INTRODUCTION
The effective removal of carbon-containing contaminants from samples prior to radiocarbon (14C) dating is vital to ensure that reliable dates are produced. The presence of even small amounts of material of a different age from the object being dated can result in an erroneous date. While many environmental contaminants, notably carbonates from sediments and hard water and humic and other organic acids, are removed during routine acid-base-acid (ABA) pretreatments, additional steps involving one or more organic solvent(s) can be required for the removal of conservation treatments such as consolidants, adhesives, waxes, and varnishes.
Identifying a suitable pretreatment to remove a conservation treatment, however, can be complicated by many different factors. In some cases, the presence of a treatment is clearly visible on the sample, or records may have been kept of prior conservation work. Where a specific treatment has been recorded, it may be possible for the 14C laboratory to tailor the pretreatment accordingly. Often, however, a treatment may only be suspected, or assumed to be present by researchers wishing to err on the side of caution when submitting a sample for dating. Multiple treatments may also have been applied to an object, possibly at different times.
The exact details of conservation treatment, even if recorded, are often unknown or imprecise. In the early days of such treatment, in particular at the start of the 20th century, thorough museum records were not always kept. The word “conserved” may be all the information available to researchers. Materials used for conservation have also changed over time, both as completely new products have been developed or become preferred as previous treatments have been observed to deteriorate over time, or as precise compositions of specific treatments have been refined by manufacturers while retaining the same brand name. Methods of application may also have varied, and some conservators may have mixed their own unique treatments to suit their needs.
Various techniques have been applied to study objects prior to dating to try to identify the presence of potential conservation contaminants, including FTIR (e.g. D’Elia et al. Reference D’Elia, Gianfrate, Quarta, Giotta, Giancane and Calcagnile2007), Raman spectroscopy (e.g. Ohlídalová et al. Reference Ohlídalová, Kučerová and Novotná2006) and pyrolysis-GC/MS (e.g. Nishimoto Reference Nishimoto2011; Ostapkowicz et al. Reference Ostapkowicz, Schulting, Wheeler, Newsom, Brock, Bull and Snoeck2017). However, these techniques are not always infallible, depending on detection limits and the fact that some conservation treatments are not chemically distinguishable from the samples to which they have been applied (e.g. fish glue on bone). Small sample sizes may also limit the amount of additional analysis that can be undertaken as well as 14C dating a specimen.
To further confuse matters, generic terms such as “Paraloid” and “PVA” are often used, despite the fact that such names can refer to a range of substances with differing chemical and physical properties, and hence potentially different requirements for successful removal. Some conservation treatments may be recorded by colloquial names, or may be brands from overseas, which have different names in different countries. Paraloid (commonly referred to as Acryloid in the USA), can refer to a number of thermoplastic acrylic polymers, such as Paraloid B-44, B-67, and B-72, and may be applied in solvent or as a prepared product that may contain additives. The term PVA is sometimes incorrectly used for a wide range of vinyl acetate-derived polymers, including poly(vinyl acetals) (PVA)—now largely discontinued; poly(vinyl acetate) (PVAc or PVAC) resins or emulsions; and poly(vinyl alcohols) (PVAL). These materials can have very different compositions and hence different properties and solubilities.
While some conservation treatments are considered to be stable, many deteriorate with time, light, heat and/or air (oxygen). All polymers can realistically be expected to oxidize over time, although the speed and mechanism vary (Horie Reference Horie2010). Such deterioration is often not observed for many years after first application. Some substances, especially the vinyl acetate-derived polymers, can cross-link (either internally or with the material they are applied to) or undergo oxidation or chain scissioning (whereby a polymer degrades in the absence of a chemical agent, in particular oxygen). All these changes can alter the solubility of the material, potentially rendering a substance soluble in a particular solvent when first applied insoluble—and potentially irremovable—with time. Likewise, a substance that may be soluble when applied to a non-porous material may not be removed with the same solvent(s) when applied to a porous substrate. Solubility may also be affected by the presence of plasticizers or other stabilizers added to some more modern consolidants. Different modes of application can result in differential ease of removal of certain substances. For example, France et al. (Reference France, Giaccai and Doney2015) observed that Paraloid B-72 was easier to remove from bones when applied with 100% acetone or 100% ethanol than when xylenes had been added to the original application to aid dissolution and transport of the mixture into the bone.
Demonstrating the effective removal of one or more specific conservation treatment can be difficult. Treatment removal protocols that are described for conservation purposes may not always be sufficient for the purposes of stable isotope analysis or 14C dating, which is even more susceptible to the effects of contamination. Studies investigating the effect of application and removal of conservation treatment on the stable isotopic signatures of bones, for example, have found varying results. Tuross and Fogel (Reference Tuross and Fogel1994) observed that while PVA (polyvinyl acetate) did not influence collagen δ13C or δ15N values, Rhoplex (an acrylic emulsion) did affect collagen δ13C values but not δ15N (although as nitrogen is not present in either PVA or Rhoplex the δ15N values were not expected to be affected). France et al. (Reference France, Giaccai and Cano2011, Reference France, Giaccai and Doney2015) observed that while the application and removal of PVAc, Paraloid B-72 and Butvar 98 (a polyvinyl butyral resin) did not influence collagen δ13C and δ15N values or those of carbonate δ13C or phosphate δ18O in hydroxyapatite, the δ18O of hydroxyapatite carbonate was affected.
Undertaking experimental work to test protocols for removing conservation treatments can also be complicated, costly and time consuming, and, in some instances, unreliable. The effects of deterioration over time are very difficult to replicate, and artificial aging procedures are only a crude approximation; Horie (Reference Horie2010) noted that approximately 50% of artificial aging protocols did not replicate natural situations. The cost of deliberately contaminating known-age material with a specific substance and then demonstrating its effective removal by 14C dating is often prohibitively high (and too time-consuming) for many dating projects. Unless a specific protocol is known to remove a particular treatment, 14C laboratories often rely on their own in-house generic sequence of solvent washes, and may then use quality control indicators such as stable isotope analysis, C:N ratio of bone, (electron or optical) microscopy or FTIR to check for the presence of remaining contaminant material. At the ORAU, a sequence of washes with acetone, methanol and chloroform is applied to all samples that are either known to be contaminated (but where the exact contaminant is often not known), or where a contaminant is suspected but not confirmed (Brock et al. Reference Brock, Higham, Ditchfield and Bronk Ramsey2010). However, whatever the combination of organic solvents used, it is always preferable to use the minimum number possible in an elutrope sequence (whereby each subsequent solvent removes the previous one, ending with water) to avoid adding contamination to the sample by incomplete removal of a solvent. Prolonged heating or ultrasonic treatment in solvents can also cause break down and loss of poorly preserved samples, especially collagen, and should be used with caution.
Bruhn et al. (Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001) undertook a valuable study, investigating the removal of a range of common conservation materials applied to known age wood. While they found that some substances (e.g. methyl cellulose and polyethylene glycol (PEG)) were removed during the ABA procedure, they recommended a sequential soxhlet treatment with 5 organic solvents (tetrahydrofuran, chloroform, petroleum ether, acetone, methanol) and finally water for others such as epoxy resin, paraffin, and unknown substances. This protocol has been applied to several other studies including the removal of glue from a reindeer mandible (Ramirez Rozzi et al. Reference Ramirez Rozzi, d’Errico, Vanhaeren, Grootes, Kerautret and Dujardin2009), and a range of conservation treatments and chain alkanes from Chinese oracle bones (Yuan et al. Reference Yuan, Wu, Liu, Guo, Cheng, Pan and Wang2007). The original study, however, is potentially limited as the conservation treatments were applied to the known-age material and then removed immediately, without allowing for aging, deterioration or cross-linking, so it is unclear how effective this protocol is for the removal of conservation substances that have aged. (The authors know from experience, for example, that aged PEG can be extremely difficult to remove completely from wood.) This process is also time-consuming, and may not be suitable for large batches of samples.
An alternative approach would be to contaminate a carbon-free analog to archaeological materials with specific conservation treatments and assess the effectiveness of a range of different pretreatment protocols in removing the contaminant by measuring the amount of carbon remaining afterwards using mass spectrometry. While this could provide a cheaper and quicker alternative to deliberately contaminating known-age archaeological materials and then dating the pretreated material, no single material could be analogous to the range of materials (and variations in their states of preservation) commonly encountered by 14C laboratories.
In 2011, Dee et al. published the results from such a study, whereby a range of substances (including epoxy resin, paraffin, and a water soluble PVAc adhesive) had been used to contaminate Chromosorb®, an absorbent, non-carbon-containing, silica-based polymer with a high surface area to volume ratio, before being artificially aged. A range of pretreatment methods was then tested to demonstrate their effectiveness at removing the contaminant(s). Chromosorb is likely to be much more porous than most well-preserved organic archaeological materials such as wood and bone, and would not form cross-links with the conservation treatment itself as some organic substrates would. However, this approach allows a wide range of potential pretreatment protocols to be tested relatively quickly and cheaply before identifying the most suitable protocols for further testing by deliberately contaminating known-age material, aging it, and then dating the pretreated material (as was done, for example when identifying the most suitable pretreatment protocol for removing pitch from wooden artefacts from Trinidad’s Pitch Lake: Brock et al., in press).
Following on from Dee et al.’s (2011) initial results—which found that the glues and adhesives tested were not removed sufficiently for 14C dating with the methods applied—it was decided to concentrate on several particular types of conservation treatments commonly encountered at the ORAU, to determine the effectiveness of routine removal protocols (or to identify more thorough ones, if possible) and to investigate the potential variation between different brands or types of the same substance.
The adhesives and consolidants selected represent a broad, but by no means exhaustive, range of some commonly used materials in conservation literature and anecdotally, spanning a wide period of use from pre-1900 to the present day, and showing a range of aging properties. The dates when these materials were first used and subsequently fell out of use have been documented by Johnson (Reference Johnson1994). However, these dates have been based on published object treatments, while anecdotal evidence from conservators suggests that, in reality, usage continued far longer than suggested.
The initial study began with 3 different types of conservation treatments commonly encountered at the ORAU: shellac (a natural resin), acrylic polymers (e.g. varieties of Paraloid), and vinyl acetate-derived polymers. Three variations of each category were chosen from different suppliers to represent a range of manufacturers’ formulae and application techniques. Some historic adhesives are no longer easily obtainable, and in these cases the closest currently obtainable substitute was used. During the course of our investigations, we received an enquiry about the possibility of dating some bones treated with Zaponlack, an early cellulose nitrate lacquer, and so additional tests were carried out on two modern equivalents.
Shellac
Shellac is a natural resin, often applied as flakes dissolved in hot alcohol (Koob Reference Koob1979). It is commonly encountered as an adhesive on old museum repairs, but went out of favor in the mid-1960s (Johnson Reference Johnson1994) due to coloration and poor aging properties. Some reports, however, suggest its use continued into the 1970s and 1980s (Koob Reference Koob1979, Reference Koob1984). Shellac is known to be soluble in a range of solvents including pyridine, dichloromethane (as the active ingredient in Nitromors), a 50:50 ethanol: acetone mix (Larney Reference Larney1971; Koob Reference Koob1979) and ethanol (e.g. Berglund et al. Reference Berglund, Hakansson and Lagerlund1976). Some anecdotal accounts suggest that methanolic potassium hydroxide is also effective in removing it. The current standard treatment for removal of shellac at ORAU consists of consecutive washes with water and methanol.
Acrylic Resins
Acrylic resins are widely used in both conservation and archaeology fields and are regarded as one of the most stable adhesive coating materials and consolidants used today. One of the most commonly used acrylic resins is Paraloid B-72 (known as Acryloid B-72 in the USA), recommended for use in archaeology since the mid-1980s (Koob Reference Koob1986; Johnson Reference Johnson1994), particularly for its ease of removability (Shelton and Chaney Reference Shelton and Chaney1993). Paraloid B-72 is a methylmethacrylate/ethylacrylate co-polymer, widely produced by several suppliers in both the UK and USA and available as resin beads or pellets and soluble for application in a range of solvents including acetone, ethanol, xylene and toluene, or in solution. However, the composition and solubility of B-72 has changed over time, when the original manufacturer slightly changed the molar ratio of ethyl methacrylate:methyl acrylate in 1978 (Chapman and Mason Reference Chapman and Mason2003). A recent study also found evidence of small quantities of cellulose nitrate and other additives in prepared tubes of HMG Paraloid B-72 that could affect long-term reversibility (Nel and Lau Reference Nel and Lau2009).
Paraloid B-67 is an alternative isobutyl methacrylate polymer supplied as solid pellets and soluble in acetone, methyl ethyl ketone, isopropanol, and Stoddard Solvent/white spirit. It is the most water resistant of the Paraloids, and is often used in conservation as an alternative to B-72 when wishing to avoid the use of polar solvents.
Several methods for removal of Paraloid B-72 have been published; for example, Caforio et al. (Reference Caforio, Fedi, Liccioli and Salvini2013) demonstrated its removal from wood with 2 days soaking and magnetic agitation in chloroform, and France et al. (Reference France, Giaccai and Doney2015) successfully used 100% acetone to remove it from bone. D’Elia et al. (Reference D’Elia, Gianfrate, Quarta, Giotta, Giancane and Calcagnile2007) reported that Paraloid B-72 applied to an osteological sample for 8 hr at room temperature was removed by routine bone pretreatment (acid demineralization and gelatinization only) without the need for an organic solvent treatment. The current ORAU method for removal of all varieties of Paraloid consists of an acetone/methanol/chloroform sequence. As Paraloid B-72 is usually applied dissolved in either toluene or acetone (Johnson Reference Johnson1994), the suitability of removal using toluene was also investigated.
Polyvinyl Acetate-Derived Polymers
As previously highlighted, the term PVA is often used when submitting samples for dating to cover a wide range of consolidants with different chemical compositions. As such, it is perhaps optimistic to believe that one specific treatment would be suitable for removing all polyvinyl acetate-derived polymers. The current ORAU in-house method is a water/acetone/water/methanol sequence first described by Stevens and Hedges (Reference Stevens and Hedges2004) for the removal of PVA from bones and teeth prior to stable isotope (δ13C, δ15N) analysis, but the origin of the method is not recorded.
Polyvinyl acetate emulsion (also known under the generic name Elmer’s carpenter’s glue) was used from the 1950s onwards and commonly applied in field archaeology (Johnson Reference Johnson1994), but is no longer used in conservation due to cross-linking of the polymer over time reducing solubility. Polyvinyl acetate emulsions are a water-based dispersion that are no longer fully soluble in water once dried. Polyvinyl acetate resins have been used since the mid-1960s by archaeologists and conservators (Johnson Reference Johnson1994), and are usually applied in solutions of acetone or ethanol.
In this study, the effectiveness of acetone for removal of polyvinyl acetate-derivatives (PVAc) was focussed on as many are applied in acetone. France et al. (Reference France, Giaccai and Cano2011) also reported that acetone was the most successful organic solvent for the removal of PVAc.
Cellulose Nitrates
Cellulose nitrate-based adhesives (nitrocellulose lacquers) have been used since the late 19th century as an adhesive and protective coating (Shashoua et al. Reference Shashoua, Bradley and Daniels1992). However, due to poor aging properties such as shrinkage, yellowing, instability, and reduced solubility (Koob Reference Koob1982), they are no longer widely used for consolidation, having mostly been replaced by Paraloid B-72 (Koob Reference Koob1986). Many modern nitrocellulose lacquers rely on plasticizers and other additives to stabilize their durability. Yuan et al. (Reference Yuan, Wu, Liu, Guo, Cheng, Pan and Wang2007) reported that nitrocellulose lacquers can generally be dissolved in Bruhn et al.’s (2001) elutrope soxhlet extraction sequence, and applied a method based on that using tetrahydrofuran, chloroform, petroleum ether, acetone, and methanol to remove contamination from oracle bones.
This study investigated a range of different organic solvent pretreatment protocols to remove 11 conservation treatments that had been applied to Chromosorb and artificially aged. A wide range of conditions are used in published artificial aging studies, so the conservation standard humidity, temperature and time conditions for accelerated corrosion testing of museum display materials were chosen for this study. These conditions were first proposed by Oddy (Reference Oddy1973), are still widely used today (e.g. Robinet and Thickett Reference Robinet and Thickett2003), and aim to represent severe aging, potentially enhancing the degradation of the conservation polymers. A range of pretreatment protocols were chosen specifically for each consolidant, based on common application techniques, published studies of their removal, and the current in-house protocols at the ORAU. Thin films of each treatment were analyzed with FTIR, to record the spectra for each conservation treatment, and to observe molecular and chemical variations between similar materials.
METHODS AND MATERIALS
Materials
A total of 11 conservation treatments were tested as follows:
Shellac (all prepared from dried flakes as a saturated solution in ethanol):
-
1. dewaxed, decolorized shellac (A.F. Suter & Co. Ltd, no. 4894).
-
2. lemon shellac (A.F. Suter & Co. Ltd, no. 4893).
-
3. dark brown shellac (unknown supplier, provided by RAMM, Exeter).
Acrylic polymers:
-
1. Paraloid B-67 10% w/v solution in white spirit, prepared from solid pellets.
-
2. Paraloid B-72, 5% w/v solution in acetone, prepared from solid pellets.
-
3. Paraloid B-72, supplied prepared by HMG Ltd.
Polyvinyl acetate (PVA) derivatives:
-
1. Common all purpose adhesive, Colourfull Ltd. Polyvinyl acetate emulsion, used in the supplied preparation.
-
2. Emultex 427. Polyvinyl alcohol stabilized, vinyl acetate/dibutyl maleate copolymer emulsion, 10% w/v suspension in distilled water.
-
3. Mowilith 50. Polyvinyl acetate resin, prepared as a 2% w/v solution in acetone.
Cellulose nitrate lacquers:
-
1. Frigilene, used as supplied in xylene.
-
2. Ercalene, used as supplied in xylene.
Chromosorb® Contamination and Artificial Aging
Individual aliquots of Chromosorb® (W/AW, mesh size 30–60, Phase Separations Ltd, UK) were contaminated with each conservation treatment in the ratio of 4:1 before being artificially aged at 60°C and 100% humidity for 1 month (as described by Dee et al. Reference Dee, Brock, Bowles and Bronk Ramsey2011). Note that this ratio represents what was applied to the Chromosorb substrate initially: some volatile contaminants may have escaped prior to or during the artificial aging process, although precautions were taken to minimize this effect. Any contaminant remaining as gas after the aging stage would certainly have dissipated during the pretreatment tests.
Chemical Methods for Removing Contaminants
A range of methods was applied to each contaminant, including the current standard treatment applied at ORAU, as well as others where suggested suitable in the literature or by conservators. All solvents used were Distol (residue analysis reagent) grade. All water washes or aqueous solutions used ultra-pure Milli-QTM (Millipore Corporation) water.
Solvent washes were carried out in triplicate with 10-20 ml solvent for ca. 10 mg contaminated Chromosorb in a precleaned glass test tube, with heating in a dri-block for 45–60 min, unless otherwise stated. All samples were left to air-dry in a fume hood for a minimum of overnight—usually longer—before being weighed and transferred into pre-cleaned tin capsules for mass spectrometry. Where a base wash was tested, a subsequent acid wash was added to remove any atmospheric CO2 incorporated into the sample during the base step.
A total of 17 discrete pretreatment protocols were tested as appropriate (although not all on each category of conservation treatment), as follows:
-
A. “Standard” treatment applied to Paraloid and any unknown contaminants at ORAU: sequential washes for each with acetone (45°C), methanol (45°C), chloroform (room temperature).
-
B. 0.2 M NaOH (80°C) followed by 1 M HCl (80°C) with 3 water rinses after both steps.
-
C. 1 M NaOH (80°C) followed by 1 M HCl (80°C), with 3 water rinses after both steps.
-
D. 5×dichloromethane (60 min each, room temperature)
-
E. 1 M KOH in methanol (45°C, 60 min), followed by 3 water rinses.
-
F. Water (45°C), followed by methanol (45°C). (ORAU standard treatment for shellac removal)
-
G. Petroleum ether (40°–60°C fraction) (45°C)
-
H. Toluene (45°C)
-
I. Water (80°C)
-
J. Acetone soxhlet (3 hr)
-
K. Water (45°C), acetone (45°C), water (45°C), methanol (45°C). (ORAU standard treatment for PVA removal).
-
L. Acetone (45°C)
-
M. Methanol (45°C)
-
N. Chloroform (room temperature)
-
O. 2×water (80°C)
-
P. 3×20 min water rinses with ultrasonication (room temperature)
-
Q. 1×60 min water rinse with ultrasonication (room temperature)
Mass Spectrometry
The amount of carbon remaining on each aliquot of Chromosorb after pretreatment was measured by combusting ca. 5–10 mg quantities in cleaned tin capsules in an elemental analyzer coupled to a mass spectrometer, as described by Brock et al. (Reference Brock, Higham, Ditchfield and Bronk Ramsey2010). In order to produce a baseline of the concentration of carbon on any given amount of contaminated stock material, triplicate aliquots of 10 mg were also taken directly from uncontaminated Chromosorb and each contaminated Chromosorb stock and analyzed in the same way.
For each of the contaminants applied, a degree of variation was observed in the amount of carbon remaining (ppm) on the Chromosorb across the triplicate results. This variation was represented by a dimensionless quantity called “heterogeneity” (h). It was calculated by taking the standard deviation (σ) of the results (n=3, unless otherwise stated) and expressing it as a percentage of the average carbon remaining (μ)
Higher heterogeneity values meant greater variation in the amounts of contamination remaining. To determine the effectiveness of each pretreatment procedure, the average carbon remaining for each contaminant (μ) was expressed as a percentage of the average concentration of the original stock.
FTIR Spectroscopy
Films of each of the adhesive stocks were cast to prepare samples for FTIR analysis. Melinex® polyester film was folded into individual trays measuring approximately 10 cm×5 cm and 1 cm high. A quantity of each stock was decanted to cover the base of each tray and these were left in ambient conditions under the fume hood to dry and any solvents to evaporate. When completely dry, the adhesive films were peeled from each tray.
The samples were analyzed by Fourier transform infrared spectroscopy using attenuated total reflectance mode with a diamond crystal (FTIR-ATR, Agilent Technologies Cary 640 FTIR with GladiATRTM, Pike Technologies). Each sample was scanned 64 times. The background was subtracted and a baseline correction was carried out using Agilent Resolution Pro software, and the spectra were normalized to the highest peak for presentation purposes.
RESULTS AND DISCUSSION
Each of the 11 conservation treatments contained different concentrations of carbon and each also delivered varying proportions of that carbon to the Chromosorb during the artificial aging process. The amount of carbon present on the Chromosorb stock samples and remaining after pretreatment for each of the conservation treatments is given in Table 1. The sample heterogeneity of the contaminated Chromosorb stock samples indicate that the conservation treatment was fairly evenly mixed throughout the stock for all samples, with the exception of Frigilene. The results show differing degrees of success in removing the different types of conservation treatments. In general, the sample heterogeneity (Table 1) values are low, demonstrating fairly consistent contamination remaining on the Chromosorb where tests were performed in triplicate. In most cases where heterogeneity was high, the percentage remaining carbon was very low (see below).
Table 1 Treatments applied to each individual contaminated batch of Chromosorb, including the sample heterogeneity (i.e. the variation in carbon present between replicate samples, as detected by mass spectrometry) and the % remaining carbon after pretreatment. All analyses were undertaken in triplicate, except for those marked * which were undertaken only once, and hence no data are available for sample heterogeneity.

FTIR spectra of thin films of all 11 conservation treatments tested in this study are presented in Figure 1. The spectra exhibit the characteristic peaks associated with each type of conservation treatment, but also highlight differences—albeit often subtle ones—between individual varieties of nominally the same treatment. While the spectra can be useful to aid identification of an unknown substance applied to a museum artifact, care must be taken to allow for these variations, the spectra of the artifact itself (e.g. wood, bone, parchment etc.), potential alterations to the chemistry of both the conservation treatment and the sample material caused by processes such as aging, thermal or UV decomposition, or oxidation, and the effect of different sampling geometries, collection methods, and instruments. For all samples, it should be noted that peaks in the 2400–2200 cm–1 range indicate the presence of atmospheric CO2 and are hence not diagnostic of any characteristics of any of the substances analyzed.
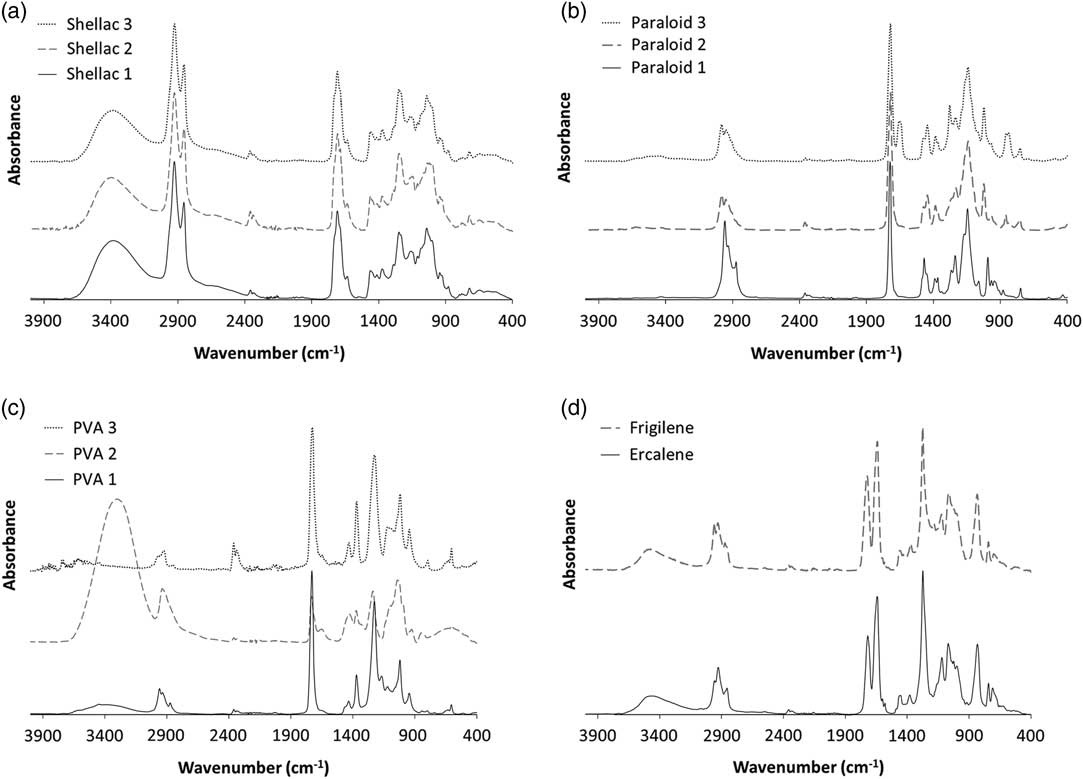
Figure 1 FTIR spectra of films of each of the conservation treatments tested. Sample numbers refer to specific treatments, as listed in Table 1: (a) shellac; (b) Paraloid; (c) polyvinyl acetate derivatives; (d) cellulose nitrate treatments.
Shellac
All three varieties of shellac were completely removed with the routine pretreatment applied for removing shellac at the ORAU, consisting of sequential washes with water and methanol at 45°C. Sodium hydroxide, both at 0.2 M and 1 M, was also successful to differing extents for the different types, removing nearly all the shellac from sample 1 (dewaxed and decolorized shellac), but with 15–18% C remaining with 0.2 M NaOH and 5–9% remaining with 1 M NaOH for samples 2 and 3. However, this is still encouraging given that most pretreatments would include a solvent wash prior to routine ABA treatment, thus providing an additional step to remove shellac without prolonging the treatment. Methanolic KOH also removed most of the shellac, leaving just 2–3% remaining contamination for all three samples. This remaining carbon may just have been modern atmospheric carbon dioxide absorbed from the atmosphere during the testing, as the base wash was not followed by an acid wash to remove it.
The use of dichloromethane to remove shellac was tested as it is the active ingredient in Nitromors, reported by Larney (Reference Larney1971) as suitable for removing aged shellac from ceramics (although Koob (Reference Koob1979) found its use to remove shellac both slow and messy). However, neither dichloromethane nor the acetone/methanol/chloroform sequence removed a significant amount from any of the shellac samples, and would not be suitable for pretreating samples for 14C dating.
The three samples of shellac produced almost identical FTIR spectra (Figure 1a), being dominated by a broad peak at around 3400 cm–1 (hydroxyl O-H), two peaks in the hydrocarbon C-H region (3000–2800 cm–1), and a distinct peak at 1710 cm–1 (C=O stretching of esters) (Khairuddin et al. Reference Khairuddin, Utomo, Wulandari, A’an Zahrotol and Clegg2016).
Acrylic Resins (Paraloid)
All three types of Paraloid were effectively removed from the Chromosorb using the acetone/methanol/chloroform sequence. Both petroleum ether and toluene were tested for sample 1 (B-67) only, and both were ineffective at removing it, leaving 25% and 17% contamination remaining, respectively. Note that, despite the very high sample heterogeneity values (591 and 239, respectively), the maximum remaining contamination for samples 2 and 3 (both B-72) after acetone/methanol/chloroform treatment was negligible (0.06% and 0.05%, respectively).
The FTIR spectra of all three samples of Paraloid (Figure 1b) are characterized by peaks at ca. 1720 cm–1 (C-O carbonyl stretching) and a strong unconjugated ester band at 1140 cm–1 (Nel et al. Reference Nel, Lonetti, Lau, Tam, Sagona and Sloggett2010). Paraloid 3, the pre-prepared HMG Paraloid B-72, also appears to contain cellulose nitrate (as previously observed by Nel and Lau Reference Nel and Lau2009), characterized by the nitrate-stretching peaks at 1645 cm–1 and 1280 cm–1 (Nel et al. Reference Nel, Lonetti, Lau, Tam, Sagona and Sloggett2010). Paraloid B-67 has much higher absorbance in the hydrocarbon C-H stretching region between 3000–2800 cm–1 than the two Paraloid B-72 samples.
Polyvinyl Acetate-Derived Polymers
The polyvinyl acetate-derived treatments (PVA) proved to be far harder to remove than either the shellac or the Paraloid samples, with no individual treatment successfully removing any of the varieties, and with significant differences in the results between the three types tested. The most successful treatment for samples 1 (common all purpose adhesive, a polyvinyl acetate) and 2 (Emultex 427) was the method applied routinely at Oxford, a sequence of water, acetone, water and methanol, but that still left 33% and 19% contamination remaining, respectively. In fact, several methods appeared to increase the amount of carbon absorbed by the Chromosorb for sample 1 (Table 1), suggesting that this particular polyvinyl acetate emulsion was not suitable for removal by organic solvents. For sample 3 (Mowilith 50, a polyvinyl acetate resin), the water-based treatments were most successful, but still left 15–23% contamination remaining. However, it is possible that a subsequent ABA pretreatment may remove additional PVA that remains after a solvent wash, during the numerous water washes applied.
The failure of any of the pretreatments tested to successfully remove any of the three polyvinyl acetate derivatives is not unexpected, and is in agreement with Dee et al.’s (2011) study, where 27% C remained after the routine ORAU water/acetone/water/methanol sequence had been applied to Chromosorb contaminated with a water soluble PVAc adhesive. Our results are in contrast to those of France et al. (Reference France, Giaccai and Cano2011) who demonstrated complete removal of PVAc with acetone. However, there were two key differences between this study and that of France et al. (Reference France, Giaccai and Cano2011). Firstly, the Chromosorb is much more porous, and has a much higher surface area to volume ratio, than bone does. However, these characteristics of Chromosorb do not appear to have affected the ability of organic solvents to remove shellac or Paraloid. Secondly, while the contaminants were artificially aged onto Chromosorb for the purposes of this study, France et al. (Reference France, Giaccai and Cano2011) only submerged bone discs in PVAc solution for 30 min before air-drying prior to removing the PVAc. Although Chromosorb is unlikely to cross-link with any of the conservation treatments studied here like wood or bone may do, the artificial aging may have resulted in cross-links forming within the polyvinyl acetate derivatives themselves, and/or may have resulted in oxidation or other degradation of the conservation treatment itself. Both the internal cross-linking and the degradation of the polymer could have potentially altered its solubility (Horie et al. 2010 and references therein).
Several studies have suggested that organic solvent pretreatments may not be necessary to remove polyvinyl acetate derivatives from bone samples prior to dating as they will be removed during gelatinization (France et al. Reference France, Giaccai and Cano2011) and/or filtration (e.g. Moore et al. Reference Moore, Murray and Schoeninger1989). However, at ORAU, heavily contaminated bone specimens have, on occasion, yielded collagen with elevated C:N atomic weight ratios indicating residual contamination, even after thorough solvent washing as well as routine gelatinization and filtration. It is therefore advised that such samples are avoided for dating if sampling away from PVA is not possible or if techniques such as single amino acid dating of bones are not available.
The FTIR spectra of PVA 2, the polyvinyl alcohol-stabilized, vinyl acetate/dibutyl maleate copolymer Emultex 427, is clearly distinct from the spectra of the two polyvinyl acetate derivatives, PVA 1 (common all purpose adhesive) and PVA3 (Mowilith 50) (Figure 1c). The polyvinyl acetates are characterized by a strong carbonyl peak at 1720 cm–1 (Nel et al. Reference Nel, Lonetti, Lau, Tam, Sagona and Sloggett2010; France et al. Reference France, Giaccai and Cano2011) and a lesser one at 1230 cm–1, likely caused by the C=O acetate group (Law et al. Reference Law, Housley, Hammond and Hedges1991; Nel et al. Reference Nel, Lonetti, Lau, Tam, Sagona and Sloggett2010). These peaks are much smaller in the polyvinyl alcohol spectrum. In contrast, the polyvinyl alcohol, PVA 2, has a broad characteristic peak at 3600–3400 cm–1, indicative of the –OH alcohol group (Law et al. Reference Law, Housley, Hammond and Hedges1991). All three PVA samples demonstrate differences in C-H stretching bands between ca. 3000–2800 cm–1, C-H bending between ca. 1450–1375 cm–1 and C-O stretching between 1300–1000 cm–1. This highlights the variation in molecular structure and sample composition between the three varieties of polyvinyl acetate derivatives studied here, which may in turn indicate different solubilities of the treatments, hence reinforcing the likelihood that there is no “one-size-fits-all” pretreatment protocol for removing all PVAs.
Cellulose Nitrates
Neither of the two cellulose nitrate samples, Frigilene and Ercalene, were removed with the ORAU in-house standard sequence of acetone/methanol/chloroform, with 81% and 36% remaining, respectively. This may be due to cross-linking, but could also be due to the presence of plasticizers and other additives added by the manufacturers that are required to stabilize the durability of cellulose nitrate treatments. The use of toluene resulted in increased carbon concentrations for both the Frigilene- and Ercalene-contaminated Chromosorb (136% and 106%, respectively), demonstrating the tendency of cellulose nitrates to swell in aromatic hydrocarbons (Shashoua et al. Reference Shashoua, Bradley and Daniels1992). It should be noted that while Yuan et al. (Reference Yuan, Wu, Liu, Guo, Cheng, Pan and Wang2007) stated that nitrocellulose lacquer can be dissolved in a soxhlet sequence based on that proposed by Bruhn et al. (Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001), using tetrahydrofuran, chloroform, petroleum ether, acetone, and methane, they also concluded that several dates were “not satisfactory” and required further research.
The FTIR spectra of the two cellulose nitrate treatments (Figure 1d), are characterized by a sharp absorption band at 1640 cm–1 (asymmetric NO2 stretching), and peaks at 1270 cm–1 (symmetric NO2 stretch) and 830 cm–1 (N-O stretch) (Nel et al. Reference Nel, Lonetti, Lau, Tam, Sagona and Sloggett2010). Nel (Reference Nel2006) also described a small, sharp peak at ca. 2600 cm–1 as characteristic of cellulose nitrates, which may correspond to the very small peaks observed at ca. 2660 cm–1 here (Figure 1). However, there are still discernible differences in the relative ratio of C-H stretching bands at 3000–2800 cm–1 and C-H bending at 1450–1375 cm–1 indicating the difference in molecular composition between the two treatments.
CONCLUSION
The successful removal of a specific conservation treatment from Chromosorb after artificial aging using chemical pretreatment methods clearly does not guarantee the complete removal of the same substance from a historical or archaeological specimen that has been treated in the past, especially if it has been subjected to ongoing conservation over an extended period of time. Cross-linking of the treatment material (either internally within the polymer itself or with the sample material), interactions with other conservation treatments, the porosity of the sample material, and aging-related oxidation and degradation over time, will all affect the solubility of the treatment and hence the ease with which it can be removed from the sample.
However, this study demonstrates that three types each of artificially aged shellac and Paraloid were effectively removed from Chromosorb, instilling confidence that existing pretreatment protocols applied at ORAU (water/methanol and acetone/methanol/chloroform sequences, respectively) are sufficient to ensure reliable, accurate dates. However, it is always preferable to avoid dating such contaminated material where possible (e.g. by sampling away from contaminated areas) and to use additional forms of quality control (such as FTIR, microscopy, py-GC/MS) to provide evidence demonstrating complete removal of any contaminants.
However, no pretreatment method completely removed any of the types of polyvinyl acetate-derived treatments tested. The porosity of the Chromosorb means that it is not an accurate analog for archaeological organic materials such as wood and bone, given that these substances are liable to form cross-links with the materials they are applied to over time, as well as internally. However, even taking this into consideration, the results of this study suggest that it is unlikely that polyvinyl acetate derivatives can be successfully removed from bones, wood or other materials to which they have historically been applied prior to 14C dating, and thus are likely to provide an erroneous date. Similarly, neither of the cellulose nitrate treatments were removed by the methods tested here. It is therefore advised that samples known to be treated with polyvinyl acetate derivatives or cellulose nitrate lacquers are not submitted for 14C dating, unless alternative methods (e.g. single amino acid dating of bones) can be applied, or if it is possible to sample well away from the contaminated region.
However, if a date on a particular object is highly desirable and no alternative method for dating is possible, testing a range of pretreatment protocols to remove a known conservation treatment artificially aged onto Chromosorb could identify the most effective pretreatment which could then be tested on known-age material contaminated with the same substance.
This study highlights the importance for researchers submitting samples for dating to provide as much information regarding potential conservation treatments to the laboratory as possible, so that suitable pretreatment protocols can be applied, and to avoid adding additional carbon to samples, for example with the use of aromatic hydrocarbons such as toluene which lead cellulose nitrate treatments to swell.
ACKNOWLEDGMENTS
Authors Brock and Snoeck were members of the Oxford Radiocarbon Accelerator Unit at Oxford University at the time when this work was undertaken there. T Higham, L Reynard, H Richardson, and R Schulting are thanked for their contributions to discussions during the initial stages of this project. L Reynard and S Lee undertook valuable literature searches prior to the experimental work. P Ditchfield and C Budd assisted with laboratory work. C Dyer is thanked for useful discussions in FTIR spectra interpretation.




