INTRODUCTION
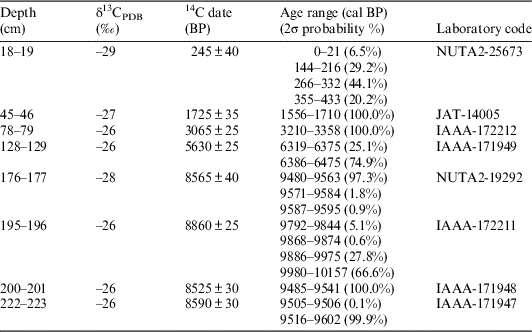
The volcanic Aleutian Islands form an arc from the Alaska Peninsula to the Kamchatka Peninsula of Russia, as shown in Figure 1. Adak Island of the Andreanof Islands group lies in the central Aleutians. Studies of vegetation changes in the Aleutian Islands have previously been conducted on Adak Island (Heusser Reference Heusser1978), Atka Island (Heusser Reference Heusser1990), and Attu Island (Heusser Reference Heusser1990). Heusser (Reference Heusser1978) described a paleovegetation change on Adak Island by identifying three major pollen assemblages: (1) Cyperaceae-Salix-Empetrum-Lycopodium (before 10 cal ka BP), (2) Cyperaceae-Poaceae (10–3 cal ka BP), and (3) Empetrum (after 3 cal ka BP). The climate of the early and late Holocene was cold and windy, whereas the middle Holocene was characterized by a warm climate; the author of that study also concluded that the change in vegetation resulted from climatic variation, not volcanic ash fall. Many dated tephra layers are interspersed within the Adak sediments; therefore, it is possible to conduct a detailed chronological analysis (Black Reference Black1976).
Figure 1 Map showing location of sampling point.
To examine vegetation changes on Adak Island, a 233-cm-long sediment core (ADK13083002) was collected from Haven Lake in the northern part of the island in August 2013. Accelerator mass spectrometry (AMS) radiocarbon dating of a peat sample was conducted, and the pollen sequences and charcoal particle contents of the sediments were examined. Here, we present these data and discuss the paleovegetation and human activities on Adak Island.
STUDY SETTING AND STRATIGRAPHY
Climate and Vegetation
Adak Island has an oceanic climate, with summer temperatures averaging 7–11°C and winter temperatures near freezing. The average annual precipitation is 1525 mm, with high humidity. Fog, low clouds, and storms frequently occur during the winter months due to the influence of the Aleutian Low (van der Leeden and Troise Reference van der Leeden and Troise1974). Trees are practically nonexistent on Adak Island, while dwarf shrubs such as Salix arctica and S. reticulata occur (Hultén Reference Hultén1960). The current grassland vegetation is characterized by herb and heath (Bank Reference Bank1952; Hultén Reference Hultén1960). The herb vegetation consists primarily of Poaceae, such as Poa arctica, Agrostis borealis, and Trisetum spicatum; Cyperaceae, such as Carex dioica, C. saxatilis, and Juncus arcticus; Ranunculaceae, such as Coptis trifolia, Anemone narcissiflora, and Ranunculus reptans; and Apiaceae, such as Ligusticum scoticum, Angelica lucida, and Conioselinum chinensis. The heath vegetation consists largely of the dwarf shrubs Empetrum nigrum and Phyllodoce aleutica subsp. aleutica; and furthermore Loiseleuria procumbens, Cassiope lycopodioides, Vaccinium uliginosum subsp. microphyllum, and V. vitis-idaea subsp. minus also occur (Heusser Reference Heusser1978).
Stratigraphy
We used a peat sampler to collect a 233-cm-long peaty sediment core (ADK13083002) near Haven Lake (51°54'23.4''N, 176°38'38.4''W; Figure 1), and detected the following six tephra layers: T1 tephra from 14–18 cm, T2 tephra from 40–44 cm, T3 tephra from 72–79 cm, T4 tephra from 121–128 cm, T5 tephra from 154–176 cm, and T6 tephra from 196–200 cm (Figure 2).
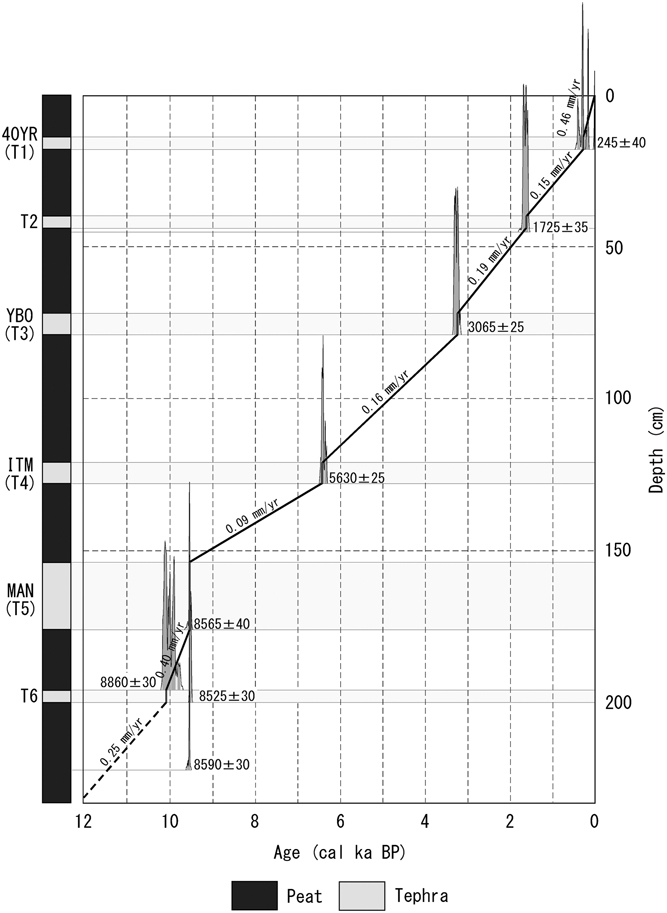
Figure 2 Columnar section showing the stratigraphy and age-depth profile of ADK13083002 core.
ANALYTICAL METHODS
AMS 14C Dating
Seven vascular plant samples were dated using the AMS 14C method. Samples were chemically cleaned with acid-alkali-acid (AAA) treatment and combusted to CO2. The resulting CO2 was cryogenically purified and catalytically converted to graphite (Kitagawa et al. Reference Kitagawa, Masuzawa, Nakamura and Matsumoto1993). The 14C age of each sample was measured along with standards using the HVEE Tandetron AMS system at Nagoya University (Nakamura et al. Reference Nakamura, Niu, Oda, Ikeda, Minami, Ohta and Oda2004), the NEC 15SDH-2 AMS system (JAEA–AMS–TONO) at Tono Geoscience Center, JAEA (Saito-Kokubu et al. 2015), or the NEC Peletron 9SDH-2 AMS system at the Institute of Accelerator Analysis, Ltd. (IAA). The systems measured all three carbon isotopes in both samples and the NIST oxalic acid (HOxII) standard.
The 14C content of dead graphite was also measured to estimate the 14C background level. The 14C contents of the samples and the standard were calculated by subtracting the 14C concentration from the background 14C concentration. We corrected for carbon isotopic fractionation using the 13C/12C ratio (δ13CPDB) measured by AMS. The 14C error was evaluated by the 14C reproducibility of repeated sample and standard measurements and errors in 14C background removal calculations. Conventional 14C dates were calibrated to a calendar year timescale using the IntCal13 dataset (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) and the CALIB 7.1 computer program (Stuiver and Reimer Reference Stuiver and Reimer1993).
Pollen and Charcoal Analyses
We collected several grams of sediment for pollen analysis at 10-cm increments along the length of the core. Fossil pollen and spores were extracted by treatment with 10% KOH, a ZnCl2 solution, and Erdtman’s acetolysis method (Erdtman Reference Erdtman1934). Following acetolysis, samples were dehydrated using an ethanol series (30, 60, and 99.5%) and treated with xylene for pollen analysis. The samples were then mounted in Eukitt medium (Fujiki et al. Reference Fujiki, Okuno, Moriwaki, Nakamura, Kawai, McComack, Cowan and Maoate2014) for observation under a light microscope. Over 500 pollen grains (excluding spores) were counted in each sample. The percentage of each taxon was based on the total pollen count. Identification of fossil pollen was based on Nakamura (Reference Nakamura1980a, Reference Nakamura1980b) and Fujiki et al. (Reference Fujiki, Miyoshi and Kimura2016), and that of fossil spores was based on Nasu and Seto (Reference Nasu and Seto1986a, Reference Nasu and Seto1986b). The species-level identification of trilete type fern spores, such as Lycopodium and Selaginella, was based on Heusser and Pettet (Reference Heusser and Peteet1988). Charcoal particles in the sample were also counted and their cross-sectional areas were measured using a Nikon DS Camera Control Unit DS-L3.
RESULTS
Radiocarbon Dating and Sedimentation Rate
The AMS 14C dating of eight samples yielded ages of 8590 ± 30 BP (IAAA-171947) for 223–222 cm, 8525 ± 30 BP (IAAA-171948) for 201–200 cm, 8860 ± 30 BP (IAAA-172211) for 196–195 cm, 8565 ± 40 BP (NUTA2-19292) for 177–176 cm, 5630 ± 25 BP (IAAA-171949) for 128–129 cm, 3065 ± 25 BP (IAAA-172212) for 79–78 cm, and 1725 ± 35 BP (JAT-14005) for 46–45 cm, and 245 ± 40 BP (NUTA2-25673) for 18–19 cm (Table 1; Figure 2). Their calendar ages were ca. 9.5, 9.5, 10.1, 9.5, 6.4, 3.3, 1.6 and 0.3 cal ka BP, respectively (Table 1).
Table 1 Results of AMS 14C dating using plant fragments.

We compared this stratigraphic description with the obtained 14C ages as well as the existing stratigraphic description (Black Reference Black1976; O'Leary Reference O’Leary2001; Okuno et al. Reference Okuno, Wada, Nakamura, Gualtieri, Brenn, Dixie and Torii2012; Krawiec et al. Reference Krawiec, Kaufman and Vaillencourt2013) and correlated the four tephras as follows: T1 tephra from 14–18 cm is Forty Years (40YR), T3 tephra from 72–79 cm is YBO, T4 tephra from 121–128 cm is Intermediate (ITM), and T5 tephra from 154–176 cm is Main (MAN) (Figure 2). Okuno et al. (Reference Okuno, Wada, Nakamura, Gualtieri, Brenn, Dixie and Torii2012) and Krawiec et al. (Reference Krawiec, Kaufman and Vaillencourt2013) reported eruption ages of 0.4, 3.6, 7.2, and 9.5 cal ka BP for 40YR, YBO, ITM, and MAN, respectively.
Because the tephra was deposited rapidly, the sedimentation rate of the core was calculated using the same age between, above, and below the tephra (Figure 2). The sedimentation rate between the T6 and MAN tephras is ca. 0.40 mm/yr. The sedimentation rate decreased from the MAN to ITM tephras (ca. 0.09 mm/yr). The sedimentation rate increased from the ITM to 40YR tephras (ca. 0.15–0.19 mm/yr). The sedimentation rate further increased from 40YR to the upper layer (0.46 mm/yr). The average sedimentation rate above the T6 tephra (ca. 0.25 mm/yr) was applied as the sedimentation rate below the T6 tephra.
Pollen Assemblages
We identified 35 varieties of fossil pollen grains and spores, which were classified as non-arboreal pollen (NAP) and spores (S). The NAP included Pinus, Betula, Alnus, Salix, Ilex, Empetrum, Poaceae, Cyperaceae, Fritillaria, Rosaceae, Myriophyllum, Linnaea, Geranium, Onagraceae, Polygonum, Caryophyllaceae, Anemone, Coptis, other Ranunculaceae, Apiaceae, Artemisia, and other Asteraceae, while the S included monolete type, Lycopodium alpinum, L. annotinum, L. clavatum, L. complanatum, L. inundatum, L. selago, L. sitchense, Selaginella selaginoides, S. wallacei, S. watsonii, and Sphagnaceae.
Figure 3 shows the pollen and spore data. Empetrum and Poaceae pollen grains and Lycopodiaceae spores were dominant in all layers. The core was divided into four zones (HL-1, HL-2, HL-3, and HL-4) based on pollen composition.

Figure 3 Pollen and spore diagram for ADK13083002 core.
HL-4 (233–176 cm; 11.8–9.5 cal ka BP)
Alnus pollen and pollen of the dwarf shrub Salix were minimal, and Salix pollen disappeared above the T6 tephra layer. The Alnus pollen concentration was very low in all layers. Empetrum pollen increased near the T6 layer, but decreased above it. Conversely, Poaceae pollen decreased near the T6 layer, but increased above it. Cyperaceae pollen was dominant throughout HL-4. Asteraceae pollen, including Artemisia, decreased, while monolete type spores rapidly increased above the T6 layer. Lycopodium annotinum and L. clavatum spores were present in high concentrations, while Selaginella selaginoides spores were present in low percentages beneath the T6 layer. The number of L. selago spores decreased near the T6 layer. Sphagnaceae spores increased beneath the T6 layer.
HL-3 (154–124 cm; 9.5–6.4 cal ka BP)
Betula pollen appeared in this zone, and was present in low amounts in the remaining pollen zones. Cyperaceae pollen, monolete-type Lycopodium annotinum, and L. clavatum spores, decreased. Apiaceae pollen increased slightly, but then decreased throughout the HL-3 zone. Ranunculaceae pollen concentrations, including those of Anemone and Coptis, were low, but increased over time. The numbers of Lycopodium spores decreased rapidly, while those of Sphagnaceae spores decreased slightly. Selaginella selaginoides spores were not present.
HL-2 (121–60 cm; 6.4–2.3 cal ka BP)
Pinus and Salix pollen grains reappeared in low amounts, but Salix pollen disappeared above the YBO layer. Empetrum pollen drastically decreased just above the ITM tephra layer, but then began to increase. Cyperaceae pollen increased above the ITM tephra layer, but decreased above 100 cm. Ranunculaceae pollen, including those of Anemone and Coptis, increased above the ITM tephra layer, but decreased above the YBO layer. Apiaceae pollen increased rapidly at 70 cm and was found in higher concentrations than in the HL-3 zone. Monolete-type and Lycopodium spores increased above the ITM tephra layer, and then decreased above the YBO layer.
HL-1 (60–0 cm; 2.3 cal ka BP–present)
Empetrum pollen decreased, while other herb pollen grains and spores increased above the T2 tephra layer. Lycopodium selago and Selaginella selaginoides spores appeared above the T2 tephra layer.
Charcoal Analysis
Charcoal particles with a cross-sectional area of approximately 10 μm2 were present in all layers. There were 25–50 charcoal particles in the HL-4 zone and approximately 50 particles in the HL-3 zone. The numbers of charcoal particles increased to more than 100 above the HL-2 zone (Figure 4). Furthermore, charcoal particles with an area of 400 μm2 or larger appeared above the HL-2 zone. The total cross-sectional areas of charcoal particles were 100–500 μm2 in the HL-4 zone, 500–1000 μm2 in the HL-3 zone, and 1000–2000 μm2 in the HL-2 zone; areas increased suddenly to 1500–4500 μm2 above the HL-1 zone (Figure 4).

Figure 4 Charcoal particle diagram for ADK13083002 core.
DISCUSSION
Vegetation Changes
The presence and percentages of pollen grains changed above and below the tephra layers. Lycopodium selago and Selaginella selaginoides spores, indicative of a cold climate (Heusser and Peteet Reference Heusser and Peteet1988; Takiya and Hagiwara Reference Takiya and Hagiwara1997), appeared in the HL-4 and HL-1 zones. These results support findings from Heusser (Reference Heusser1978) that imply the climate was relatively dry and windy until 9.5 cal ka BP and after 1.5 cal ka BP, but relatively warm from 9.5 to 1.5 cal ka BP. As in Heusser (Reference Heusser1978), no Salix pollen was detected from ca 9.5 to 4.7 cal ka BP. This phenomenon is thought to be because of the disappearance of willows as dwarf shrubs that grow in lowland, such as Salix barclayi, due to transgression in the warm period (Heusser Reference Heusser1978), and the Salix currently distributed in Adak Island is thought to be a species that cannot grow at low altitude (Heusser Reference Heusser1978). Empetrum plants are short, have small, needle-like ericoid leaves, and can tolerate strong and cold winds, fog, and freezing temperatures. Therefore, this plant can grow in very windy environments where other species cannot survive (Koizumi Reference Koizumi2009). In the period when the percentage of Empetrum pollen was high, we think that strong winds from the Aleutian Low dominated. It is thought that the increase in Poaceae pollen in the middle of the HL-1 zone was an effect of T2 tephra fall (Heusser Reference Heusser1978). The same phenomenon occurred at Tashiro Mire at Aomori, Japan (Kito et al. Reference Kito, Ohtsuki, Tsuji and Tsuji2017), and Utasai Bog in Hokkaido, Japan (Hughes et al. Reference Hughes, Mallon, Brown, Essex, Stanford and Hotes2013). Regarding the cause of this phenomenon, Kito et al. (Reference Kito, Ohtsuki, Tsuji and Tsuji2017) suggested that deposition of volcanic ash formed an impermeable layer that raised the water level. Hughes et al. (Reference Hughes, Mallon, Brown, Essex, Stanford and Hotes2013) speculated that tephra supplied nutrients and minerals to the water. Pinus, Betula, and Alnus plants do not currently grow on Adak Island. These fossil pollen grains were also detected by Heusser (Reference Heusser1978), as well as in our analysis. These fossil pollen grains have also been detected on other islands where the plants do not now grow naturally, and we think that westerlies blew the pollen grains to Adak Island. The percentages of Pinus, Betula, and Alnus pollen grains were slightly higher on Attu Island (Heusser Reference Heusser1990) than on Atka and Adak Islands. Therefore, it is possible that the pollen grains were transported from the Kamchatka Peninsula and Commander Islands (Heusser Reference Heusser1990). The Pinus pollen is considered to be from Pinus pumila distributed in the Kamchatka Peninsula and Commander Islands, and the Betula and Alnus pollen grains are thought to be pollen grains of Betula nana subsp. exilis and Alnus crispa subsp. Sinuate distributed in the Kamchatka Peninsula (Hultén Reference Hultén1960; Heusser Reference Heusser1990).
Charcoal Particles and Aleut Settlement
Aleuts, the indigenous people of the Aleutian Islands, migrated from east to west across the Aleutian Islands (Laughlin et al. Reference Laughlin, Jorgensen and Frohlich1979). Aleuts settled in the eastern Aleutians approximately 9.0 cal ka BP (Davis and Knecht Reference Davis and Knecht2010), arrived in the central Aleutians by approximately 6.0 cal ka BP (O’Leary Reference O’Leary2001; Savinetsky et al. Reference Savinetsky, West, Antipushima, Khassanov, Kiseleva, Krylovich and Pereladov2012), and reached the far western Aleutians by approximately 3.2 cal ka BP (West et al. Reference West, Lefevre, Corbett and Savinetsky1999). We hypothesize that the 10-μm2 charcoal particles in the HL-4 and HL-3 zones were transported to Adak Island from a distance. The numbers of charcoal particles, and especially of those with a cross-sectional area of 500–1000 μm2, increased suddenly above the ITM tephra layer (Figure 4). Aleut shell mounds were also discovered just above the ITM tephra layer (Okuno et al. Reference Okuno, Gualtieri, West, Wilmerding and Nakamura2007; Savinetsky et al. Reference Savinetsky, West, Antipushima, Khassanov, Kiseleva, Krylovich and Pereladov2012); therefore, it is possible that the large charcoal particles were transported to Adak Island from nearby Aleut settlements. These results support the earlier evidence that Aleuts settled on Adak Island after deposition of the ITM tephra at locations such as the Tutiakoff site (village ADK-171; Savinetsky et al. Reference Savinetsky, West, Antipushima, Khassanov, Kiseleva, Krylovich and Pereladov2012). Therefore, we think that the vegetation also changed from 6.4 cal ka BP due to anthropogenic activity.
CONCLUSIONS
We conducted pollen and charcoal analyses on a peat sediment core collected from Haven Lake on Adak Island in the central Aleutian Islands. Our results imply changes in paleovegetation that correspond with the settlement of Aleuts on the island. Heath and grassland were the dominant vegetation, and four zones, HL-1, HL-2, HL-3, and HL-4, were identified based on the composition of pollen. These results support the earlier evidence that Aleuts settled on Adak Island approximately 7.2 cal ka BP.
ACKNOWLEDGMENTS
This study was partly supported by a Grant-in-Aid (23501254) for Scientific Research from the Japan Society for the Promotion of Science (JSPS), Japan. AMS 14C dating was conducted partly by AMS (JAEA-AMS-TONO) under the Common-Use Facility Program of JAEA. Permission to work on Adak Island was granted by the Aleut Corporation and the Alaska Maritime National Wildlife Refuge. We thank the U.S. Fish and Wildlife Service and VECO Polar Resources for logistical field support. We also thank the City of Adak, Alaska, and its citizens for welcoming us into their community during the survey.