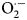Introduction
Vegetative growth and yield of crops are largely determined by the germination capacity of seeds and the hardiness of seedlings. Germination begins with imbibition and ends when the radicle breaks through the surrounding tissues, usually including the endosperm and testa, after which seedling growth begins (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013; Penfield, Reference Penfield2017). Seed germination is an extremely complex process regulated by many internal and external factors. Among them, abscisic acid (ABA) and gibberellin (GAs) are the most important phytohormones regulating germination (Díaz-Vivancos et al., Reference Díaz-Vivancos, Barba-Espín and Hernández2013; Shu et al., Reference Shu, Liu, Xie and He2016; Shuai et al., Reference Shuai, Meng, Luo, Chen, Zhou, Dai, Qi, Du, Yang, Liu, Yang and Shu2017; Tuan et al., Reference Tuan, Kumar, Rehal, Toora and Ayele2018). In many seeds, ABA content decreases rapidly during imbibition, while GAs content gradually increases (Yamaguchi et al., Reference Yamaguchi, Kamiya, Nambara, Bradford and Nonogaki2007). Phytohormones may regulate seed germination by affecting the activity of hydrolytic enzymes, or by regulating the accumulation of non-enzymatic factors such as reactive oxygen species (ROS) (Ye et al., Reference Ye, Zhu, Liu, Zhang, Li, Liu, Shi, Jia and Zhang2012; Bailly, Reference Bailly2019). Likewise, some phytohormones may also alter endo-β-mannanases, other endoglucanases, and expansins involved in modifying the polysaccharides of the cell wall matrix of the endosperm and radicle (Rodríguez-Gacio et al., Reference Rodríguez-Gacio, Iglesias-Fernández, Carbonero and Matilla2012; Yan et al., Reference Yan, Wu, Yan, Hu, Ali and Gan2014; Cosgrove, Reference Cosgrove2016). These cell wall hydrolases and ROS are critical for cleaving the glycosidic bonds of cellulose and hemicellulose polysaccharides that constitute the skeleton of the cell wall, thus loosening the cell wall during seed germination (Sampedro et al., Reference Sampedro, Valdivia, Fraga, Iglesias, Revilla and Zarra2017). For example, regulation of lettuce seed germination by ABA and ethylene is achieved by inhibiting or enhancing the activity of cellulase in the micropylar endosperm and radicle, respectively (Chen et al., Reference Chen, Ma, Xu and Wang2016a). When rice seeds are imbibed in ABA solution, both the germination rate and endogenous ROS generation are reduced (Ye et al., Reference Ye, Zhu, Liu, Zhang, Li, Liu, Shi, Jia and Zhang2012; Bailly, Reference Bailly2019). In addition, chemical agents such as sodium dichloroisocyanurate (SDIC) and guazatine inhibit ROS accumulation and germination in lettuce and rice seeds, while germination is restored with exogenous ROS (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b; Chen et al., Reference Chen, Li, Gao, Chen, Zhang, Liu and Chen2016b).
Coumarin is an unsaturated lactone and plant allelochemical. The relationship between coumarin and seed germination has been studied since the middle of the last century, when it was found that it could effectively inhibit lettuce seed germination and seedling growth (Khan and Tolbert, Reference Khan and Tolbert1966). Although the concentration of GAs in lettuce seeds and seedlings is inhibited by coumarin, the addition of exogenous GAs cannot reverse the inhibitory effect of coumarin on germination and seedling growth (Khan and Tolbert, Reference Khan and Tolbert1966; Berrie et al., Reference Berrie, Parker, Knights and Hendrie1968). Imbibing sorghum seeds in the presence of coumarin strongly inhibits catalase (CAT), superoxide dismutase (SOD) and ascorbic acid peroxidase (APX), the main enzymes responsible for ROS detoxification (Wang et al., Reference Wang, Yao, Xu, Wu, Zhao and Hua2017). Furthermore, as ROS production is prevented by inhibitors such as diphenylene iodonium chloride, adenine, SDIC among others, seed germination is also delayed (Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009; Zhang et al., Reference Zhang, Zhang, Yang, Wang, Sun, Li, Cao, Weeda, Zhao, Ren and Guo2014a). Previously, we found that coumarin inhibits rice germination by suppressing ABA catabolism (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019).
Although the underlying mechanisms for seed dormancy and germination have been well studied in model plants such as Arabidopsis thaliana, there are many unsolved questions about these mechanisms in crop seeds. Examples of pressing agricultural problems include a low germination rate and poor seedling vigour in the field following direct sowing of crop seeds and preharvest sprouting of cereal crops that are exposed to high temperature and humidity during maturation (Paulsen and Auld, Reference Paulsen, Auld, Benech-Arnold and Sánchez2004). We previously found that coumarin effectively delayed preharvest sprouting in cereal crops and improved crop seedling vigour (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). As one of the vegetables widely planted in South China, the seed germination characteristics of Brassica parachinensis and the underlying mechanism of coumarin regulating germination are largely elusive. In this work, we investigated the changes in phytohormones, ROS and enzyme activities of B. parachinensis seeds imbibed in water and coumarin, and showed that GAs promoted germination by increasing ROS accumulation, while coumarin delayed seed germination by inhibiting GAs and ROS production.
Material and methods
Plant material and growth conditions
B. parachinensis L. cv. Lvbao seeds were provided by the vegetable research institute, Guangdong academy of agricultural sciences, Guangdong Province, China. For germination assays, seeds were placed in a transparent plastic germination box (12 cm × 12 cm × 6 cm) containing two layers of filter paper soaked in 25 ml water or test solution [200 μM coumarin, 0.1 μM GA3, 0.1 μM GA4+7, 10 μM fluridone, 5 μM paclobutrazol (PC) and their combinations]. By definition, the process before the radicle breaks through the seed coat belongs to germination and the process afterwards belongs to the seedling growth stage (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013). In this study, 10 h was the time point for seed germination, after which the germinated seeds gradually grew into seedlings. Therefore, we sampled seeds and seedling at 10 h and 4 d after imbibition, respectively. To monitor seedling growth, individual germinated seeds were placed in separate holes of seed germination bags, and then 25 ml water or test solution was added to the bag. Seeds and seedlings were incubated in a growth chamber at 28 ± 1°C under a 16-h light/8-h dark photocycle (10,000 lux).
Evaluation of germination and seedling growth
After the start of imbibition, germinated seeds were counted at 2-h intervals from 8 to 24 h. The number of germinated seeds at each time point was converted to a percentage, and the mean value ± SE of three biological replicates of 100 seeds was calculated. Seeds were photographed using a stereomicroscope (SteREO Lumar V12, Zeiss, Germany, https://www.zeiss.com/microscopy/int/products/stereo-zoom-microscopes/stereo-discovery-v12.html). Germinated seeds were grown in germination bags for 7 d. Seedlings were photographed, root and seedling lengths were measured with a ruler, and fresh and dry weights were measured with an electronic balance (accuracy 0.0001 g).
Determination of seed viability and seedling vigour
The triphenyl tetrazolium chloride (TTC) test was used to determine seed viability (Li et al., Reference Li, Chen, Chen, Gao, Chen and Liu2017). Seeds were incubated in water or 200 μM coumarin for 10 h, and then ten seeds with intact testa or testa removed were selected and stained with 0.5% TTC at 35°C for 30 min, washed three times with water and photographed as described above.
Seedling vigour was evaluated by staining with trypan blue (Xiao et al., Reference Xiao, Wang, Hu, Zhou and Huang2019 with slight modifications). The trypan blue is taken up only by dead cells (Duan et al., Reference Duan, Zhang, Li, Wang, Li, Han, Zhang and Li2010). Trypan blue dye was prepared at 1% in distilled water and stored at 4°C. Five seedlings incubated in water or 200 μM coumarin for 72 h were placed in 10-ml glass tubes, trypan blue dye (5 ml) was added and seedlings were stained on a vortex shaker for 15 min. Seedlings were rinsed with tap water and photographed as described above.
Measurement of endogenous phytohormone levels
Hormone extraction and quantitative analysis were carried out by MetWare (http://www.metware.cn/). B. parachinensis seeds imbibed in water or 200 μM coumarin for 10 h and 4 d were sampled for hormone determination. Seeds or seedlings (1.5 g) were frozen in liquid nitrogen, ground to powder and extracted with 1 ml of methanol/water/formic acid (15:4:1, v/v/v) containing the corresponding internal standard [d6-ABA for ABA and ABA glucose ester (ABA-GE), d2-GA4 for GA1, GA3, GA4, GA7, GA19, GA20 and GA24, d2-GA9 for GA9, d2-GA15 for GA15, d2-GA53 for GA53, respectively]. All of the internal standards were purchased from Olchemim Ltd. (Olomouc, Czech Republic). The combined extracts were evaporated to dryness under nitrogen gas stream, reconstituted in 100 μl of 80% methanol (v/v) and filtered through a 0.22 μm filter. The supernatant was collected for analysis using an UPLC-ESI-MS/MS system (UPLC, ExionLC™; MS, Applied Biosystems 6500 Triple Quadrupole). Standard curves and representative ion chromatograms of samples are shown in supplementary Fig. S7.
In situ detection of  ${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase in seeds
${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase in seeds
Nitroblue tetrazolium (NBT), 3,3′-diaminobenzidine hydrochloride (DAB) and 3,3′,5,5′-tetramethylbenzidine (TMB) were used to stain seeds for ![]() ${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase activity (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Seeds were imbibed in water, 200 μM coumarin, 5 μM PC, 0.1 μM GAs, or a combination of GAs + coumarin, for 10 h. Then, five seeds (10 h) with intact testa and with testa removed were incubated in 1 mM NBT in 10 mM Tris–HCl (pH 7.0), 1 mg/ml DAB in acetate buffer (pH 3.8) or 0.2% TMB in 20 mM potassium phosphate (pH 6.5) at room temperature for 10 min, washed with double-distilled water and photographed as described.
${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase activity (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Seeds were imbibed in water, 200 μM coumarin, 5 μM PC, 0.1 μM GAs, or a combination of GAs + coumarin, for 10 h. Then, five seeds (10 h) with intact testa and with testa removed were incubated in 1 mM NBT in 10 mM Tris–HCl (pH 7.0), 1 mg/ml DAB in acetate buffer (pH 3.8) or 0.2% TMB in 20 mM potassium phosphate (pH 6.5) at room temperature for 10 min, washed with double-distilled water and photographed as described.
Quantification of  ${\rm O}_2^{{\cdot}{-}} $ and H2O2 in seeds
${\rm O}_2^{{\cdot}{-}} $ and H2O2 in seeds
The rate of ![]() ${\rm O}_2^{{\cdot}{-}} $ production (nmol
${\rm O}_2^{{\cdot}{-}} $ production (nmol ![]() ${\rm O}_2^{{\cdot}{-}} $ min−1 g−1 fresh weight) and the concentration of H2O2 (μmol g−1 fresh weight) were measured spectrophotometrically as described before (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b; Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Seeds (100) were imbibed in each of the aforementioned solutions for 6–14 h, and mean values ± SE of three biological replicates were calculated.
${\rm O}_2^{{\cdot}{-}} $ min−1 g−1 fresh weight) and the concentration of H2O2 (μmol g−1 fresh weight) were measured spectrophotometrically as described before (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b; Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Seeds (100) were imbibed in each of the aforementioned solutions for 6–14 h, and mean values ± SE of three biological replicates were calculated.
Determination of enzyme activities and soluble sugars and proline content
Frozen seeds (0.5 g) were homogenised on ice with 1 ml of 50 mM potassium phosphate (pH 7.0), 1 mM EDTA and 1% polyvinylpyrrolidone (PVP). Each homogenate was centrifuged at 12,000g for 30 min at 4°C, and the supernatant was used for enzyme assays. The activities of SOD, CAT and APX were determined spectrophotometrically according to Ye et al. (Reference Ye, Li and Zhu2014). The activities of α- and β-amylase were determined according to Nandi et al. (Reference Nandi, Das and Sen-Mandi1995). Proline, soluble sugars and malondialdehyde (MDA) content were, respectively, determined by the sulfosalicylic acid, anthrone and thiobarbituric acid methods (Zhang, Reference Zhang2009).
Statistical analysis
Data are presented as the mean ± SE of three replicates. One-way analysis of variance was used to compare mean values, and when significant, differences between individual means were compared with the Fisher's least-significant difference test (P < 0.05).
Results
Germination of B. parachinensis seeds is inhibited by coumarin and restored by GAs
The germination of B. parachinensis seeds was determined with and without testa. When intact seeds were imbibed in water, the seed coat (testa) first ruptured near the radicle and endosperm cap, and then the radicle broke through the endosperm cap, thus completing germination (Fig. 1a). In contrast, when we removed the testa of seeds before imbibition, the micropylar endosperm is also inevitably destroyed, for it is tightly adjacent to the testa. As a result, the radicle breaking through the endosperm was invisible for seeds with removed testa during germination (Fig. 1b).

Fig. 1. Morphology of B. parachinensis seeds imbibed in water for 10 h. (a) Intact seed and (b) seed with testa removed.
As shown in Fig. 2, radicle emergence begins at 10 h of imbibition and accelerated in the next 4 h, reaching >80% germination at 14 h. Emergence of the remaining seeds was relatively slow, requiring an extra 6 h (total 20 h of imbibition) to reach 99%. Coumarin inhibited emergence and had a significant dose effect (supplementary Fig. S1), similar to its effect on rice seed germination (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Seeds imbibed in 200 μM coumarin started their radicle emergence at ~10 h, similar to control, but the subsequent rate of emergence was significantly reduced (Fig. 2a). The time required for seeds to reach 50% emergence increased from 13 to 17.5 h, and the final emergence percentage fell from 99 to 94% (Table 1). Low concentrations of GA3 and GA4+7 showed promotion of seed germination, of which a concentration 0.1 μM showed the better effect, whereas high concentration of GAs reduced the germination rate to some extent (supplementary Figs S2 and S3). The presence of 0.1 μM GA3 or GA4+7 partially restored the rate of radicle emergence, GA4+7 being the most effective. The effects of fluridone (an inhibitor of ABA synthesis) and PC (an inhibitor of GA synthesis) on the coumarin-mediated inhibition of radicle emergence showed that fluridone failed to reverse the inhibitory effect while PC promoted it (Fig. 2a). In addition, we plotted a corresponding probability of germination distribution curve (Fig. 2b) based on the observed germination rates. As described by Bewley et al. (Reference Bewley, Bradford, Hilhorst and Nonogaki2013), this can reveal the timing, uniformity and extent of germination in a seed population. For example, of the seeds imbibed in water, 0.1 μM GA3 and 0.1 μM GA4+7, most of the seeds completed germination around 14 h, and the probability of seed germination at other time points was small (Fig. 2b).

Fig. 2. Germination time course (a) and probability of germination (b) for B. parachinensis seeds imbibed in water, 200 μM coumarin alone or 200 μM coumarin plus 0.1 μM GA4+7, 0.1 μM GA3, 10 μM fluridone or 5 μM PC. Germinated seeds were counted every 2 h for 24 h, and the results are presented as percent cumulative germination. Data represent the mean ± SE of three biological replicates of 100 seeds each.
Table 1. Effect of exogenous ROS and inducers of ROS generation on the time required to obtain 50% of testa rupture and the final germination percentage of B. parachinensis seeds

Data are means ± SE of three biological replicates of 100 seeds each.
Seedling root growth is inhibited by coumarin and partially restored by GA4+7
To observe the effect of coumarin and GAs on germinated seeds and seedlings, we used seed germination bags to monitor seedling growth in real time (Fig. 3a–f). The average root and sprout lengths of control seedlings were 3.2 and 2.1 cm, respectively (Fig. 3g). Coumarin significantly suppressed root growth (average length, 1.5 cm) and increased the number of root hairs on the main root, but had only a slight effect on sprout growth (Fig. 3b, g). On the other hand, GA3 had no obvious effect on seedling growth, whereas GA4+7 significantly promoted root growth (Fig. 3a, c, e, g). Moreover, GA4+7 but not GA3 had a restorative effect on coumarin-suppressed roots (Fig. 3d, f, g). All treatments increased the fresh weight of seedlings but had no detectable effect on dry weight (Fig. 3h).

Fig. 3. Changes in morphology (a–f) and physiological indices (g,h) of B. parachinensis seedlings incubated in water, 200 μM coumarin, 0.1 μM GA3, 0.1 μM GA4+7 or combinations of coumarin and GA3 or GA4+7. Data for seedling length and weight were collected at 7 d after germination and represent the mean ± SE of three biological replicates of 100 seeds each. Means denoted by the same letter did not significantly differ at P < 0.05 according to the Fisher's least-significant difference test.
Seed viability is decreased by coumarin, yet seedling vigour is retained
In the imbibed control seeds, the embryo, radicle and cotyledons were all stained by TTC (Fig. 4a, b). However, the staining of the radicle and endosperm cap was reduced in the presence of 200 μM coumarin (Fig. 4d, e), demonstrating that coumarin specifically decreased the viability of the radicle and endosperm cap but not of other parts of the seed. This result is similar to changes in the viability of lettuce seeds imbibed in SDIC, a seed germination inhibitor (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b). Although coumarin inhibited root elongation of the seedlings, there was no significant difference in the degree of trypan blue staining in 4-d-old seedlings between water and coumarin treatments, indicating that coumarin had little effect on seedling viability (Fig. 4c, f).

Fig. 4. Changes in seed viability (a,b,d,e) and seedling vigour (c,f) after imbibition in water (a–c) or 200 μM coumarin (d–f). Whole seeds and seeds with the testa removed were stained with TTC (a,b,d,e). Seedlings were stained with trypan blue (c,f).
GAs and ABA are dominant in seeds, and GA4 content is decreased by coumarin
We assayed changes in the levels of endogenous GAs, ABA and ABA-GE in seeds and seedlings after imbibition in water or 200 μM coumarin. The contents of ABA and ABA-GE, an inactive ABA conjugate, were significantly higher in seeds than in seedlings. Coumarin had no obvious effect on the content of ABA and ABA-GE in seeds, but reduced their content in seedlings (Fig. 5a, b). Since the synthesis and metabolism of GAs in higher plants are divided into two pathways starting from GA12 (Thomas and Hedden, Reference Thomas and Hedden2018), we detected the content of most of the GAs in the downstream metabolic pathway of GA12. However, only GA20, GA3, GA15, GA24, GA9 and GA4 were detected (orange oval, Fig. 5a), while GA53, GA19, GA1 and GA7 could not be detected (grey oval, Fig. 5c). GA contents were high in germinating seeds (10 h) and were relatively low in seedlings (5 d) (Fig. 5d–i). Moreover, GA9 and GA4 could not be detected in seedlings at all (Fig. 5h–i). The contents of GA20, GA3, GA15, GA24 and GA9 were increased by 200 μM coumarin, but they did not change significantly in seedlings in the presence or absence of coumarin (Fig. 5d–h). Interestingly, unlike other types of GAs, coumarin significantly reduced the content of GA4 in seeds (Fig. 5i).

Fig. 5. Changes in content of ABA (a,b) and GAs (d–i) of B. parachinensis seeds and seedlings incubated in water or 200 μM coumarin. Seeds were sampled after being imbibed for 10 h; seedlings were sampled at 4 d. Data represent the mean ± SE of three biological replicates of 100 seeds each. Means denoted by the same letter did not significantly differ at P < 0.05 according to the Fisher's least-significant difference test. (c) Schematic diagram of GAs metabolism.
During germination, ROS accumulate in the radicle and their levels are inhibited by coumarin and promoted by GAs
Many studies have shown that ROS play an important role in seed germination and are specifically expressed in the radicle and micropylar endosperm during the germination of lettuce, tomato and cress seeds (Morohashi, Reference Morohashi2002; Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009; Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b). Here, by using histochemical staining and spectrophotometry, we have confirmed this in B. parachinensis seeds (Fig. 6a and supplementary Fig. S6). In the radicle, coumarin inhibited the accumulation of ![]() ${\rm O}_2^{{\cdot}{-}} $ and H2O2, whereas both GA3 and GA4+7 promoted the production of each of these ROS. Notably, GA3 and GA4+7 promoted not only ROS accumulation at the radicle but also
${\rm O}_2^{{\cdot}{-}} $ and H2O2, whereas both GA3 and GA4+7 promoted the production of each of these ROS. Notably, GA3 and GA4+7 promoted not only ROS accumulation at the radicle but also ![]() ${\rm O}_2^{{\cdot}{-}} $ accumulation at the cotyledon (Fig. 6a). On the other hand, PC significantly inhibited accumulation of
${\rm O}_2^{{\cdot}{-}} $ accumulation at the cotyledon (Fig. 6a). On the other hand, PC significantly inhibited accumulation of ![]() ${\rm O}_2^{{\cdot}{-}} $ and H2O2 as well as peroxidase activity in the radicle and in whole seeds throughout the imbibition period (Fig. 6a–d). More notably, GA3 and GA4+7 partially reversed the coumarin-induced decrease of ROS accumulation in the radicle (Fig. 6a). In the control seeds,
${\rm O}_2^{{\cdot}{-}} $ and H2O2 as well as peroxidase activity in the radicle and in whole seeds throughout the imbibition period (Fig. 6a–d). More notably, GA3 and GA4+7 partially reversed the coumarin-induced decrease of ROS accumulation in the radicle (Fig. 6a). In the control seeds, ![]() ${\rm O}_2^{{\cdot}{-}} $ gradually increased, reached a peak at 12 h and then gradually decreased (Fig. 6b). GA3 and GA4+7 increased the peak
${\rm O}_2^{{\cdot}{-}} $ gradually increased, reached a peak at 12 h and then gradually decreased (Fig. 6b). GA3 and GA4+7 increased the peak ![]() ${\rm O}_2^{{\cdot}{-}} $ content but also shortened the time to peak by 2 h (Fig. 6b). This is consistent with the observation that GAs promoted seed germination (Fig. 2). On the other hand, both PC and coumarin could significantly decrease the accumulation of
${\rm O}_2^{{\cdot}{-}} $ content but also shortened the time to peak by 2 h (Fig. 6b). This is consistent with the observation that GAs promoted seed germination (Fig. 2). On the other hand, both PC and coumarin could significantly decrease the accumulation of ![]() ${\rm O}_2^{{\cdot}{-}} $ in seeds. Furthermore, during the period from 6 to 10 h, GA4+7 partially reversed the effect of coumarin on
${\rm O}_2^{{\cdot}{-}} $ in seeds. Furthermore, during the period from 6 to 10 h, GA4+7 partially reversed the effect of coumarin on ![]() ${\rm O}_2^{{\cdot}{-}} $ production (Fig. 6b). Similarly, coumarin inhibited H2O2 production and GAs promoted H2O2 production throughout the imbibition period, demonstrating an antagonistic effect on the production of H2O2 (Fig. 6c). The peroxidase activity was low for all treatments until 10 h, after which the activity increased sharply except in the presence of PC (Fig. 6d). These results seem to suggest that this enzyme may be more involved in seedling development than in seed germination.
${\rm O}_2^{{\cdot}{-}} $ production (Fig. 6b). Similarly, coumarin inhibited H2O2 production and GAs promoted H2O2 production throughout the imbibition period, demonstrating an antagonistic effect on the production of H2O2 (Fig. 6c). The peroxidase activity was low for all treatments until 10 h, after which the activity increased sharply except in the presence of PC (Fig. 6d). These results seem to suggest that this enzyme may be more involved in seedling development than in seed germination.

Fig. 6. In situ detection of ROS in seeds of B. parachinensis. (a) Histochemical staining of ![]() ${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase using NBT, DAB and TMB, respectively. (b,c,d) Determination of superoxide radical content (b), H2O2 content (c) and peroxidase activity (d). For each experiment, seeds were imbibed in water, 200 μM coumarin, 5 μM PC, 0.1 μM GA3, 200 μM coumarin plus 0.1 μM GA3, 0.1 μM GA4+7 or 200 μM coumarin plus 0.1 μM GA4+7. Data represent the mean ± SE of three biological replicates of 100 seeds each.
${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase using NBT, DAB and TMB, respectively. (b,c,d) Determination of superoxide radical content (b), H2O2 content (c) and peroxidase activity (d). For each experiment, seeds were imbibed in water, 200 μM coumarin, 5 μM PC, 0.1 μM GA3, 200 μM coumarin plus 0.1 μM GA3, 0.1 μM GA4+7 or 200 μM coumarin plus 0.1 μM GA4+7. Data represent the mean ± SE of three biological replicates of 100 seeds each.
Finally, we examined the effects of exogenous ROS and inducers of ROS on seed germination. Compared with seeds imbibed in water, the ![]() ${\rm O}_2^{{\cdot}{-}} $ generation promotor paraquat and exogenous H2O2 both increased the rate of germination, i.e., time to 50% testa rupture was shortened by 1.5 and 2 h, respectively (Table 1). In contrast, ⋅OH produced by the Fenton reaction had no obvious effect on the rate of germination. However, paraquat, H2O2 and ⋅OH were found to accelerate germination in seeds imbibed in coumarin. None of the treatments had a significant effect on the final percentage of germination, which were all >90% (Table 1).
${\rm O}_2^{{\cdot}{-}} $ generation promotor paraquat and exogenous H2O2 both increased the rate of germination, i.e., time to 50% testa rupture was shortened by 1.5 and 2 h, respectively (Table 1). In contrast, ⋅OH produced by the Fenton reaction had no obvious effect on the rate of germination. However, paraquat, H2O2 and ⋅OH were found to accelerate germination in seeds imbibed in coumarin. None of the treatments had a significant effect on the final percentage of germination, which were all >90% (Table 1).
Changes in physiological indices of seeds and seedlings in water and coumarin
Many physiological indices can be used to directly and accurately reflect the adaptation of seeds to the germination environment (Bewley and Black, Reference Bewley and Black1982). Here, we examined the content of MDA, soluble sugar and proline as well as the activities of SOD, CAT, APX, α-amylase and β-amylase in seeds and seedlings imbibed in water or 200 μM coumarin. MDA is the final decomposition product of membrane lipid peroxidation, and its content may reflect the degree of stress suffered by plant cells (Koźmińska et al., Reference Koźmińska, Al Hassan, Wiszniewska, Hanus-Fajerska, Boscaiu and Vicente2019). As shown in Fig. 7a, coumarin-treated seeds and seedlings had lower levels of MDA than the control, indicating that coumarin treatment did not cause significant stress. The activities of the ROS-degrading enzymes SOD and APX were lower during germination than seedlings, and the activities were reduced by coumarin at both stages (Fig. 7b, d). In contrast, CAT activity was higher during germination than seedlings and was significantly inhibited by coumarin during germination and slightly elevated during seedling establishment (Fig. 7c). Coumarin had no significant effect on α-amylase activity in seeds or seedlings but caused a significant decrease in β-amylase activity at both stages (Fig. 7e, f). Soluble sugar was higher in germinating seeds than in seedlings, and their content was slightly increased in seeds and not significantly affected in seedlings by coumarin (Fig. 7g, h). Proline levels were similar in seeds and seedlings when imbibed in water, and coumarin notably decreased and increased proline content in germinating seeds and seedling, respectively (Fig. 7i).

Fig. 7. Coumarin-induced changes in physiological indices in seeds and seedlings of B. parachinensis. Seeds were imbibed in water or 200 μm coumarin. At 10 h or 4 d, the activities of SOD (b), CAT (c), APX (d), α-amylase (e) and β-amylase (f), and the contents of MDA (a), soluble sugar (g), soluble protein (h) and proline (i) were assayed spectrophotometrically. Data represent the mean ± SE of three biological replicates of 100 seeds or seedlings each. Means denoted by the same letter did not significantly differ at P < 0.05 according to the Fisher's least-significant difference test.
Discussion
Positive roles of ROS in regulating seed germination of B. parachinensis
Although researchers in seed biology have traditionally emphasised the deleterious effects of ROS, the positive roles of ROS in alleviation of seed dormancy and acceleration of germination are now the focus of many studies worldwide (Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008; Díaz-Vivancos et al., Reference Díaz-Vivancos, Barba-Espín and Hernández2013). The concept of a ‘ROS window’ is helpful in explaining what ROS accumulation level, seen as a ‘double-edged sword’, demarcates the beneficial versus harmful effects on seeds (Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008; Lambeth and Neish, Reference Lambeth and Neish2014; Bailly, Reference Bailly2019). The final events of germination in dicotyledonous seeds (i.e., those with an endosperm) include relaxation and degradation of the cell wall in the micropylar endosperm (also called endosperm cap) and the radicle (Nonogaki et al., Reference Nonogaki, Gee and Bradford2000; Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b; Steinbrecher and Leubner-Metzger, Reference Steinbrecher and Leubner-Metzger2017, 2018). Loosening of the endosperm cell wall leads to weakening of the endosperm cap, and relaxation of the radicle cell wall leads to elongation of the radicle (Rodríguez-Gacio et al., Reference Rodríguez-Gacio, Iglesias-Fernández, Carbonero and Matilla2012). The process of cell wall weakening involves cleavage of cell wall polymers or cleavage of bonds between polymers. As a part of this process, ROS are thought to play a prominent role in the degradation of cell wall polysaccharides (Müller et al., Reference Müller, Hess, Leubner-Metzger, Adkins, Ashmore and Navie2007; Majda and Robert, Reference Majda and Robert2018; Rose et al., Reference Rose, Catalá, Gonzalez-Carranza and Roberts2003). During the germination of tobacco, tomato, pepper, Lepidium and Arabidopsis, ROS are specifically expressed in the endosperm cap, radicle and hypocotyl (Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008). During the germination of B. parachinensis seeds, ![]() ${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase specifically accumulated at the radicle and endosperm cap (Fig. 6a and supplementary Fig. S6). However,
${\rm O}_2^{{\cdot}{-}} $, H2O2 and peroxidase specifically accumulated at the radicle and endosperm cap (Fig. 6a and supplementary Fig. S6). However, ![]() ${\rm O}_2^{{\cdot}{-}} $ accumulation in B. parachinensis seeds might be higher than that of H2O2 and peroxidase since
${\rm O}_2^{{\cdot}{-}} $ accumulation in B. parachinensis seeds might be higher than that of H2O2 and peroxidase since ![]() ${\rm O}_2^{{\cdot}{-}} $ accumulated not only in the radicle and hypocotyl but also in the cotyledon when induced by GAs (Fig. 6a). In contrast, our previous research on lettuce seed germination showed that the H2O2 content in the embryo is significantly higher than that of
${\rm O}_2^{{\cdot}{-}} $ accumulated not only in the radicle and hypocotyl but also in the cotyledon when induced by GAs (Fig. 6a). In contrast, our previous research on lettuce seed germination showed that the H2O2 content in the embryo is significantly higher than that of ![]() ${\rm O}_2^{{\cdot}{-}} $ (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b). During rice seed germination, the accumulation of H2O2 in the coleorhiza and radicle is also significantly higher than the accumulation of
${\rm O}_2^{{\cdot}{-}} $ (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b). During rice seed germination, the accumulation of H2O2 in the coleorhiza and radicle is also significantly higher than the accumulation of ![]() ${\rm O}_2^{{\cdot}{-}} $ in these tissues (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Interestingly, despite the high content of
${\rm O}_2^{{\cdot}{-}} $ in these tissues (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). Interestingly, despite the high content of ![]() ${\rm O}_2^{{\cdot}{-}} $ during germination,
${\rm O}_2^{{\cdot}{-}} $ during germination, ![]() ${\rm O}_2^{{\cdot}{-}} $ did not accumulate substantially in the endosperm cap, where significant H2O2 was generated (supplementary Fig. S6). Because weakening of the endosperm cap and elongation of the radicle are prerequisites for seed germination, our results suggest that
${\rm O}_2^{{\cdot}{-}} $ did not accumulate substantially in the endosperm cap, where significant H2O2 was generated (supplementary Fig. S6). Because weakening of the endosperm cap and elongation of the radicle are prerequisites for seed germination, our results suggest that ![]() ${\rm O}_2^{{\cdot}{-}} $ may play a major role in radicle elongation, whereas H2O2 plays a leading role in endosperm cap weakening in B. parachinensis. Furthermore, peroxidase activity was low before 10 h, the time at which the first seeds complete germination. In the cell wall of plant, a Fenton-type reaction can take place in the presence of peroxidases, leading to the formation of ⋅OH (Schopfer et al., Reference Schopfer, Plachy and Frahry2001; Müller et al., Reference Müller, Hess, Leubner-Metzger, Adkins, Ashmore and Navie2007). Therefore, the activity of peroxidases might indirectly reflect the level of ⋅OH (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b). The above results indicated that the content of ⋅OH was at a low level during seed germination, an observation that is inconsistent with ⋅OH accumulation during the germination of cress seeds (Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009). Combined with the relative abundance and increased activity of peroxidase in germinating seeds and seedlings, ⋅OH may play a more predominant role in seedling establishment.
${\rm O}_2^{{\cdot}{-}} $ may play a major role in radicle elongation, whereas H2O2 plays a leading role in endosperm cap weakening in B. parachinensis. Furthermore, peroxidase activity was low before 10 h, the time at which the first seeds complete germination. In the cell wall of plant, a Fenton-type reaction can take place in the presence of peroxidases, leading to the formation of ⋅OH (Schopfer et al., Reference Schopfer, Plachy and Frahry2001; Müller et al., Reference Müller, Hess, Leubner-Metzger, Adkins, Ashmore and Navie2007). Therefore, the activity of peroxidases might indirectly reflect the level of ⋅OH (Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b). The above results indicated that the content of ⋅OH was at a low level during seed germination, an observation that is inconsistent with ⋅OH accumulation during the germination of cress seeds (Müller et al., Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009). Combined with the relative abundance and increased activity of peroxidase in germinating seeds and seedlings, ⋅OH may play a more predominant role in seedling establishment.
Coumarin delays B. parachinensis germination mainly by decreasing production of GA4, thereby reducing ROS accumulation
Coumarin is a phenolic acid compound that inhibits germination in lettuce, wheat, sudangrass, ryegrass and other species by inhibiting the elongation of the radicle and hypocotyl. The mechanism by which coumarin inhibits seed germination is now less understood, although previous studies have provided some answers (Khan and Tolbert, Reference Khan and Tolbert1966; Berrie et al., Reference Berrie, Parker, Knights and Hendrie1968; Wang et al., Reference Wang, Yao, Xu, Wu, Zhao and Hua2017; He, Reference He2019). Our examination of coumarin and seed germination in B. parachinensis gave similar results to previous studies, that is, that increased concentrations of coumarin correlated with increased delay in germination (supplementary Fig. S1). Fluridone failed to reverse the inhibitory effect of coumarin, yet PC aggravated the inhibitory effect (Fig. 2 and supplementary Figs S4 and S5). Combined with the observation that GA3 and GA4+7 could effectively restore germination, we speculate that coumarin may inhibit the germination of B. parachinensis seeds by inhibiting or reducing the generation of endogenous GAs rather than by increasing ABA production.
GAs play an important role in regulating seed germination and restoring germination under adverse conditions (Llanes et al., Reference Llanes, Andrade, Masciarelli, Alemano and Luna2016). There are many types of GAs, but the most relevant forms are GA1, GA3, GA4 and GA7, which have biological activity and are highly expressed in plants (Plackett and Wilson, Reference Plackett and Wilson2018; Thomas and Hedden, Reference Thomas and Hedden2018). We detected the contents of ten GAs in the process of GA metabolism, of which six were detected, including the bioactive GA3 and GA4 (Fig. 5c–i). Since the content of detectable GAs in germinating seeds was higher than that in seedlings, we speculated that GAs may play a more crucial role in seed germination than that in seedling growth. This speculation was also confirmed by a seedling growth test, that is, exogenous GA3 and GA4+7 could hardly or slightly promoted seedling growth (Fig. 3a, c, e, g, h). Coumarin delayed seed germination, reduced the contents of bioactive GA4 in seeds (Fig. 5f) and exogenous GA4+7 could also restore the delayed germination caused by coumarin to a certain extent (Fig. 2). Therefore, we believe that coumarin may delay seed germination by decreasing the GA4 level during seed germination of B. parachinensis. In addition to GAs, ABA, strigolactones and ethylene are often considered to be involved in regulating seed germination (Bewley et al., Reference Bewley, Bradford, Hilhorst and Nonogaki2013; Brewer et al., Reference Brewer, Koltai and Beveridge2013; Ahammed et al., Reference Ahammed, Gantait, Mitra, Yang and Li2020). The ABA content in germinating seeds was obviously higher than that in seedlings (Fig. 5 a, b), which was similar to the result of changes in ABA levels during rice seed germination. The gradual decrease in endogenous ABA indicated its negative regulatory effect on germination (Zhu et al., Reference Zhu, Ye and Zhang2009; Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). During rice seed germination, coumarin inhibits germination by inhibiting the synthesis of endogenous ABA and the expression of metabolic genes related to ABA signalling (Chen et al., Reference Chen, Peng, Gao, Zhang, Liu, Fu and Liu2019). In B. parachinensis, however, coumarin had almost no effect on ABA content in seeds and decreased ABA content in seedlings (Fig. 5a, b).
Compared with the effect of coumarin on rice seeds, this difference may be caused by the difference in species, sampling time or concentration of coumarin. In addition, the content of 5-deoxystrigol and 1-aminocyclopropanecarboxylic acid was low in seeds but high in seedlings (data not shown), indicating that seedling establishment was more closely related to strigolactone and ethylene. Due to the crosstalk in plant hormone signalling pathways, the relationships among GAs, ABA, ethylene and strigolactone are worthy of our further investigation during seed germination (Iglesias-Fernández and Matilla, Reference Iglesias-Fernández and Matilla2009; Arc et al., Reference Arc, Sechet, Corbineau, Rajjou and Marion-Poll2013; Ahammed et al., Reference Ahammed, Gantait, Mitra, Yang and Li2020).
Interactions between plant hormones and ROS during plant growth and development have been widely reported (Gomes et al., Reference Gomes, Smedbol, Carneiro, Garcia, Juneau and Ahmad2014; Jeevan Kumar et al., Reference Jeevan Kumar, Rajendra Prasad, Banerjee and Thammineni2015; Mhamdi and Van Breusegem, Reference Mhamdi and Van Breusegem2018; Waszczak et al., Reference Waszczak, Carmody and Kangasjärvi2018). During pea germination, ABA reduced the content of endogenous H2O2, and the application of exogenous H2O2 reduced the content of endogenous ABA, increased the content of endogenous GA and upregulated transcription of the ABA metabolism gene CYP707A and the GA synthesis genes GA3OX and GA20OX (Liu et al., Reference Liu, Ye, Liu, Chen and Zhang2010; Barba-Espin et al., Reference Barba-Espin, Nicolas, Almansa, Cantero-Navarro, Albacete, Hernández and Díaz-Vivancos2012). It is unclear, however, how exogenous GAs affect the accumulation of endogenous ROS in seeds. We analysed the accumulation of ROS in single seed by histochemical staining and determined the ROS content of a population of seeds by spectrophotometry. We found that GAs significantly promoted the production of endogenous ROS in seeds, that the GA synthesis inhibitor PC significantly inhibited endogenous ROS production, and that GAs could effectively reverse the decrease of ROS production caused by coumarin (Fig. 6). In view of these results, combined with previous studies on the relationships among GA, ROS, and seed germination (Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008; Díaz-Vivancos et al., Reference Díaz-Vivancos, Barba-Espín and Hernández2013; Zhang et al., Reference Zhang, Chen, Xu, Shi, Chen, Huang, Chen and Wang2014b), we propose a model for the relationships among GAs, coumarin and ROS in the regulation of germination in B. parachinensis (Fig. 8). During germination, GAs accelerate the production of ROS, which facilitate cell wall relaxation (Liszkay et al., Reference Liszkay, van der Zalm and Schopfer2004; Müller et al., Reference Müller, Hess, Leubner-Metzger, Adkins, Ashmore and Navie2007, Reference Müller, Linkies, Vreeburg, Fry, Krieger-Liszkay and Leubner-Metzger2009). This promotes endosperm cap weakening and radicle elongation and accelerates the germination process. Coumarin specifically reduces the synthesis of endogenous GA4 in germinating seeds, which decrease ROS levels in seeds. Consequently, the germination process is delayed (Fig. 7).

Fig. 8. Crosstalk among GAs, coumarin and ROS in the regulation of germination of B. parachinensis seeds.
As mentioned above, coumarin is an allelochemical widely distributed throughout the plant kingdom (Williams et al., Reference Williams, Peal, Bartholomew and Williams2005). Although it has long been reported that coumarin inhibits germination of lettuce seeds, in-depth studies of coumarin and seed germination are still very limited (Berrie et al., Reference Berrie, Parker, Knights and Hendrie1968). At 200 μM coumarin did not increase the MDA content or the activities of ROS-degrading enzymes in seeds or seedlings (Fig. 7), indicating that coumarin at this concentration does not cause significant stress on germinating seeds. This differs from studies involving abiotic stressors, such as salt and heavy metals (Zhang et al., Reference Zhang, Wang, Lou and Dong2007; Gill and Tuteja, Reference Gill and Tuteja2010). In addition, coumarin inhibited germination only by delaying its time course, having no significant effect on the final germination percentage of the seed population. Furthermore, although coumarin reduced seed viability slightly, it had the opposite effect on seedling vigour – increasing the number of root hairs and thus allowing growth of a more robust root system (Fig. 4b). Similar results were reported for sudangrass and ryegrass seeds imbibed in coumarin (Wang et al., Reference Wang, Yao, Xu, Wu, Zhao and Hua2017). The molecular mechanisms underlying the inhibition of seed germination remain to be explored. The use of multi-omics association analysis and molecular biology technologies may provide further assistance in revealing the interplay of coumarin, GAs and ROS with respect to seed germination.
Supplementary material
To view supplementary material for this article, please visit: https://doi.org/10.1017/S0960258521000167.
Author Contributions
J.L. and B.-X.C. designed the research and interpreted results. Y.-X.P. performed the majority of the experiments. X.-Q.Y. performed the quantification of ![]() ${\rm O}_2^{{\cdot}{-}} $ and H2O2. B.-X.C. wrote the manuscript. J.L. and B.-X.C. critically revised the manuscript.
${\rm O}_2^{{\cdot}{-}} $ and H2O2. B.-X.C. wrote the manuscript. J.L. and B.-X.C. critically revised the manuscript.
Acknowledgements
We are very grateful to Mr. Zhangyan Dai and Dr. Xiumei Li for their assistance in revising the paper. This work is funded by the Key-Area Research and Development Program of Guangdong Province (Grant Nos. 2018B020202010 and 2018B020202007), the Natural Science Foundation of Guangdong Province (2020A1515011535) and the Science and Technology Planning Project of Guangzhou (202002030403, 201807010114, 201909020001), which are gratefully acknowledged. We are grateful to Hancai Chen (the Vegetable Research Institute, Guangdong Academy of Agricultural Sciences) for providing us sufficient experimental materials.
Conflicts of Interest
The authors declare no conflict of interest