain more information on the impact of C-lignin versus G/S-lignin, we need studies where a known germination niche provides a background for setting up realistic environmental experiments, challenging the seeds for instance by weathering and microbial attacks, monitoring seed coat changes chemically, and recording germination responses. Our unsuccessful quest for functional implications may also suggest that germination ecology depends on a syndrome of chemical characters, and not just the type of lignin developed.
With the recent discovery of C-lignin, studies of its molecular properties and functional implications are only beginning, and many questions are unanswered. We have demonstrated its involvement in seed coat compositional development for several orchid species, and in that context we have identified several promising future investigation paths.
Acknowledgements
We thank John Ralph for donating a C-lignin sample for this study. We thank Mr Sheng-Kun Yu for providing the photograph of the natural habitat of Phalaenopsis aprodite subsp. formosana, and Dr Tim Wing Yam for providing the photograph of the natural habitat of Neuwiedia veratrifolia. This work was supported by the funding from the National Museum of Natural Science, Taiwan to Y.-I.L.
Supplemental material
Supporting information: Figures S1 to S3. To view supplementary material for this article, please visit https://doi.org/10.1017/S0960258517000344.