Introduction
The eradication of hunger and the promotion of sustainable agriculture make up the second place in the list of objectives of the United Nations (UN) for the transformation of the world. The outlook is that by 2030 there may be sustainable food production systems with increased productivity, coupled with resilient agricultural practices that preserve ecosystem quality (ONU, 2015). In order to meet this demand, new production models are required. Organic agriculture, a cropping system where agrochemicals and practices are reduced, has been proposed as an innovative solution (Brasil, 2003; Rahmann et al., Reference Rahmann, Ardakani, Barberi, Boehm, Canali, Chander, David, Dengel, Erisman, Galvis-Martinez, Hamm, Kahl, Köpke, Kühne, Lee, Løes, Moos, Neuhof, Nuutila, Olowe, Oppermann, Rembiałkowska, Riddle, Rasmussen, Shade, Sohn, Tadesse, Tashi, Thatcher, Uddin, Niemsdorff, Wibe, Wivstad, Wenliang and Zanoli2016).
Due to important benefits to the system, such as reduction in environmental impact; biodiversity preservation; mitigation of climate change; social and food safety issues (Rahmann et al., Reference Rahmann, Ardakani, Barberi, Boehm, Canali, Chander, David, Dengel, Erisman, Galvis-Martinez, Hamm, Kahl, Köpke, Kühne, Lee, Løes, Moos, Neuhof, Nuutila, Olowe, Oppermann, Rembiałkowska, Riddle, Rasmussen, Shade, Sohn, Tadesse, Tashi, Thatcher, Uddin, Niemsdorff, Wibe, Wivstad, Wenliang and Zanoli2016), organic agriculture is an already well-established model in Europe and the USA. In Brazil, its application more than doubled in the last 3 years (Estruzani and Cavichioli, Reference Estruzani and Cavichioli2016).
The transition to a new agricultural model is considered a challenging task. The citrus crop, which despite being the most productive in the world (Brasil, 2014), faces phytosanitary challenges, causing farmers to give up the crop or migrate to alternative production systems (CDA, 2016). Practices of gradual reduction and/or replacement of fertilizers and agrochemicals, combined with soil and organic matter management, add to these challenges. A shift to the organic management make the plants more dependent on soil microbial interactions and the soil assumes a central role (Santos et al., Reference Santos, Araújo, Leite, Nunes and Melo2012).
Soil is an extremely complex ecosystem where the greatest microbial diversity can be found; associating with its physical and chemical characteristics, soil becomes a key factor in controlling productivity and quality in agricultural ecosystems (Kennedy, Reference Kennedy, Collins and Qualset1999; Mendes et al., Reference Mendes, Reis Junior, Hungria, Fernandes, Chaer, Mercante, Zilli, Faleiro, Andrade and Reis Junior2011). Among the microorganisms known for their beneficial performance in a variety of crops, fungi make up the bulk of the microbial biomass. They are directly involved in vital processes such as nutrient cycling and availability, soil particle stabilization, plant growth promotion, control of pathogens and insect pests and degradation of chemical molecules (Pfenning, Reference Pfenning, Moreira, Cares, Zanetti and Sturmer2013). Fungi are also less affected by drought-related climate change when compared with bacteria (De Vries et al., Reference De Vries, Liiri, Bjørnlund, Bowker, Christensen, Setala and Bardgett2012), as they have a greater capacity to withstand disturbances (Pimm, Reference Pimm1984), which explains their contribution to the construction of a more sustainable, efficient and optimized agricultural model (Ellouze et al., Reference Ellouze, Taheri, Bainard, Yang, Bazghaleh, Navarro-Borrell, Hanson and Hamel2014; Lange, Reference Lange2014).
The understanding of the diversity and ecological interactions of fungi with soil, plants and other microorganisms in transitional agricultural systems remains limited (Bedini et al., Reference Bedini, Avio, Sbrana, Turrini, Migliorini, Vazzana and Giovannetti2013). In this study, the influence of transitional management was investigated through the characterization, occurrence and diversity of the soil fungal community under citrus. The present work aims to contribute to the development of a sustainable agricultural technique.
Materials and methods
Study area
The study site was a citrus commercial orchard (Citrus sinensis L. Osbeck) located at Santo Antônio do Lageado farm (22°08′49,4″S and 47°10′47,6″W), Mogi Guaçú, São Paulo, Brazil. The trees were from the Westin cultivar inserted in the stocks of lemon (Citrus limonia L. Osbeck) and citrumelo tree (Citrus paradisi MACF × Poncirus trifoliata (L) RAF). It was planted in 2007, on a sandy-clay Oxisoil (Santos et al., Reference Santos, Jacomine, Anjos, Oliveira, Lumbreras, Coelho, Almeida, Cunha and Oliveira2013). Climate is Cwa type (Köppen climate classification), mesothermal, with hot and rainy summers and dry winters. During the study period, rainfall range was 0 mm in August and 113 mm in January and temperatures varied between 21.3 and 28.1°C, respectively.
Experimental design and management descriptions
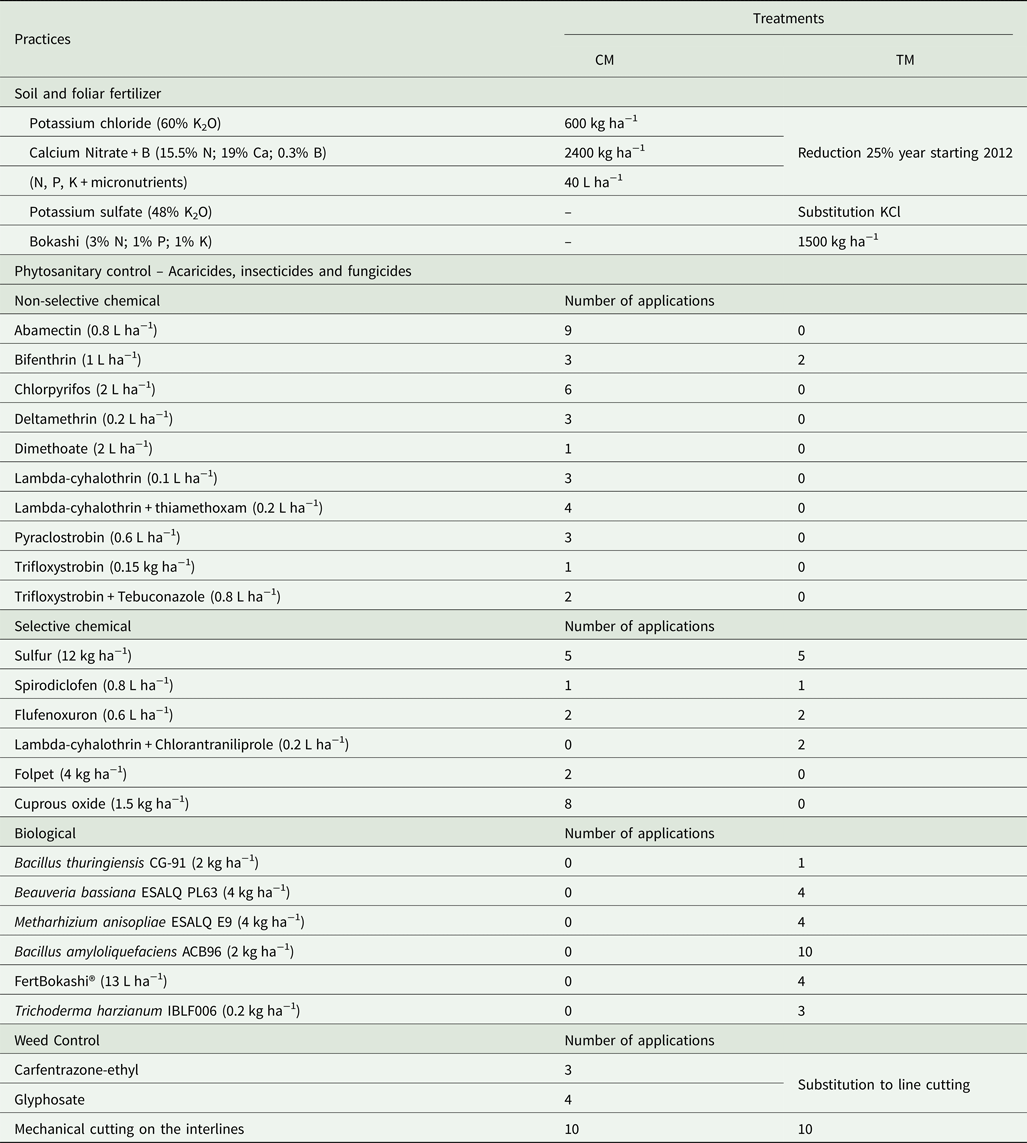
The experimental design was a completely randomized design model, where an area of 4.5 ha was divided into 10 plots with eight citrus trees of adult size each and the following treatments were installed: a conventional management (CM) based on farming practices with agrochemicals and fertilizers use, in five plots randomly selected, and another, called transition management (TM) based on the 25% reduction per year of the chemical substances used in the CM, in another five plots. The reductions were applied in the growing seasons of 2012/2013, 2013/2014 and 2014/2015, totaling a 75% reduction of chemicals and fertilizers (Table 1). From 2012 on, a soil conditioner known as bokashi (Fundação Mokiti Okada – MOA, Brazil) was introduced in TM; it was obtained from controlled fermentation of vegetal bran, a mixture of bacterial and fungal inoculum and prebiotics.
Table 1. Management practices applied to Conventional (CM) and Transitional (TM) managements
Soil sampling
Samples were obtained during the dry and rainy seasons (August 2014 and January 2015, respectively), around citrus plants. For each treatment, samples were collected from five experimental plots. In each plot, one composite sample was obtained from four equidistant subsamples (considering North, South, East and West, 0.4 m distant from the trees) collected at 0.15 m depth, using a sterilized garden shovel. Thus, in each treatment and period, five soil samples were collected, totalizing at the end of the experiment 20 soil samples. The soil samples were homogenized and wrapped on identified plastic bags. These were sealed with elastic bands and a paper towel to allow gas exchange. Then they were packed in a styrofoam box to the laboratory, where it was refrigerated at 0–10°C until analysis.
The soil temperature was measured with a digital thermometer in all the sample spots. It was registered an average of 20 and 26°C in dry and rainy periods, respectively, in both treatments. The moisture determination of the soil (EMBRAPA, 1997) varied between 10.6 and 10.0% on the dry period, on the rainy period, 7.4 and 7.6% to TM and CM, respectively.
Table 2 shows the chemical characterization of soil samples, as described by van Raij et al. (Reference van Raij, Andrade, Cantarella and Quaggio2001), in both periods.
Table 2. Chemical analysis of the soil during dry (August, 2014) and rainy (January, 2015) periods on the Conventional Management (CM) and Transitional Management (TM) treatments at the citrus orchard

OM, Organic matter.
Fungal isolation and characterization
Ten grams of homogenized and sieved soil (2 mm mesh) was submitted up to dilution 10−3, followed by 0.1 mL on spread-plate method. Isolation was performed on Potato Dextrose Agar (PDA), Sabouraud Dextrose Agar (SDA) and Oat Agar (OA) with 100 µg mL−1 chloramphenicol with five replicates. Plates were incubated at 26°C for 7 days (Moreira and Siqueira, Reference Moreira and Siqueira2006). Later, the number of colony forming unities (CFU) per gram of dry soil was recorded, according to the calculation (Gaviria, Reference Gaviria1978): CFU g−1 dry soil = (counts × dilution selected × 10).
Preliminary fungal identification was realized upon macro and microscopic characteristics, based on observations of slide mountings with lactophenol (Domsch et al., Reference Domsch, Gams and Anderson1993; Samson et al., Reference Samson, Houbraken, Thrane, Frisvad and Andersen2010). For identification, the dilution plates 103 were used, whose count reached 104 CFU of fungi. Colonies were transferred and purified on PDA.
Statistical analysis
The frequency of fungal groups was calculated upon the ratio between the number of isolates per taxon and a total number of fungal isolates, multiplying by 100. To evaluate fungal diversity, the indexes proposed by Magurran (Reference Magurran2013) of the richness of Margalef (D mg), of the diversity of Shannon (H′) and reverse Simpson (D) were calculated by the EstimateS program (Colwell, Reference Colwell2013).
Data were first submitted to the normality test of Shapiro–Wilk at 5% of significance. Those with abnormal distribution were transformed by Box-Cox. Then, the data were submitted to the t test at 5% by SISVAR program (Ferreira, Reference Ferreira2010). The principal component analysis (PCA), by the PAST program version 2.17c was also applied (Hammer et al., Reference Hammer, Harper and Ryan2001). For this, the data were transformed by the Min–Max normalization.
Results
Fungal analysis
Morphological characterization revealed 11 fungal groups. Both treatments presented nine taxa in the dry period, with no statistical difference (P ⩾ 0.05). In the rainy period, 11 taxa were recorded in the TM and 10 in the CM (Table 3); the genera Phialophora spp. (PHI) and Neurospora spp. (NEU) were exclusively present in this period. In the wet period, Paecilomyces spp. (PAE) was significantly higher in CM (P = 0.05) and Penicillium spp. (PEN) in TM (P = 0.02); the same pattern for the total number of isolates per treatment was observed (P = 0.04; P = 0.01, respectively). In terms of frequency, Paecilomyces spp. (PAE) represented 45% of the total fungi isolated in the CM against 29% in the TM. Penicillium spp. (PEN) represented 4% and 9% of the total fungi in the CM and TM, respectively (Table 3).
Table 3. Number of fungal groups isolated from a suspension of 104 during dry and rainy periods, in the transitional (TM) and conventional management (CM) of a citrus orchard

Average of 5 replicates, compared by t test.
*5% significance.
Diversity indexes
TM presented higher richness and diversity indexes during both studied periods (Table 4). The Margalef index (D mg), which expresses richness, was higher in TM, especially in the wet period. Concerning the H' and D indexes, although the same total number of CFU was found between CM and TM, the latter differed from CM in the percentage and composition of taxa, directly reflecting on the diversity result.
Table 4. Analysis of Margalef (D mg) richness index and diversity indexes [Shannon (H') and Simpson (D)], in the dry and rainy periods, transitional (TM) and conventional (CM) management in the citrus orchard

Principal component analysis
The PCA showed differences between management and periods studied (Fig. 1). The treatments, separated by component 1 explained 49.9% of the data, while the periods, to a lesser extent, explained 16.5%. Among the factors that distinguished the treatments, the non-selective chemical applications of fungicides (NSC_Fun), herbicides (NSC_Herb), insecticides (NSC_Ins) and acaricides (NSC_Aca), in addition to the fertilization (represented by NPK), soil temperature (Tem), humidity (Hum) and some nutrients (Ca, Fe and B) were significant for CM. Biological fungicides (Bio_Fun), insecticides (Bio_Ins), selective chemical insecticides (SC_Ins), acaricides (SC_Aca) and the micronutrients Mn and Zn were the most evident in TM.
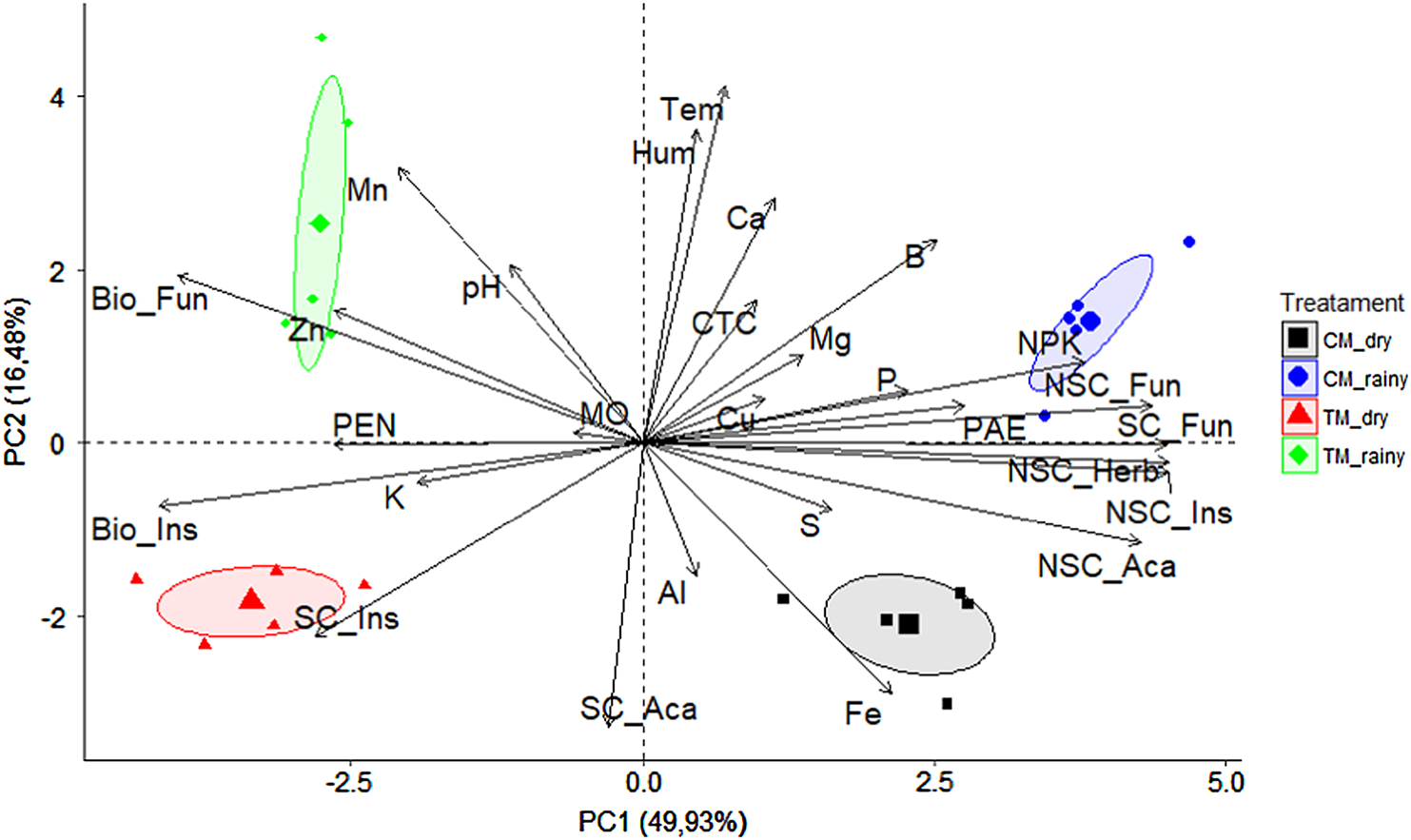
Fig. 1. Principal component analysis (PCA) among treatments, transitional (TM) and conventional management (CM), and dry and rainy periods.
For the periods, the dry season was characterized by all insecticide, acaricide and herbicide applications, as well as the presence Fe element. The wet period humid was characterized by the higher number of fungicide application (chemical no selective, selective and biological), fertilization with NPK, B, Ca, Mn and Zn accompanied by higher temperature and humidity.
Discussion
Fungal analysis
Despite the identified groups being common inhabitants of the soil and also present in areas of agricultural management (Domsch et al., Reference Domsch, Gams and Anderson1993; Webster and Weber, Reference Webster and Weber2007), differences were found in two, of the 11 fungal groups observed (Table 3).
Phialophora spp. and Neurospora spp. were more sensitive to moisture and occurred exclusively during the rainy season. The wet period favored their isolation (Arundel et al., Reference Arundel, Sterling, Biggin and Sterling1986). Studies conducted in the southern region of Brazil showed a higher number of CFUs in summer, corresponding to more constant rainfall (Borges et al., Reference Borges, Lazzari, Pimentel and Vila Nova2011). Another report did not discuss the influence of moisture on the number of fungal CFUs, the effect of this variable on the composition of some fungal species, which were more abundant in the wet period, indicated qualitative changes (Oliveira et al., Reference Oliveira, Cavalcanti, Fernandes and Lima2013).
Paecilomyces showed the highest occurrence in soil in both periods (between 29 and 45% of the total isolates), although a certain reduction of its frequency was observed in the TM system, where the application of chemicals for phytopathogenic control was decreased (Table 3). Several species of Paecilomyces have already demonstrated the potential for degradation of herbicides and fungicides (Martinez et al., Reference Martinez, Silva, Fay, Abakerli, Maia and Durrant2008; Slaba et al., Reference Slaba, Różalska, Bernat, Szewczyk, Piątek and Długoński2015; Sharma and Malaviya, Reference Sharma and Malaviya2016), using the compounds as an important source of carbon. So, particularly for this genus, the agrochemical applications and its extreme adaptation and thermotolerance (Houbraken et al., Reference Houbraken, Samson, Frisvad, Hocking, Pitt, Samson and Thrane2006) may possibly explain its high occurrence in the present study. The genus is also frequently found in tropical agricultural soils (Prade et al., Reference Prade, Matsumura, Ott and Porto2007; Borges et al., Reference Borges, Lazzari, Pimentel and Vila Nova2011), where its population is generally 10% in relation to other fungi present.
The CFU count of Penicillium isolates was significantly higher in TM, which may be also associated with the reduced chemical applications (75%), mainly from the second year of harvest. The genus is considered the main threat in citrus post-harvest (Fischer et al., Reference Fischer, Zanette, Spósito and Amorim2011), but its manifestation also depends on the practices used in the conduction of the crop to the field, harvesting, transportation and storage (Fischer et al., Reference Fischer, Palharini, Spósito and Amorim2013).
With the reduction of the chemical inputs in the TM, fungi of the genus Penicillium benefited, causing their population to increase in relation to CM. Several studies carried out in Brazilian agricultural systems show the predominance of fungi of the genera Aspergillus and Penicillium (Coutinho et al., Reference Coutinho, Cavalcanti and Yano-Melo2010; Borges et al., Reference Borges, Lazzari, Pimentel and Vila Nova2011; Pinotti et al., Reference Pinotti, Santos, Klauberg Filho and Castro2011; Costa et al., Reference Costa, Souza-Motta and Malosso2012). In cultivated soils of India, a dominance of Aspergillus, Penicillium and Mucor spp. was reported (Chandrashekar et al., Reference Chandrashekar, Pai and Raju2014). Among the fungi identified by Grantina et al. (Reference Grantina, Kenigsvalde, Eze, Petrina, Skrabule, Rostoks and Nikolajeva2011a, Reference Grantina, Seile, Kenigsvalde, Kasparinskis, Tabors, Nikolajeva, Jungerius and Muiznieks2011b) in systems with 6 years of implantation in Latvia, Penicillium was the most abundant in organic management, while the Verticillium population was higher in conventional management.
It is important to mention that Metarhizium spp., a widely known entomopathogen (Alves, Reference Alves and Alves1998), was introduced in the TM area in the second crop year of the experiment, as a biological insecticide for the control of fruit flies (Ceratitis spp., Anastrepha spp., Ecdytolopha aurantiana), larva-miner (Phyllocnistis citrella) and psyllium (Diaphorina citri). There was a commercial use of Metarhizium in the period of March 2014 (data not shown), which explains the higher number of isolates under TM during the dry period (Table 3).
Our work showed that the management regime under in TM reduced this selective effect on soil fungi, decreasing the population of Paecilomyces spp. by 36% in relation to CM. The conventional monoculture cassava favored generalist groups, selecting them and making them predominate in the system, corroborating the results found in citrus culture (Kalia and Gosal, Reference Kalia and Gosal2011; Costa et al., Reference Costa, Souza-Motta and Malosso2012).
Diversity indexes
Greater differences among the samples were obtained by the Margalef index (Table 4), probably because this is intended to compensate the effects of the sampling, taking into account the total number of individuals identified in its calculation method (Magurran, Reference Magurran2013). The higher difference attributed to the wet season strongly suggests that this season may benefit the biological processes in soil (Thies and Grossman, Reference Thies, Grossman, Uphoff, Ball, Fernandes, Herren, Husson, Laing, Palm, Pretty, Sanchez, Sanginga and Thies2006).
Despite the similar CFU numbers found in TM and CM, H' and D indexes showed differences in the percentage and composition of the taxa identified between both treatments, consequently reflecting on the diversity of the fungal community. This was mainly demonstrated by the results obtained with the D index (Table 4).
The elimination of fungicides and the reduction of other chemical inputs (Table 1) combined with the bokashi application were decisive to obtain this result in the transition practices. Organic materials, such as the bokashi compound, need to go through the process of mineralization to supply nutrients to plants, unlike soluble fertilizers. Thus, the nutrients are released gradually, which avoids large losses and can be more efficient by synchronizing with the need of the plant (Boechat et al., Reference Boechat, Santos and Accioly2013; Vann et al., Reference Vann, Bennett, Fisher, Reberg-Horton and Burrack2017). In addition, it favors soil biological activity that plays a key role in the dynamics of cycling and nutrient solubilization.
According to Prade et al. (Reference Prade, Matsumura, Ott and Porto2007), the cultural practices of agroecosystems interfere in the qualitative and quantitative distribution of soil fungi, allowing a greater diversity in agroecological management to be evidenced. The same result was reported by Costa et al. (Reference Costa, Souza-Motta and Malosso2012) when comparing agroecological with conventional management, demonstrating that the later had a reduction in the diversity of fungi, favoring of generalist species that predominated in this system.
In practice, TM presents higher stability and efficiency in the use of available resources. The larger diversity exerts a buffer effect on the soil against environmental and anthropic stresses, ensuring that a certain function is performed by other species, called functional redundancy (Mendes et al., Reference Mendes, Reis Junior, Hungria, Fernandes, Chaer, Mercante, Zilli, Faleiro, Andrade and Reis Junior2011).
A reduction in the biological community of the soil with the possible extinction of species can result in drastic losses for the resilience of agricultural systems. Therefore, management effects should count not only the increase in agricultural productivity but also the effects on soil biota over time (Moreira et al., Reference Moreira, Cares, Zanetti and Stürmer2013).
Principal component analysis
PCA confirmed the influence of agrochemical applications on the difference of fungal groups observed between treatments (Fig. 1). Likewise, it is known that fertilizers of high mineral solubility can alter the composition and diversity of microbial populations in the soil of long-term agricultural systems (Jangid et al., Reference Jangid, Williams, Franzluebbers, Sanderlin, Reeves, Jenkins, Endale, Coleman and Whitman2008). This study showed that Paecilomyces spp. found favorable conditions for its development in CM. The well-known resistance of the genus to various xenobiotics (Slaba et al., Reference Slaba, Różalska, Bernat, Szewczyk, Piątek and Długoński2015; Sharma and Malaviya, Reference Sharma and Malaviya2016) may explain the high occurrence of their representatives which were able to use the chemical compounds as a source of carbon and energy (Hussain et al., Reference Hussain, Siddique, Saleem, Arshad and Khalid2009; Usman et al., Reference Usman, Kundiri and Nzamouhe2017). This hypothesizes the strong association between the population of Paecilomyces isolates with chemical applications, especially fungicides and herbicides.
Comparatively, in both harvests evaluated (2013/2014 and 2014/2015), 60 sprays of different chemical substances were carried out in CM vs 12 in TM (Table 1). It is assumed that the modifications active microbial groups represented by Penicillium and Paecilomyces, important to the good functioning of the agroecosystem, was the result of the reduction/replacement of non-friendly chemical among other inputs, accompanied by the integrated management described in this study, in agreement with the data found by Postma-Blaauw et al. (Reference Postma-Blaauw, De Goede, Bloem, Faber and Brussaard2010).
The substitution of chemicals by biological ones diminished the impact and the selectivity on the soil biota (Kalia and Gosal, Reference Kalia and Gosal2011; Sethi and Gupta, Reference Sethi and Gupta2013), favoring the population of Penicillium. This genus is an important producer of several secondary metabolites and enzymes, with action in biocontrol and mycoparasitism (Petit et al., Reference Petit, Lucas, Abreu, Pfenning and Takahashi2009). Other roles include phosphate solubilization, antagonistic action against phytopathogens and plant growth promotion from the production of indole acetic acid (Guijarro et al., Reference Guijarro, Melgarejo, Torres, Lamarca, Usall and De Cal2008; Efthymiou et al., Reference Efthymiou, Granlund, Müller-Stöver and Jakobsen2018). With the increase of Penicillium occurrence, these functions may also be intensified reflecting in improvements in the system as a whole (Lehman et al., Reference Lehman, Cambardella, Stott, Acosta-Martinez, Manter, Buyer, Maul, Smith, Collins, Halvorson, Kremer, Lundgren, Ducey, Lin and Karlen2015).
Surprisingly, even with the fertilizer reduction, higher concentrations of Mn and Zn were detected in the TM (P < 0.05, data not shown), correlating with the change on the fungal population in the soil. These micronutrients may cause adverse effects on living organisms when in excess, however, they are essential to biological systems participating in their metabolic, enzymatic (Nogueira and Soares, Reference Nogueira, Soares, Siqueira, Souza, Cardoso and Tsai2010; Ballou and Wilson, Reference Ballou and Wilson2016) and nutritional processes (Gonçalves et al., Reference Gonçalves, Souza, Rocha, Medeiros and Jacob2014). The levels of Mn and Zn in the studied soil were 16.5 and 17.0 mg dm3, respectively, indicating a synergistic effect on Penicillium spp., since these concentrations are not toxic.
With the high temperature and humidity of the wet seasons, fungal diseases become the highest level of phytosanitary pressure, which concentrates sprays of fungicides. In the present study, the black spot (Phyllosticta citricarpa) posed the greatest threat to the orchard. The control, which covers up to six sprays per year, is usually carried out between October and April (Fundecitrus – Fundo de Defesa da Citricultura, 2017), precisely during the periods of greatest rainfall. Citrus fertilization occurs in a piecemeal manner between spring and summer (months of higher rainfall) (Malavolta et al., Reference Malavolta, Prates, Casale and Leão1994; Vitti and Cabrita, Reference Vitti and Cabrita1998), which explains the higher concentration of the nutrients Ca, B, Mn and Zn in relation to the dry period.
In addition to the positive changes already demonstrated, an efficient and sustainable agricultural system must encompass aspects other than microbiological aspects. The productivity of the culture, for example, is what actually moves farmers to new practices. Although critics point out that organic systems produce an average of 25% less than the conventional (Reganold and Wachter, Reference Reganold and Wachter2016), in our work the productive levels in both treatments were equal or higher with TM. In the 2013/2014 crop, the fruit yield was 108 and 110 kg per plant in TM and CM, respectively. The quality of marketable oranges in the same period was higher in TM, with a ratio of 16.1%, against 13.4% in CM. In the following harvest 2014/2015, TM was more productive, with 132 kg per plant vs 109 kg in CM, but fruit quality did not follow the higher TM productivity (data not shown). This fact associated with the higher microbiological quality of the soil can be the incentive for management migration and system sustainability.
Few studies have focused on the evaluation of fungal groups in transitional management to organic. In Pennsylvania fields, there was no consistency in the increase of entomopathogenic fungi after 3 years of agricultural transition (Jabbour and Barbercheck, Reference Jabbour and Barbercheck2009). In contrast, 2-year transition in a tropical climate was sufficient to alter biological soil properties (Santos et al., Reference Santos, Araújo, Leite, Nunes and Melo2012). In our work, 4 years of transition were sufficient to modify the soil mycobiota reflecting positively also on other aspects. Therefore, from a microbiological point of view, after this period the soil will be able to sustain the organic system production. In addition, the management proposed in our work proved to be competitive and sustainable and could serve as a model for the supply of global food demand allied to ecosystem conservation.
Conclusions
In Citrus cultivation areas, both in dry and rainy periods, the gradual replacement of chemical fertilizers and agrochemicals, quantitatively modified the fungal community, increasing its diversity when compared with CM.
The biological properties of the soil under Citrus cultivation were positively influenced by a 4-year TM, since this period is sufficient to favor an important microbial group which is crucial for nutrient recycling and soil stabilization, reflecting on productivity and similar fruit quality or even higher than CM.
The genera Penicillium and Paecilomyces presented different behaviors during the transition from one management to another. The TM favored the occurrence of the first and reduced the second one, however, both endured the process.
The genera Paecilomyces is highly resistant to agrochemical applications demonstrated great potential in bioremediation of soils contaminated with pesticides.
The fertility parameters, Mn and Zn were more associated with TM, while Ca, Fe and B were associated with CM.







