Introduction
Seeds with morphological dormancy (MD) contain small, under-developed embryos that must grow prior to radicle emergence, while seeds with physiological dormancy (PD) have a physiological impedance which restricts radicle emergence (Nikolaeva, Reference Nikolaeva and Kahn1977; Baskin and Baskin, Reference Baskin and Baskin2014). When found in combination, seeds with morphophysiological dormancy (MPD) have both under-developed embryos and a physiological restriction on germination (Baskin and Baskin, Reference Baskin and Baskin2014). MPD is hypothesized to be the ancestral dormancy trait and is thought to have evolved in moist or wet environments (Willis et al., Reference Willis, Baskin, Baskin, Auld, Venable, Cavender-Bares, Donohue and de Casas2014). As such, MPD is predominantly found in seeds of mesic or herbaceous species that occur in temperate forests of the northern hemisphere, although it is also known to occur in a number of species occupying Mediterranean and tropical biomes (Baskin and Baskin, Reference Baskin and Baskin2014). Within arid and semi-arid climatic regions, MPD occurs far less frequently (Kos et al., Reference Kos, Baskin and Baskin2012; Baskin and Baskin, Reference Baskin and Baskin2014). Information regarding suitable conditions for overcoming MPD is predominantly northern-temperate centric, with relatively few studies focusing on warm or arid biomes (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012).
Hibbertia is a large genus (ca 225 species) of the family Dilleniaceae that is widely distributed throughout Australia, with a smaller number of species also occurring in Madagascar, New Guinea, New Caledonia and Fiji (Wheeler et al., Reference Wheeler, Rye, Koch and Wilson1992; Horn, Reference Horn and Kubitzki2007). Previous studies investigating seed dormancy and germination characteristics in Hibbertia species from the south-west Western Australia (Mediterranean climate) and New Caledonia (tropical climate) have noted that their seeds are often deeply dormant, are slow to germinate, and possess MD or MPD (Schatral, Reference Schatral1996; Schatral et al., Reference Schatral, Osborne and Fox1997; Allan et al., Reference Allan, Adkins, Preston and Bellairs2004; Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012; Wulff et al., Reference Wulff, Turner, Fogliani and L'Huillier2012). For example, seeds of H. pancheri from New Caledonia only germinated after incubation for 50 days at 30°C, irrespective of whether seeds were exposed to a germination stimulant (Wulff et al., Reference Wulff, Turner, Fogliani and L'Huillier2012), while seeds of H. commutata from Western Australia did not germinate for at least 18 weeks, even under favourable temperatures (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012). In the Mediterranean-type climate of south-west Western Australia, seeds of H. commutata, H. huegelii, H. hypericoides and H. racemosa are naturally exposed to a hot, dry summer period, followed by a cooler and wetter autumn and winter (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012). Under laboratory conditions, exposure of seeds to a series of drying and wetting cycles at warm temperatures, prior to incubation at cooler conditions (18/7°C), elicited the highest germination. Notably, when seeds were moved through a sequence of temperatures (33/18°C → 26/16°C → 18/7°C), simulating the seasonal shifts experienced by seeds in the natural environment, germination was also observed to occur at 18/7°C over two successive ‘seasons’ (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012).
Many Hibbertia species occurring in fire-prone ecosystems have also been shown to produce seeds that germinate in response to heat or smoke. Germination of seeds of both Western Australian (e.g. H. amplexicaulis, H. commutata, H. huegelii, H. hypericoides, H. lasiopus, H. quadricolor and H. racemosa) and New Caledonian (e.g. H. pancheri) Hibbertia species increases in response to the application of aerosol smoke or its derivative compounds such as smoke-water or karrikinolide (KAR1) (Dixon et al., Reference Dixon, Roche and Pate1995; Roche et al., Reference Roche, Koch and Dixon1997; Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012; Wulff et al., Reference Wulff, Turner, Fogliani and L'Huillier2012).
Hibbertia glaberrima F. Muell. is a small, woody shrub found in skeletal soils on rocky outcrops, slopes and gorges in the arid regions of Australia, and is the only member of the Dilleniaceae found in Australia's central arid zone (Western Australian Herbarium, 1998; Moore, Reference Moore2005). Seeds of H. glaberrima have been previously reported to possess MPD (Erickson et al., Reference Erickson, Muñoz-Rojas, Kildisheva, Stokes, White, Heyes, Dalziell, Lewandrowski, James, Madsen, Turner and Merritt2017) – one of few records of MPD in seeds of an arid-zone shrub – but the requirements for dormancy break and germination are yet to be fully described. In Western Australia, the species is restricted to the Hamersley Ranges in the Pilbara bioregion, an area characterized by hot summers and warm winters, with erratic, often cyclonic rainfall occurring sporadically throughout the year, but most commonly in the summer or early autumn months of December through to March (Erickson et al., Reference Erickson, Barrett, Merritt and Dixon2016a). Flowering in H. glaberrima occurs during July to September, with seed formation and dehiscence occurring during October to December (Erickson et al., Reference Erickson, Barrett, Symons, Turner, Merritt, Erickson, Barrett, Merritt and Dixon2016b). Thus, the environmental conditions seeds are exposed to between seed set and the growing season are warm-hot, with inconsistent rainfall. During the summer period, conditions are likely to be suitable for warm stratification, with semi-predictable, but potentially sporadic rainfall events. As such, seeds of H. glaberrima may respond favourably to cycles of drying and wetting, similar to the responses observed in other Western Australian Hibbertia species (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012). Furthermore, fire plays an important role in shaping the ecological communities in the region (Burbidge, Reference Burbidge1943; Muñoz-Rojas et al., Reference Muñoz-Rojas, Erickson, Martini, Dixon and Merritt2016) and smoke is likely to be an important cue for seed germination and plant regeneration (Erickson et al., Reference Erickson, Merritt, Turner, Erickson, Barrett, Merritt and Dixon2016c).
Hibbertia glaberrima represents a species with an uncommon dormancy type in semi-arid environments and is a species of interest for post-mining restoration in the Pilbara region as its distribution coincides with several areas that are under active ecological restoration (Erickson et al., Reference Erickson, Barrett, Merritt and Dixon2016a). Our aim here is to more fully describe the germination ecology of H. glaberrima through understanding the timing and extent of embryo growth and the degree of physiological dormancy. Our hypothesis is that MPD will be overcome in seeds of H. glaberrima through a period of warm stratification in conjunction with cycles of drying and wetting, and that germination will be stimulated by the application of KAR1.
Materials and methods
Seed collection, seed handling and viability assessment
Mature seeds of H. glaberrima were collected in October 2011 from approximately 150 individuals on the southern slope of Mt Robinson ca 100 km north of Newman in Western Australia (23°02′16.0′′S, 118°51′32.5′′E), and a second collection was made in the following 2012 season (Fig. 1). Seeds were placed in calico bags for transport, and upon arrival at Kings Park and Botanic Garden in Perth, Western Australia, were placed in a controlled environment facility (CE: 15°C and 15% relative humidity) prior to experimentation. Seeds collected in 2011 were used experimentally in February 2012, and seeds collected in 2012 were used experimentally in February 2013. Seeds were separated from chaff using a vacuum aspirator (SELECTA BV Gravity Seed Separator ‘Zig Zag’, Netherlands) or by hand. In order to confirm that seeds were filled prior to experimentation, all seeds were X-rayed (Faxitron Specimen Radiography System MX-20 Cabinet) to confirm the presence of viable internal structures. Seeds that appeared uniformly white/grey under X-ray (i.e. had a fully-formed endosperm) were deemed viable, assumed to contain an intact embryo, and were used experimentally. Seeds that were partially filled, empty or damaged (i.e. obvious areas of insect predation or visible cracks across the endosperm/embryo) were deemed non-viable and discarded.
Fig. 1. Map of Western Australia showing the location of seed collection, Mt Robinson, and the nearest residential town of Newman.
Laboratory procedures
To reduce the impact of fungal contamination prior to germination experiments, seeds were imbibed (either in sterilized water or sterilized water with the addition of KAR1, as below) for 24 h before the aril was removed with a scalpel blade. Seeds were then surface sterilized in a 2% w/v solution of calcium hypochlorite [Ca(OCl)2] with several drops of surfactant (Tween 80) for 30 min under alternating vacuum (10 min on–off–on, at –70kPA). Seeds were then rinsed three times in sterile, deionized water, prior to plating in a laminar flow cabinet. Seeds were sown into sterile 90 mm round Petri dishes containing sterilized white sand, and then irrigated with 12 ml of sterile water (control) or sterile water containing KAR1 (0.67 µM 3-methyl-2H-furo[2,3c]pyran-2-one; Flematti et al., Reference Flematti, Ghisalberti, Dixon and Trengove2004). Petri dishes were wrapped in plastic film to reduce water loss and placed in the appropriate incubator under a 12 h/12 h photoperiod of 30 μmol m–2 s–1, 400–700 nm, cool-white fluorescent light. Germination was defined as radicle emergence ≥1 mm from the seed coat and was scored weekly. Sand was also remoistened with sterile water or water containing KAR1 on a weekly basis.
Temperature stratification
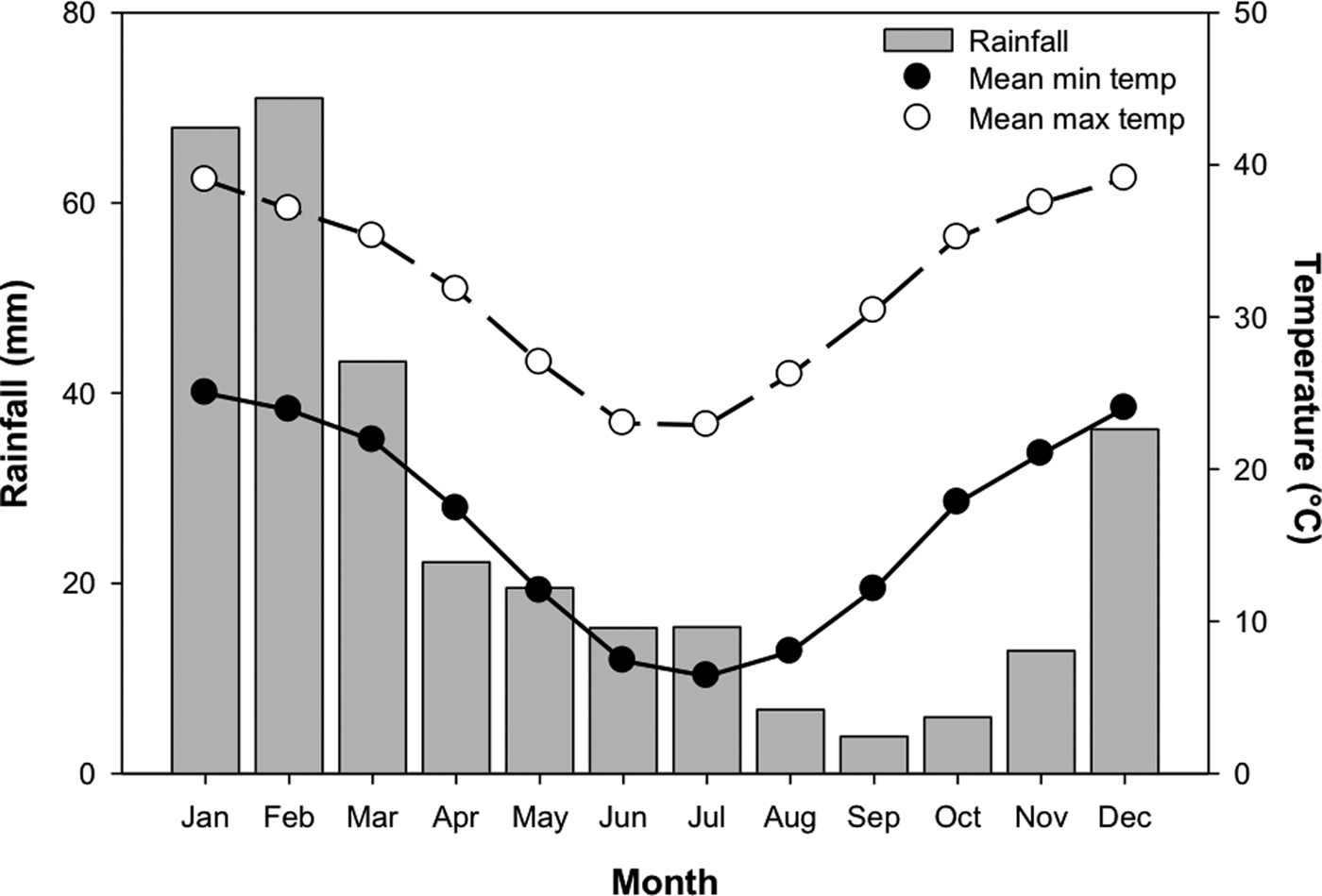
In order to test the effect of temperature stratification on germination, four separate germination treatments (GT) were devised. Temperature regimes were selected as representative of those experienced in Pilbara soils during summer and winter (Fig. 2; Newman Aero station 007176; Bureau of Meteorology, 2018), and informed by other studies of seed germination of species from the Pilbara region (e.g. see Erickson et al., Reference Erickson, Muñoz-Rojas, Kildisheva, Stokes, White, Heyes, Dalziell, Lewandrowski, James, Madsen, Turner and Merritt2017; Lewandrowski et al., Reference Lewandrowski, Erickson, Dixon and Stevens2017). Seeds were incubated at: constant 35°C for 24 weeks (GT1); constant 25°C for 24 weeks (GT2); constant 35°C for 8 weeks and transferred to constant 25°C for 16 weeks (GT3); or constant 35°C for 16 weeks and transferred to 25°C for 8 weeks (GT4). Each treatment consisted of four replicates of 20 seeds from the collection made in 2011.
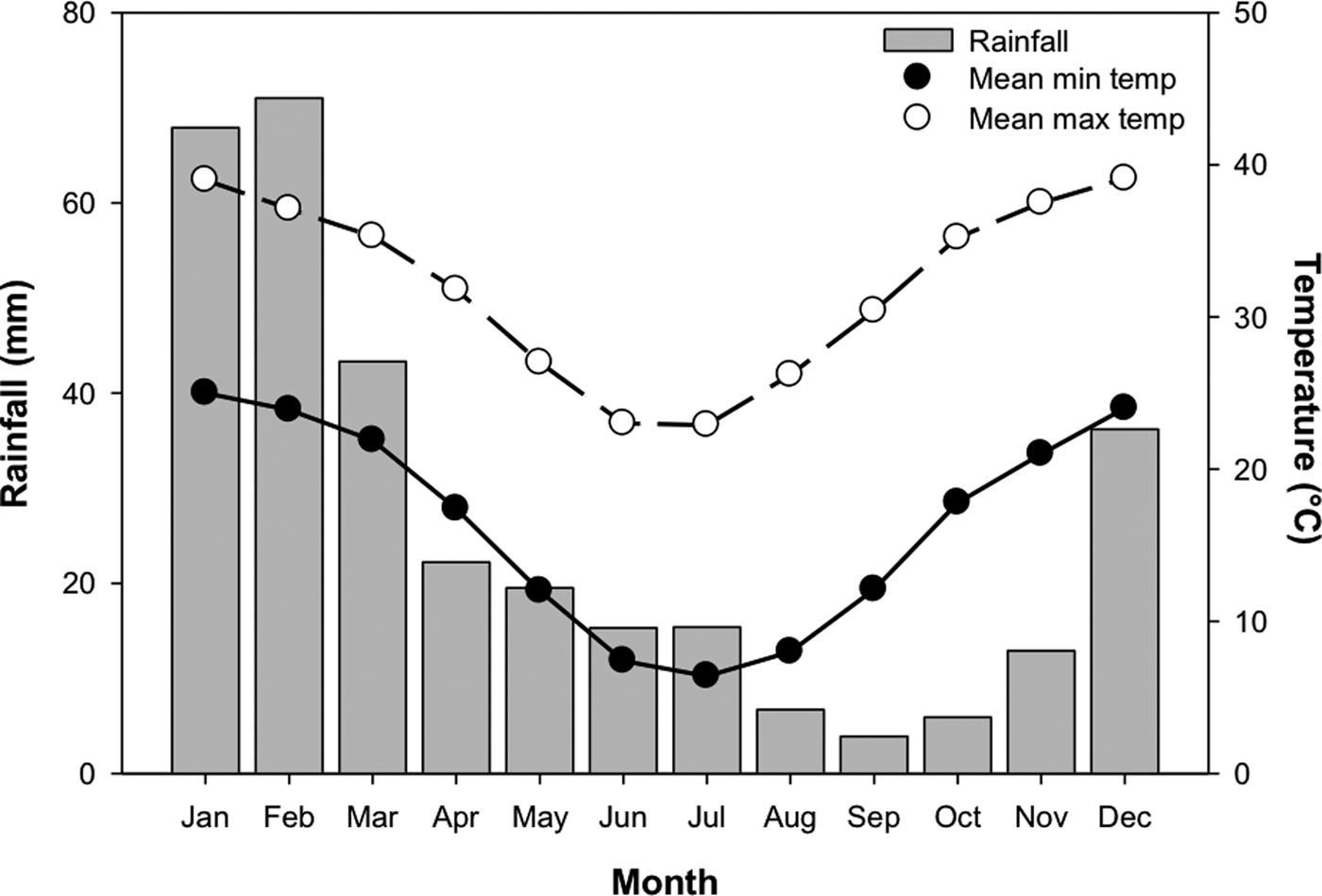
Fig. 2. Mean monthly rainfall, minimum and maximum temperatures for Newman, Western Australia (23.36°S, 119.73°E). Mean monthly rainfall was averaged between 1971 and 2018, while temperature data were averaged between 1996 and 2018. Data from the Bureau of Meteorology (2018).
Embryo growth measurements
In order to test the effect of temperature stratification on the timing of embryo growth in H. glaberrima (2011 collection), an additional 20 Petri dishes were set up in conjunction with the stratification experiment. Four dishes each were allocated to each combination of GT1 and GT2, with and without the addition of KAR1 (i.e. 16 dishes in total), while one dish was allocated to each treatment combination of GT3 and GT4, with and without the addition of KAR1 (i.e. four dishes in total). At 8-week intervals (i.e. 0, 8, 16 and 24 weeks), one dish from each of the GT1 and GT2 treatments was removed for inspection of embryo growth, while dishes in GT3 were inspected after a total incubation period of 16 weeks, and dishes in GT4 were inspected at the end of the 24-week incubation period. The thickness of the seed coat was not included in measurements of total seed length and was physically removed from the endosperm using a scalpel blade, prior to measurements. The endosperm was then dissected longitudinally using a scalpel blade, and the embryo and endosperm lengths measured using a binocular dissecting microscope (Olympus SZX16, Japan) equipped with a digital micrometer (Nikon, Digital Sight DS-L2, Japan). When necessary, the embryo length was measured using a series of the longest straight lines possible, as described by Erickson et al. (Reference Erickson, Merritt, Turner, Erickson, Barrett, Merritt and Dixon2016c, Reference Erickson, Muñoz-Rojas, Kildisheva, Stokes, White, Heyes, Dalziell, Lewandrowski, James, Madsen, Turner and Merritt2017) as the embryo grows in a curved line around the perimeter of the seed coat, as shown to occur in other Hibbertia species (see Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012). The embryo to seed length ratio (E:S) was then calculated by dividing the total length of the embryo by the total length of the endosperm. To assess when embryo growth occurred, E:S size classes were determined based on the initial E:S ratio and the critical E:S ratio attained prior to radicle emergence, as per Adams et al. (Reference Adams, Baskin and Baskin2011). The following E:S ratio size classes were selected: 0.05–0.09, 0.10–0.14, 0.15–0.19, 0.20–0.24, 0.25–0.29 and ≥0.3. Where seeds had germinated prior to measurements taking place, they were visually inspected for embryo growth (but not measured) and assigned to the largest E:S ratio size class of ≥0.3 (Adams et al., Reference Adams, Baskin and Baskin2011).
Stratification in conjunction with dry/wet cycling
To test the effect of drying and wetting cycles in combination with temperature stratification, four replicates of 10 seeds from the 2012 collection were sown in Petri dishes, with and without the addition of KAR1 and allocated to one of the following treatments: (1) constant 35°C for 24 weeks, sand kept constantly moist; (2) constant 25°C for 24 weeks, sand kept constantly moist; (3) constant 35°C for 8 weeks and transferred to constant 25°C for 16 weeks, sand kept constantly moist; or (4) constant 35°C for 8 weeks and transferred to constant 25°C for 16 weeks, with the sand moistened with 10–12 ml of sterile water or KAR1 on days 0, 14, 28, 42 and 56, and allowed to dry back. Drying back was achieved by removing the lid of the Petri dish entirely and allowing moisture to evaporate naturally over the 2-week dry period. On day 56 (i.e. 8 weeks), the Petri dish lids were replaced, and wrapped in plastic film to avoid water loss.
Statistical analyses
Final germination data (i.e. after 24 weeks) were analysed using a generalized linear model with an inbuilt ‘logit’ link function and binomial errors in R (R Core Team, 2017). Germination response was analysed through the main factors of temperature stratification, dry/wet cycling and stimulant (+/– KAR1), with interaction terms fitted. Differences in embryo to seed ratios (E:S) were determined by a general linear model on square-root transformed data to satisfy the assumptions of normality. Pairwise comparisons were determined on Holm's adjusted P-values to assess differences between temperature regimes, incubation weeks and the addition of KAR1.
Results
Temperature stratification
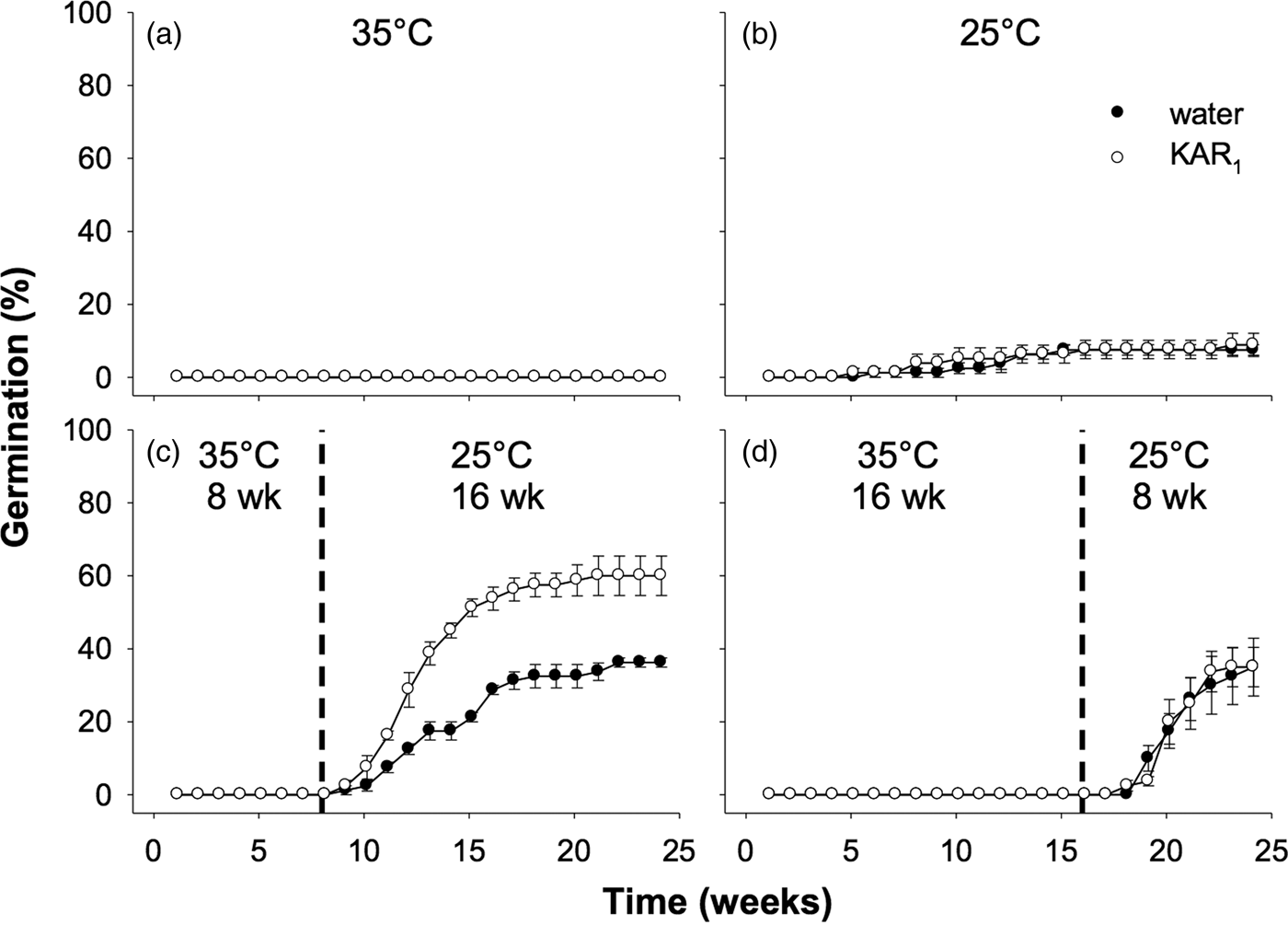
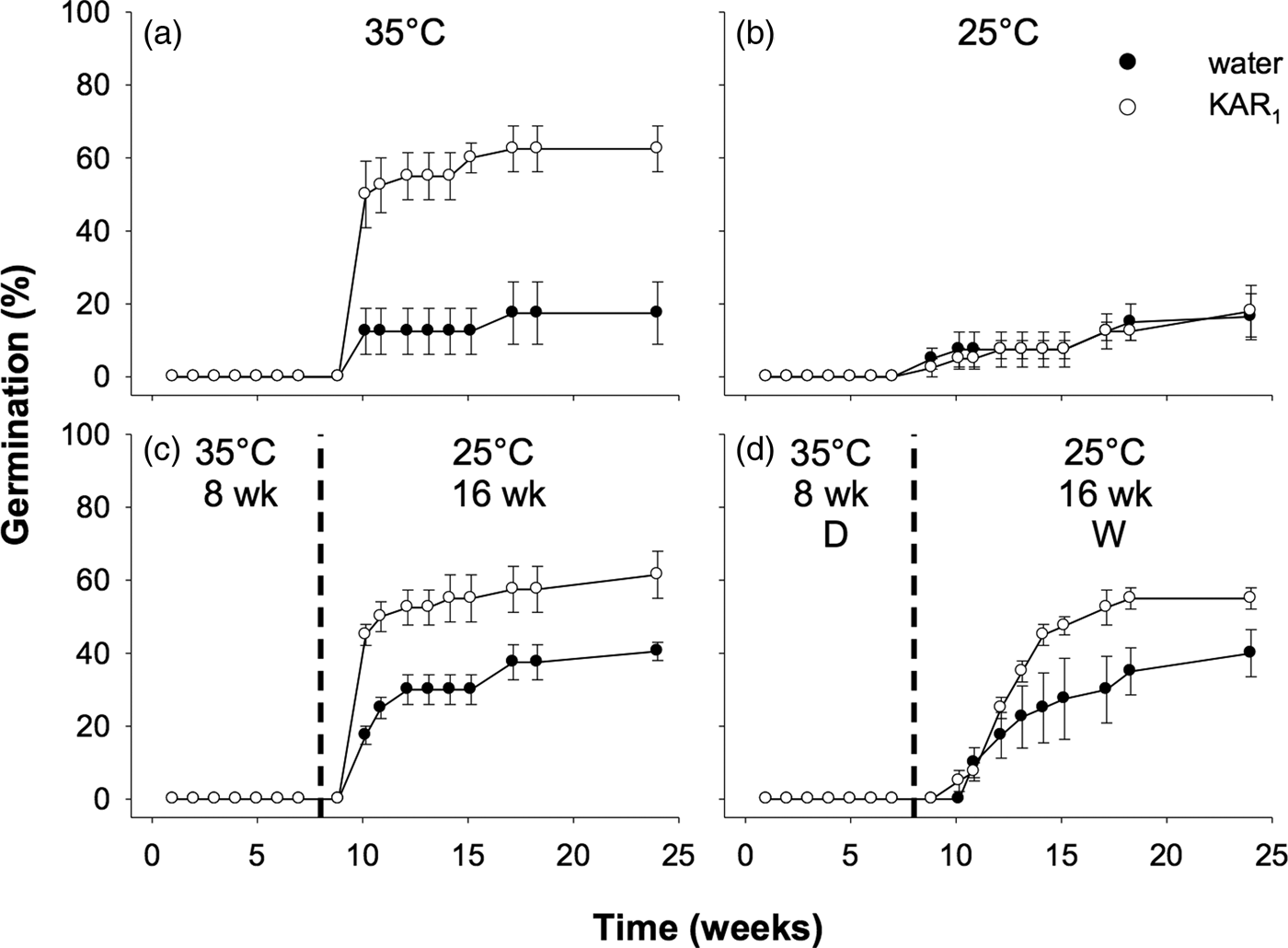
Seeds collected in 2011 were first used to examine the effects of stratification on dormancy break and germination. In the overall model describing the impact of stratification on germination, germination increased significantly following stratification of seeds for both 8 and 16 weeks (P < 0.001, Fig. 3). The overall effect of KAR1 on germination was not significant, nor was the interaction between stratification and KAR1 (both P > 0.05). Comparing the 8- and 16-week stratification treatments, for seeds that were not treated with KAR1, there was no significant difference in germination (P = 1.0); total germination was ca 40% for both treatments. However, within the 8-week stratification period, KAR1 did significantly (P < 0.01) increase germination, compared with seeds treated with water alone (KAR1 = 60 ± 5.4%, water = 36.3 ± 1.3%). Seeds did not germinate when incubated at constant 35°C and showed low total germination (<8%) when incubated at constant 25°C after 24 weeks.
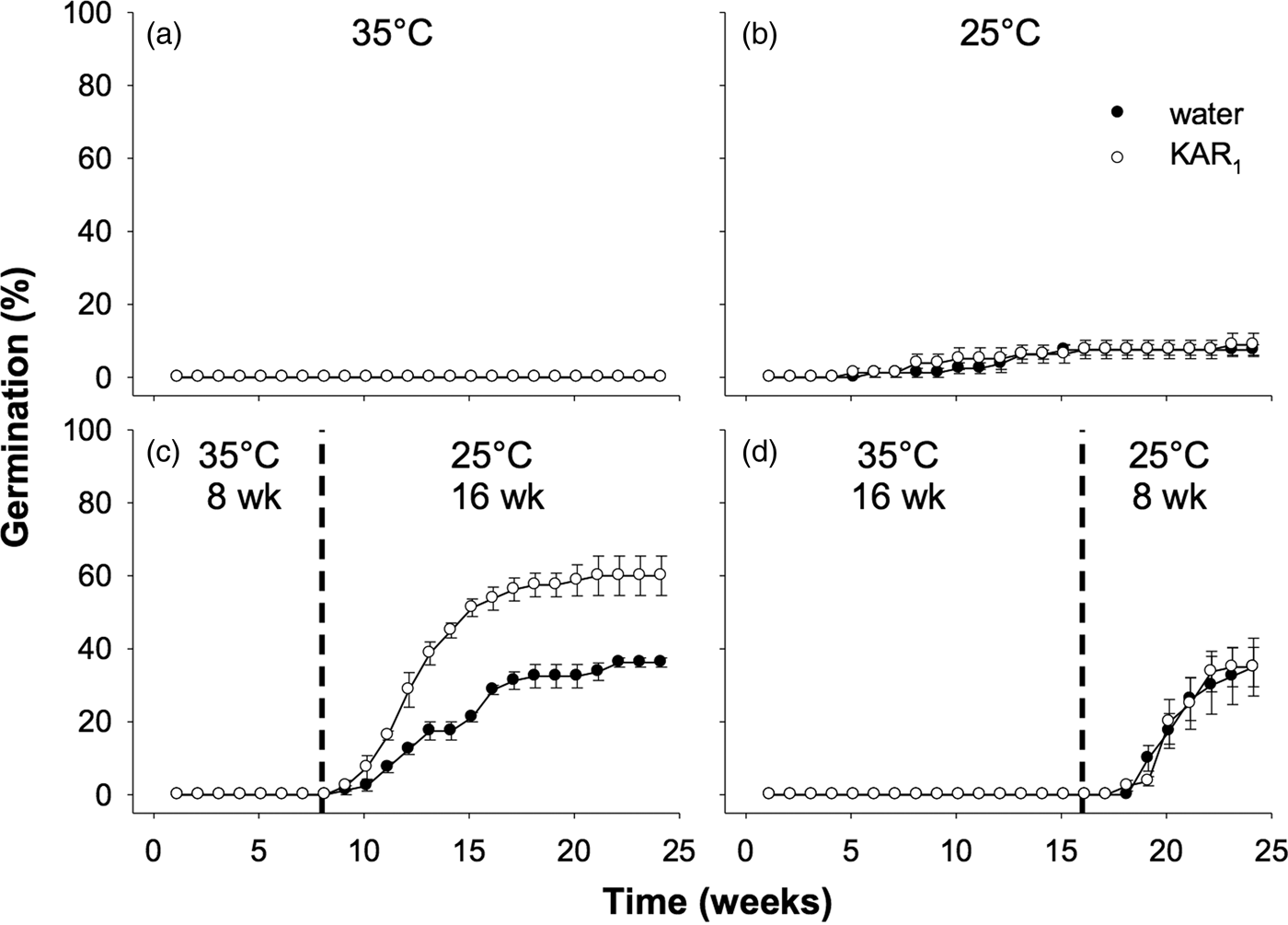
Fig. 3. Effects of temperature and KAR1 on mean germination (±s.e.m.) of Hibbertia glaberrima seeds. Seeds were placed at (a) 35°C or (b) 25°C for 24 weeks or placed at 35°C and shifted to 25°C after (c) 8 weeks or (d) 16 weeks. The 8- and 16-week time points at which treatments shown in panels c and d were shifted, respectively, are indicated by the dashed vertical lines.
Embryo growth
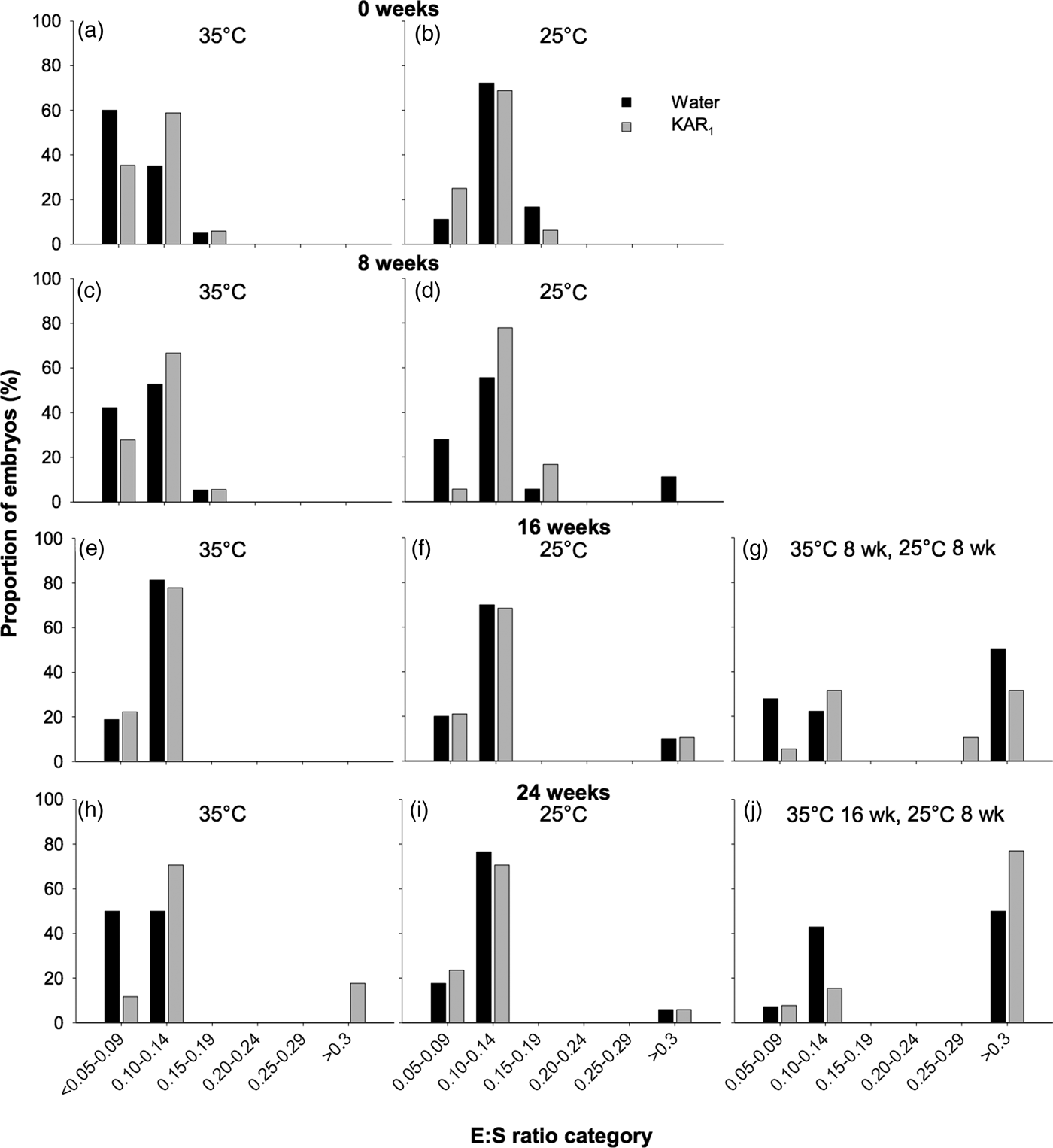
In seeds of H. glaberrima collected in 2011, embryos were found to be rudimentary (i.e. small, globular to oval-oblong with under-developed cotyledons, sensu Martin, Reference Martin1946), and underwent significant growth prior to radicle emergence. The initial embryo length (i.e. day 0) was 205 µm (±0.042) with an E:S of 0.112, which increased to as high as 2887 µm, resulting in an E:S of 1.1 prior to initiation of germination, after ≥ 8 weeks of incubation. However, the full length of the embryo prior to radicle emergence was only observed in a single seed. Prior to embryo growth occurring, most embryos (between 83.3 and 97.1%) occupied the smallest E:S size classes, which ranged in E:S from <0.05 to 0.14 (Fig. 4). After 16–24 weeks, E:S ratios were largest in seeds that underwent temperature stratification (both P < 0.001), compared with the controls (P < 0.001). Embryo growth was first observed to occur in seeds after 8 weeks at constant 25°C (–KAR1), where 11.1% of embryos had reached the >0.3 E:S size category. After 16 weeks, the greatest increase in E:S was observed in seeds stratified at 35°C (for 8 weeks) and then moved to 25°C (for 8 weeks), where 50% of seeds not treated with KAR1 had reached the >0.3 size class and 42.1% of seeds treated with KAR1 had reached size classes >0.25. No embryo growth was detected after 16 weeks in seeds incubated at 35°C, and minimal growth was detected in seeds incubated at 25°C (P = 0.09). The greatest proportion of seeds that had observable embryo growth was in those stratified at 35°C (for 16 weeks) and then moved to 25°C (for 8 weeks), where 50% (–KAR1) and 76.9% (+KAR1) of seeds had embryos within the largest size class.
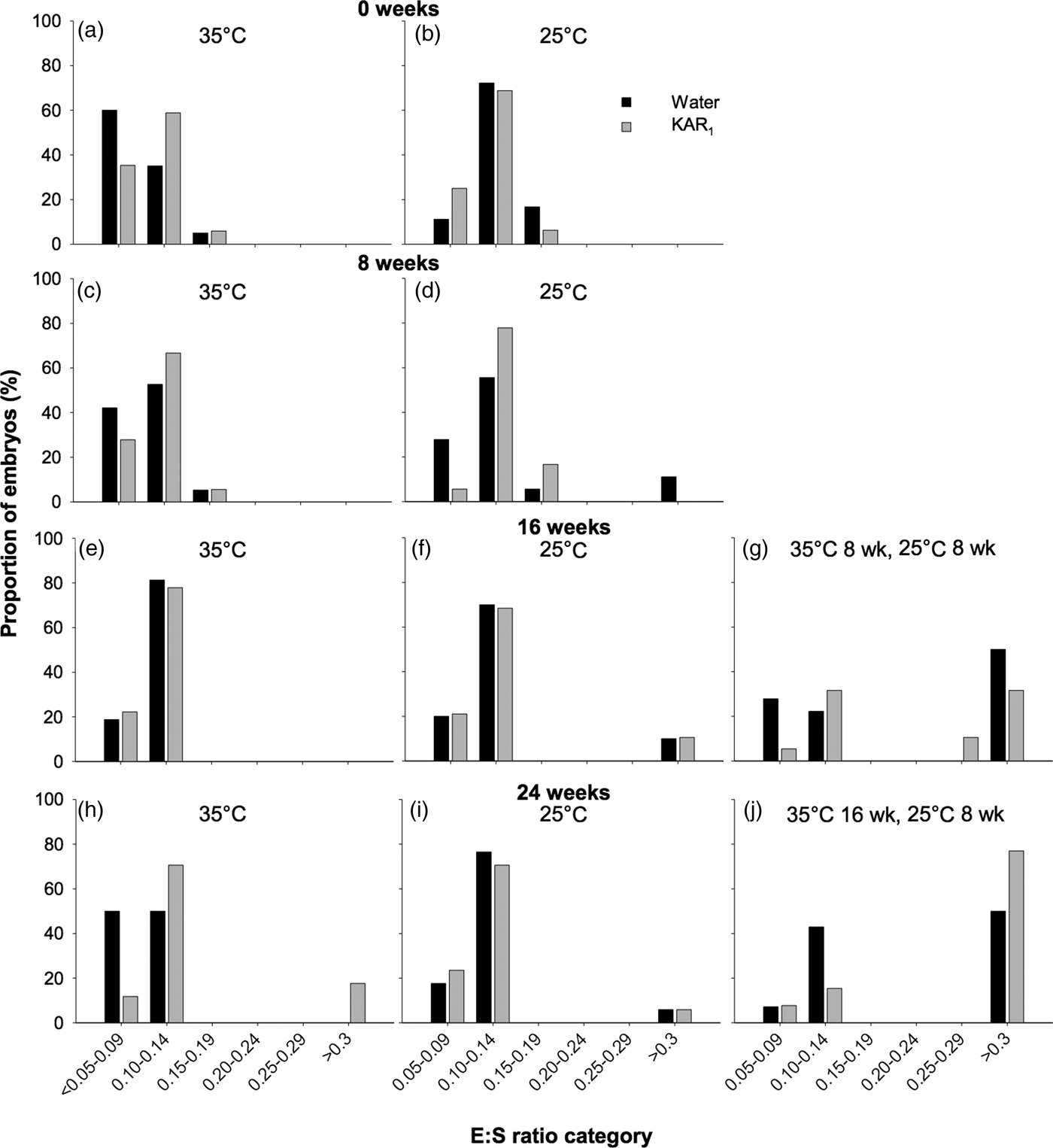
Fig. 4. Effects of temperature and KAR1 on embryo growth in seeds of Hibbertia glaberrima. Seeds were placed at either 35 or 25°C for up to 24 weeks or placed at 35°C and then shifted to 25°C after 8 or 16 weeks. Embryos and seeds were measured to calculate embryo to seed length ratio (E:S) at 0, 8, 16 and 24 weeks for seeds at 35°C (a, c, e, h) and 25°C (b, d, f, i), while seeds stratified for 8 weeks at 35°C were measured after 16 weeks (g), and seeds stratified for 16 weeks at 35°C were measured after 24 weeks (j). Seeds that had germinated during the incubation period were assigned to the largest E:S category of >0.3.
Stratification in conjunction with dry/wet cycling
Seeds collected in 2012 were used for experiments comparing the effects of stratification to those of dry/wet cycling. Overall, the effect of stratification was significant (P < 0.01), as was the stimulatory effect of KAR1 (P = 0.014). However, dry/wet cycling did not significantly increase the germination response overall, compared with seeds in other treatments that were kept constantly moist (P = 0.81, Fig. 5). Notably, for this accession of seeds, those incubated at constant 35°C for 24 weeks, and treated with KAR1, germinated to a similar percentage as seeds treated with KAR1 in the stratification and dry/wet cycling treatments (62.5 ± 6.3, 61.5 ± 6.5 and 56.0 ± 3.0%, respectively, all P = 1.0).
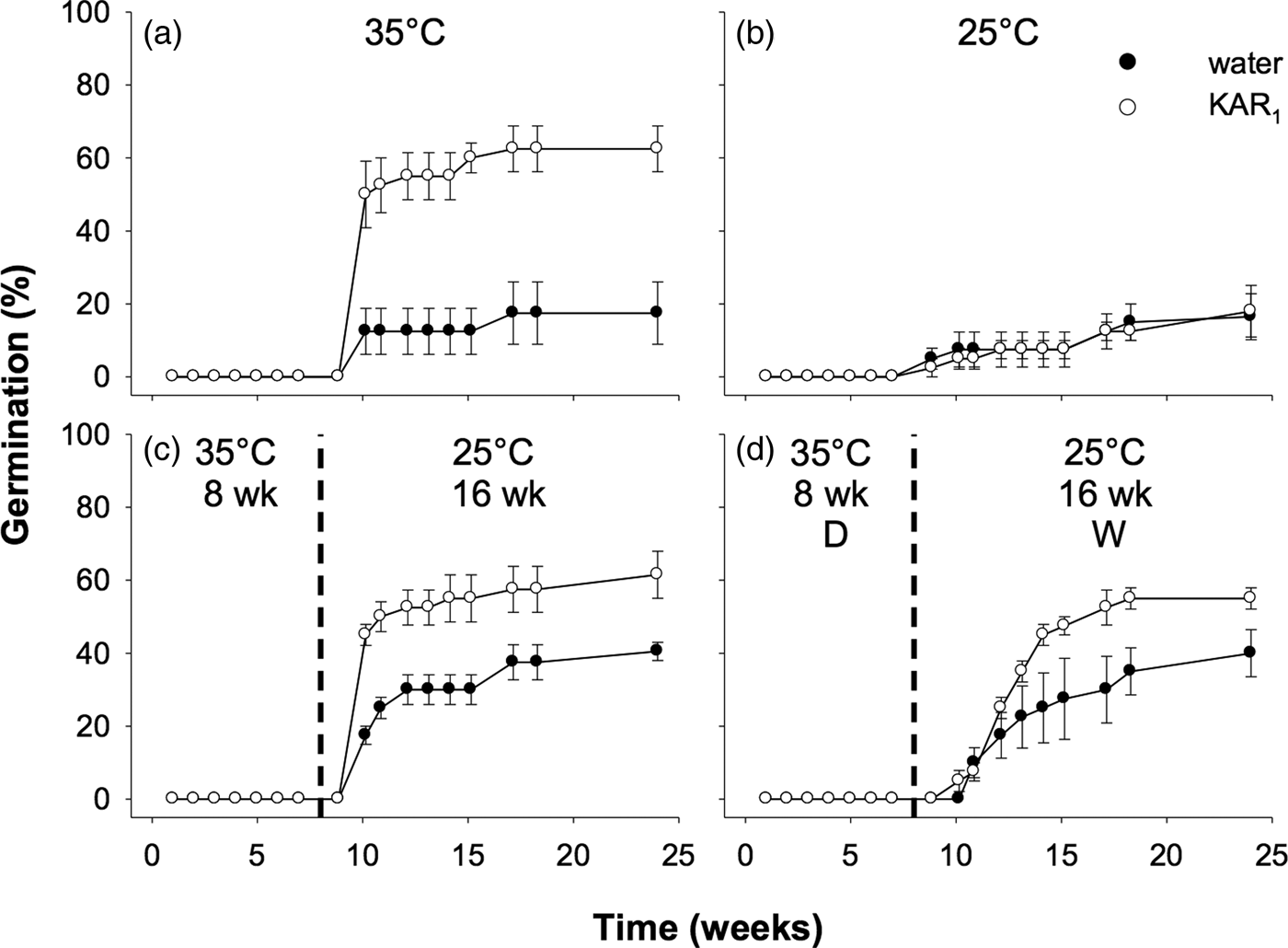
Fig. 5. Effects of temperature, KAR1 and cycles of drying and wetting on mean germination (± s.e.m.) of Hibbertia glaberrima seeds. Seeds were kept constantly moist and placed at (a) 35°C, (b) 25°C for 24 weeks, or (c) 35°C and shifted to 25°C after 8 weeks or were (d) contained in lidless Petri dishes (which allowed for water to evaporate = dry condition, ‘D’) moistened on days 0, 14, 28, 42 and 56 at 35°C for 8 weeks, then Petri dish lids were replaced, seeds moistened, and shifted to 25°C for 16 weeks (= wet condition, ‘W’). Dashed vertical lines indicate time at which seeds were moved from 35 to 25°C.
Discussion
The aim of this study was to examine the germination ecology of seeds from H. glaberrima, a rare example of a species in the arid-zone of Western Australia that produces seeds with MPD. Like the embryos in seeds of other species of Hibbertia from the Mediterranean-climate region of south-western Australia (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012), embryos of H. glaberrima are rudimentary in shape (Baskin and Baskin, Reference Baskin and Baskin2007; Martin, Reference Martin1946), are under-developed, and undergo significant growth prior to germination. Irrespective of the treatments we applied (i.e. stratification, dry/wet cycling, or KAR1) to both seed collections, the vast majority of embryos required ≥ 8 weeks to grow prior to germination, thus confirming the presence of MPD. For seeds from the 2011 collection, warm stratification at 35°C prior to incubation at 25°C overcame dormancy in a proportion of the population and resulted in significantly higher germination compared with seeds that were not stratified. Using the system for classifying the levels of MPD presented by Baskin and Baskin (Reference Baskin and Baskin2004, Reference Baskin and Baskin2014), it is likely that seeds of H. glaberrima possess simple MPD, as they germinate at temperatures suitable for warm stratification. As we did not test the effect that gibberellic acid had on germination, we cannot further define the level of MPD. However, the level is most likely to be non-deep simple MPD as no significant delay between root and short emergence was observed, thus excluding the presence of deep simple epicotyl or non-deep simple epicotyl MPD. The temperatures at which MPD is overcome in seeds of H. glaberrima are much higher than those used in the classification system. However, Baskin and Baskin (Reference Baskin and Baskin2004, Reference Baskin and Baskin2014) designed the classification system only to determine the level of MPD in seeds from temperate regions. As such, a biome-specific classification system, as suggested by Hidayati et al. (Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012), should be more appropriate for classifying the level of MPD in seeds that occur in other bioclimatic regions, such as the arid or semi-arid zones.
Unlike seeds of south-west Western Australian Hibbertia (H. commutata, H. hypericoides and H. racemosa) which showed an increase in germination response after stratification combined with cycles of wetting and drying (Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012), exposing seeds of H. glaberrima to cycles of wetting and drying did not increase total germination, when compared with seeds that were kept continuously moist. Other studies investigating the dormancy and germination mechanisms of seeds with PD from the arid or semi-arid zone of Australia have shown that short cycles of wetting and drying that act to mimic short-term, sporadic rainfall events can overcome PD and improve germination in some species: Triodia epactia, T. pungens and T. wiseana (Poaceae) (Lewandrowski et al., Reference Lewandrowski, Erickson, Dixon and Stevens2017, Reference Lewandrowski, Erickson, Dalziell and Stevens2018) and Actinobole uliginosum (Asteraceae), Goodenia cycloptera and Velleia glabrata (Goodeniaceae) (Hoyle et al., Reference Hoyle, Daws, Steadman and Adkins2008). In the Pilbara region of Western Australia, seeds of H. glaberrima are dispersed at the end of spring to early summer (October to December), and are then exposed to high summer air temperatures (≥35°C) in conjunction with sporadic or episodic rainfall events (Charles et al., Reference Charles, Fu, Silberstein, Mpelasoka, McFarlane, Hodgson, Teng, Gabrovesk, Ali, Barron, Aryal, Dawes, van Niel and Chiew2013; Erickson et al., Reference Erickson, Barrett, Symons, Turner, Merritt, Erickson, Barrett, Merritt and Dixon2016b). Given our results from both the stratification and dry/wet cycling treatments, embryo growth is likely to occur during this warm and intermittently wet summer period. However, germination may be restricted to periods of time when soil water availability is high, as is the case for many other arid-zone species (Lewandrowski et al., Reference Lewandrowski, Erickson, Dixon and Stevens2017; Merino-Martín et al., Reference Merino-Martín, Courtauld, Commander, Turner, Lewandrowski and Stevens2017). Thus, recruitment events may occur over many successive seasons, with germination likely to occur during February and March, or at other times during autumn when rainfall is high, and evaporation rates are low (Turner et al., Reference Turner, Lewandrowski, Elliott, Merino-Martín, Miller, Stevens, Erickson and Merritt2017).
Field observations indicate that adult plants of H. glaberrima predominantly occur in rocky gullies, protected outcrops, and gorges of southern-facing slopes (T. Erickson, personal observation). Such locations within the landscape may act to accumulate water during rainfall events, and retain moisture for a longer period of time, as they are often protected from direct sunlight (Erickson, Reference Erickson2015). Therefore, these microhabitats are more likely to provide sufficient moisture to allow MPD to be overcome, and support subsequent germination, compared with the surrounding landscape (Kos et al., Reference Kos, Baskin and Baskin2012). It is apparent that the seeds of H. glaberrima collected in 2012, used to test the efficacy of dry/wet cycling on dormancy break, were less dormant compared with those collected in 2011, as non-stratified seeds germinated after 8 weeks incubation at 35°C (Fig. 5). Therefore, this suggests that H. glaberrima plants produce seeds with varying levels of dormancy depth across seasons, which may be a result of differing maternal environments in each seed production season. It has been shown that production of seeds under different maternal environments can alter the dormancy status of freshly collected seeds, the sensitivity of these seeds to smoke-derived germination cues, and the germination response of seeds that have experienced differing dormancy break conditions (Gorecki et al., Reference Gorecki, Long, Flematti and Stevens2012; Dwyer and Erickson, Reference Dwyer and Erickson2016). Producing populations of seeds with varying levels of dormancy depth and germination requirements, across seasons, most likely allows seeds of H. glaberrima to spread the risk of germination and recruitment failure over multiple years.
One such example of seed responsiveness to environmental cues was observed in this study, whereby, once dormancy had been alleviated to its full extent through stratification, seeds of H. glaberrima showed an increase in germination when exposed to KAR1. This response is consistent with many previous reports of smoke-stimulated germination in seeds of other Hibbertia species (Dixon et al., Reference Dixon, Roche and Pate1995; Roche et al., Reference Roche, Koch and Dixon1997; Hidayati et al., Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012; Wulff et al., Reference Wulff, Turner, Fogliani and L'Huillier2012). However, there were differences in the KAR1 response when comparing the 8- to 16-week stratification treatments. Exposure to KAR1 increased germination in seeds stratified for 8 weeks compared with untreated seeds but did not increase germination in seeds stratified for 16 weeks (Fig. 3). Similarly, Hidayati et al. (Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012) also showed a reduced requirement for smoke in seeds of H. hypericoides after several months burial in soil (under tunnel house conditions), to the extent that seedling emergence was equal in seeds treated with and without smoke. This response can be attributed to PD being overcome, such that the window of suitable germination conditions broadens, and the requirement for smoke to stimulate germination is reduced or lost (Hidayati et al. Reference Hidayati, Walck, Merritt, Turner, Turner and Dixon2012; Erickson et al., Reference Erickson, Shackelford, Dixon, Turner and Merritt2016d). Nonetheless, our study only encompasses dormancy loss and germination over a total period of 24 weeks, and either stratification and/or drying and wetting cycles may still have an effect on dormant seeds during their second summer. Follow-up laboratory and field evaluations over multiple years are required to unravel the ecology of this species further.
From a restoration and conservation perspective, the ever-increasing need for wild-sourced seed in Western Australia, combined with the recruitment bottlenecks associated with broadcast seeding (Merritt and Dixon, Reference Merritt and Dixon2011), dictates the need to increase our knowledge of germination and dormancy syndromes. With an increasing body of knowledge now available for previously under-represented arid-zone species (Erickson et al., Reference Erickson, Muñoz-Rojas, Kildisheva, Stokes, White, Heyes, Dalziell, Lewandrowski, James, Madsen, Turner and Merritt2017) our study serves to highlight the intricacies associated with restoring species with complex dormancy types, such as MPD. Overall, we have shown that the majority of embryos in seed of H. glabberima take ≥ 8 weeks to grow prior to germinating, and MPD may be overcome in a proportion of the seed population by exposing seeds to a period of warm stratification (35°C) followed by a shift to cooler, autumn temperatures (25°C). If direct seeding is required for restoration purposes, practitioners should pre-treat seeds by keeping them warm (i.e. 35°C) and moist for 8 weeks, in conjunction with treatment with smoke-water or KAR1 prior to seeding during late summer. Alternatively, plants may be propagated from seed ex situ by exposing seeds (especially those from deeply dormant seed batches) to successive warm stratification treatments, in conjunction with smoke-water or KAR1.
Author ORCID
Emma L. Dalziell https://orcid.org/0000-0003-4463-9984
Acknowledgements
The authors would like to thank Luke Sweedman (Botanic Gardens and Parks Authority) for providing the 2012 seed collection, Wolfgang Lewandrowski for his assistance with statistical analysis, and Gavin Flematti (School of Biomedical, Biomolecular and Chemical Science, University of Western Australia) for providing us with KAR1.
Financial support
E.L.D. and T.E.E. acknowledge the financial support provided by BHP Western Australian Iron Ore, under the Pilbara Seed Atlas project (2008–2013) when this research took place. Continued financial and project support for T.E.E. was provided by a BHP Western Australian Iron Ore Community Development Project (contract no. 8600048550) under the auspices of the Restoration Seedbank Initiative (2013–2018), a partnership between BHP Western Australian Iron Ore, The University of Western Australia, and the Botanic Gardens and Parks Authority. E.L.D. was latterly supported by the Australian Research Council (LP160100381).