Introduction
The seed coat has two important but opposing roles: as a physical barrier and as a pathway of water intake. A tougher barrier makes seeds resistant to environmental impacts, including attack by pathogens and pests, but also prevents the seed from imbibing water, causing problems in food processing and crop production. Soybean (Glycine max (L.) Merrill) is problematic in this respect because of consumer preferences for a thin but undamaged seed coat.
Impermeability of the seed coat of legumes, called hardseededness (Argel and Paton, Reference Argel, Paton, Loch and Ferguson1999), causes physical dormancy (Baskin et al., Reference Baskin, Baskin and Li2000). Hardseededness in soybean improves seed quality (Kilen and Hartwig, Reference Kilen and Hartwig1978; Heatherly et al., Reference Heatherly, Kenty and Kilen1995; Tyler, Reference Tyler1997), provides resistance to fungi (Kulik and Yaklich, Reference Kulik and Yaklich1991; Roy et al., Reference Roy, Keith and Andrews1994), and protects against unstable weather conditions (Hartwig and Potts, Reference Hartwig and Potts1987). In contrast, hardseededness needs to be overcome for efficient production or processing of soybeans for food (e.g. Argel and Paton, Reference Argel, Paton, Loch and Ferguson1999). Unexpected expression of hardseededness in seeds used in food products reduces product quality (Mullin and Xu, Reference Mullin and Xu2001; Ma et al., Reference Ma, Cholewa, Mohamed, Peterson and Gijzen2004; Otobe and Yoshioka, Reference Otobe and Yoshioka2008). In a recent review of the role of the testa in establishing dormancy in legume seeds, the limited availability of information on the regulation of physical dormancy is still mentioned (Smýkal et al., Reference Smýkal, Vernoud, Blair, Soukup and Thompson2014).
Testing for hardseededness would appear to be simple. It is commonly tested by imbibition and is attributed to water repellency of the seed coat. In breeding, selection for high germination rate should have eliminated hardseededness. However, some commercial cultivars possess hardseededness that apparently escaped the selection process. One reason could be that the expression is too weak to be recognized under optimal cultivation conditions, as an occurrence of < 10% is most frequently reported (Mullin and Xu, Reference Mullin and Xu2001; Otobe and Yoshioka, Reference Otobe and Yoshioka2008), and hardseededness is easily broken by microfractures of the seed coat, which tend to occur during harvest or postharvest handling (Marbach and Mayer, Reference Marbach and Mayer1974). Slight mechanical damage to the seed coat can easily hide the phenotype, making it hard to eliminate hardseededness. Therefore, other evidence of variation in hardseededness is required.
The characteristics of the natural pathway of water imbibition by the seed have been persistently debated in connection with soybean hardseededness, because occlusion of the pathway is a convincing mechanism of hardseededness. Recent papers on the issue have mostly supported the hypothesis that the pathway is not distributed within specific seed organs (e.g. the hilum or micropyle) but occurs in the broad area of the seed coat, mainly on the dorsal side (Ma et al., Reference Ma, Cholewa, Mohamed, Peterson and Gijzen2004; Meyer et al., Reference Meyer, Steudle and Peterson2007; Koizumi et al., Reference Koizumi, Kikuchi, Isobe, Ishida, Naito and Kano2008). Macroscopically, apparent variations caused by occlusion within the seed coat are hard to detect. Scanning electron microscopy has provided some visible evidence unrelated to the imbibition test. Calero et al. (Reference Calero, West and Hinson1981) and Yaklich et al. (Reference Yaklich, Vigil and Wergin1984) observed small pores on the seed coat of permeable soybean seeds, but a smooth surface covered by a waxy material on impermeable seeds. Yaklich et al. (Reference Yaklich, Vigil and Wergin1986) concluded that pore formation is typical. Chachalis and Smith (Reference Chachalis and Smith2001) observed a high density of deep, wide pores in the seed coat of rapidly permeable soybean lines. Although these reports suggest that the pores are a water pathway, Ma et al. (Reference Ma, Cholewa, Mohamed, Peterson and Gijzen2004) and Shao et al. (Reference Shao, Meyer, Ma, Peterson and Bernards2007) proposed that small cracks in the palisade cuticle of the seed coat were associated with permeability. In terms of structural aspects of the impermeability of soybean seeds, Smýkal et al. (Reference Smýkal, Vernoud, Blair, Soukup and Thompson2014) concluded that the presence of small cracks was the only feature consistently associated with seed coat permeability. On the other hand, Baskin and Baskin (Reference Baskin and Baskin2014) pointed out that the crack hypothesis needs to be tested for further evidence. These reports all associate the presence of an impermeable layer in the seed coat with surface morphology, and they argue their results qualitatively (i.e. the structure of interest is either a water pathway or it is not). To test this association quantitatively, we set this water-pathway assumption aside and instead looked for simple associations between morphology and hardseededness. Our logical choice of a testing method was to analyse the quantitative trait loci (QTLs) involved in this association.
Several linkage maps of soybean hardseededness based on QTL analysis have been proposed (Keim et al., Reference Keim, Diers and Shoemaker1990; Sakamoto et al., Reference Sakamoto, Abe, Kanazawa and Shimamoto2004; Watanabe et al., Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004; Liu et al., Reference Liu, Fujita, Yan, Sakamoto, Xu and Abe2007; Kebede et al., Reference Kebede, Smith and Ray2014), although they were based on imbibition test scores. We therefore need to use a quantitative phenotype that reflects the morphology of the seed coat surface for a more precise understanding of hardseededness. Scala et al. (Reference Scala, Florentino and Carvalho1999) tried to quantify the morphological characteristics, but their mercury-based method posed difficulties. Instead, laser-assisted topography microscopy (LTM) allows easy micromorphological analysis since specimens need no special preparation. The LTM instrument measures variations in the height of a surface, creating a three-dimensional topographic image with a vertical precision of 0.01 μm. By using LTM, Otobe and Yoshioka (Reference Otobe and Yoshioka2008) found a quantitative association between hardseededness and reduction of the surface roughness (SR) of the seed coat, attributable to a reduction in the depth and frequency of micropores.
Here, we used a genetic linkage map covering 2663.6 cM of all 20 linkage groups (LGs) of soybean by using recombinant inbred lines (RILs) reported by Watanabe et al. (Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004). We analysed QTLs governing SR formation by using seeds derived from the same RILs, and discuss the relationship between SR measured by LTM and seed coat impermeability.
Materials and methods
Plant material
A population of RILs derived from a cross between the soybean cultivar Misuzudaizu (MI) and the line Moshidou Gong 503 (Mo) was used. The population, consisting of 156 lines derived from the F8 generation, was grown in 2009 at the National Institute of Agrobiological Sciences (NIAS), and seeds harvested from each line were used for seed surface analysis.
Genotyping and phenotyping for QTL analysis
The number of seeds that absorbed water as a percentage of the total number of seeds after 1 d of water absorption treatment, as previously determined by Watanabe et al. (Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004), was used as the seed permeability data. We also used genotype data and a linkage map obtained in a previous study (Watanabe et al., Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004) for the QTL analysis. The genetic linkage map covered 2663.6 cM of all 20 linkage groups (LGs) and included 177 restriction fragment length polymorphisms (RFLPs), 150 simple sequence repeats (SSRs), 28 amplified fragment length polymorphism (AFLP) markers and 5 phenotype markers.
Micromorphological analysis
LTM was performed with a laser three-dimensional profile microscope (VK-8500, Keyence Corporation, Japan). A topographic image covering 0.7 mm × 0.5 mm of the seed surface was obtained through a 20 × objective lens, with a vertical precision of 0.01 μm. Each seed was scanned in the centre of a cotyledon. The image was reshaped to remove detection errors caused by uneven scattering of the laser and the influence of surface folds by using a noise-reduction algorithm and a flattening algorithm. The root mean square of the vertical fluctuations in a 0.3-mm square in the centre of the image (to eliminate the edge effect of the flattening algorithm) was then calculated as a quantitative indicator of SR. The formula for calculating the indicator was based on the root mean square height of the surface, defined in ISO 25 178–2:2012(en) (https://www.iso.org/obp/ui/#iso:std:42 785:en) in relation to the analysis of three-dimensional areal surface texture. These calculations were performed automatically by the microscope. Ten seeds of each RIL were scanned. The median value was calculated to minimize the effects of faint fractures or scratches of the surface. Eight RILs were omitted because of severe damage to their seed coats.
Statistical analysis
The correlation between SR and the proportion of imbibed seeds reported by Watanabe et al. (Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004), used as a measure of hardseededness, and the proportion of phenotypic variance explained, based on analysis of variance (ANOVA), were calculated in R software v. 3.10. (http://www.r-project.org/).
QTL analysis for seed surface roughness
R/qtl software (Broman et al., Reference Broman, Wu, Sen and Churchill2003) was used to identify the loci associated with hardseededness and SR. We used the method of composite interval mapping with 1000 permutations to find QTLs, and the ‘fitqtl’ command to estimate the genetic parameters (additive effect and proportion of variance explained) for each QTL.
Results and discussion
Seed coat surface characteristics
In laser reflection images, bloom (a thin film of the pod endocarp) on the seed coat (Fig. 1A) was consistent with that reported previously (e.g. Kulik and Yaklich, Reference Kulik and Yaklich1991; Qutob et al., Reference Qutob, Ma, Peterson, Bernards and Gijzen2008). Removal of the bloom reduced the surface roughness (SR) of one seed from 8.9 μm to 1.6 μm, revealing small depressions on the surface (Fig. 1B). Bloom was buffed off with a paper towel before measurement, since it artificially inflated the SR by scattering the laser reflections. This removal procedure was conducted as follows: a double-layered paper towel, folded in four, was softly pressed on the table and the seed held between the forefinger and thumb. The centre of the cotyledon was pressed softly on the towel and then wiped back and forth until the bloom in the wiped area was removed.

Figure 1 Laser reflectance images of a seed with bloom. (A) Seed coat surface with bloom (surface roughness (SR) value = 8.9 μm). Arrowheads point to depressions detected through the bloom. (B) Clear depressions (indicated by arrowheads) appeared on the surface after buffing with a paper towel (SR = 1.6 μm). Scale bars = 100 μm.
Optical images showed differences in surface morphology between parental lines: MI had scattered depressions, whereas Mo had a smooth surface (Fig. 2). The corresponding values of SR were significantly different (0.1 < P < 0.05): MI, 1.92 ± 0.44 μm (median ± interquartile range); Mo, 1.11 ± 0.69 μm. The distribution of depressions ranged from none to frequent (data not shown), and was independent of bloom deposition. Heritability of SR (proportion of phenotypic variance explained by genetic variance) was estimated to be 0.47 (see supplementary Table S1). SR showed transgressive segregation in RILs (Fig. 3). These results suggest that SR is genetically controlled, and it is possible that the parental line has several genetic loci that have effects in opposite directions.

Figure 2 Laser reflectance images of seed coat surface with contrasting surface roughness (SR). (A) High-SR seed coat of Misuzudaizu soybean. Clear depressions (indicated by arrowheads) are apparent on the surface (SR = 3.075 μm). (B) Low-SR seed coat observed in Moshidou Gong 503 soybean. Depressions are absent (SR = 0.574 μm). Scale bars = 100 μm.

Figure 3 Phenotypic distribution of surface roughness (SR) in a population of recombinant inbred lines. The SR value (mean with interquartile range) of P1 (Misuzudaizu; rough surface) was 1.92 ± 0.44 μm; that of P2 (Moshidou Gong 503; smooth surface) was 1.11 ± 0.69 μm. Bars represent interquartile ranges.
Comparison of the surfaces of impermeable and permeable seeds suggested an association between low SR score (i.e. smooth seed surface) and impermeability (Otobe and Yoshioka, Reference Otobe and Yoshioka2008). Consistent with this, hardseededness (Watanabe et al., Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004) ( = low imbibition ratio) and SR showed a significant correlation (r= 0.41, P < 0.05; Fig. 4). Lines with high permeability had a wide range of SR, but as permeability decreased, so did SR. Although these data were obtained in different experiments, the genetic structure of this RIL population is almost fixed, so this correlation reflects a real genetic relationship between the traits. We therefore compared their genetic architectures by QTL analysis.

Figure 4 Phenotypic correlation between surface roughness (SR) and ratio of seeds absorbing water. A ratio of 1.0 indicates that all of the seeds in a line absorbed water, whereas a value of 0 indicates complete seed impermeability (hardseededness).
QTL analysis
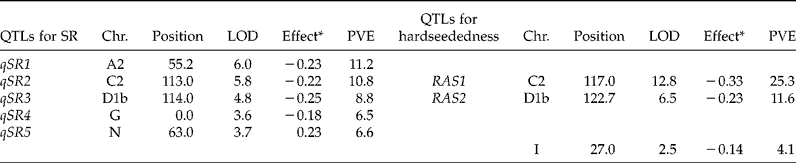
Five SR-related QTLs (hereafter, QTLs for seed roughness are designated as qSR plus a number representing the genetic effects of each locus) were detected at the threshold value of 3.56 (P < 0.1) calculated by permutation test (Fig. 5, supplementary Fig. S1; Table 1). Two of these, qSR2 and qSR3, were detected close to the previously identified hardseededness QTLs for ratio of seeds absorbing water, namely RAS1 and RAS2 (Watanabe et al., Reference Watanabe, Tajuddin, Yamanaka, Hayashi and Harada2004). The Mo allele of each decreased SR. The sum of the effects of these QTLs explained half of the genetic variance of SR (22.0% of 43.9%; Table 1). We also identified a major QTL, qSR1 (consensus linkage group A2 corresponding to chromosome number Gm08), near the genetic locus influencing soybean seed colour, locus I (inhibitor), which inhibits seed coat pigmentation (reviewed by Senda et al., Reference Senda, Kurauchi, Kasai and Ohnishi2012), and two minor QTLs, qSR4 (LG G; Gm18) and qSR5 (LG N; Gm03). The MI allele of qSR5 decreased SR, resulting in the transgressive distribution in the RILs. The total variance explained by these QTLs (43.9%) coincided well with the heritability estimated by ANOVA (0.47; see supplementary Table S1). Although qSR2 and qSR3 coincided with RAS1 and RAS2, closely linked but different genes could control SR and hardseededness independently. Therefore, the genes responsible for these QTLs must be identified.

Figure 5 Results of quantitative trait locus (QTL) analysis, showing logarithm of odds (LOD) score curves with composite interval mapping for seed surface roughness (SR; ——) and hardseededness (- - - -). Soybean chromosomes (horizontal axis) labelled with the consensus names of linkage groups (Song et al., Reference Song, Marek, Shoemaker, Lark, Concibido, Delannay, Specht and Cregan2004) and chromosomes named according to the soybean genome database (SoyBase: http://soybase.org/) are distinguished by alternating bands of shading. Black arrowheads indicate QTLs for SR. The horizontal line indicates the threshold value for SR with 1000 permutations (threshold value 3.56, P < 0.1).
Table 1 The list of QTLs for SR and positional relationship for that of hardseededness (ratio of absorbed seeds)

Chr., chromosome; LOD, logarithm of odds scores; PVE, phenotypic variance explained.
* Effect of QTLs indicated the Mo homozygous allele for MI homozygous allele.
The other QTLs regulating SR identified here also have suggestive relationships with other QTLs previously reported. Keim et al. (Reference Keim, Diers and Shoemaker1990) identified a QTL for hardseededness in the same position as the I locus in molecular LG A2 (Gm08). Sakamoto et al. (Reference Sakamoto, Abe, Kanazawa and Shimamoto2004) identified QTLs for hardseededness in a population derived from wild soybean (Glycine soja) and cultivated soybean: a seed coat colour QTL including the I locus was associated with hardseededness, and a QTL detected in LG D1b (Gm02) and positioned close to qSR3 and RAS2 had the greatest effect on hardseededness. The involvement of these different QTLs suggests the contribution of diverse QTLs and their interactions to the expression of seed permeability in soybean. Liu et al. (Reference Liu, Fujita, Yan, Sakamoto, Xu and Abe2007) also found QTLs for seed permeability in LG D1b (Gm02), as well as in LG C2 (Gm06). Most of the loci reported in these studies are consistent with our results.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0960258515000318.
Acknowledgements
We thank Dr Chikako-Kiribuchi Otobe for her helpful comments, and Ms Keiko Ninomiya for her continuous technical support.
Financial support
This work was supported by the Ministry of Agriculture, Forestry, and Fisheries, Japan.
Conflict of interest
None.