INTRODUCTION
The transition from the Paleolithic to the Neolithic period is characterized by biological and cultural changes accompanied by the transformation of human societies to stable and organized social structures. In more detail, humans gradually shifted to intensive cultivation, with specific production and food storage systems, as well as the permanent use of available natural resources abandoning hunting activities as secondary (Barker Reference Barker2006). During the transition to societies with integrated production systems, agriculture and farming practices were first applied to wild (non-domesticated) plant and animal species, resulting in typical local plant varieties and native livestock breeds. With the domesticated species as heritage, the farmers extended to several places. Most researchers agree that this social transformation in the last phase of prehistory contributed to the specialization of labor, as evidenced by the marked presence of diverse, inspired, and specialized tools for land cultivation and the exploitation of land use and natural resources. The root causes that led to this transformation are still being debated. The evolution of the ecosystem (flora and fauna) (Rindos Reference Rindos1984), the homogenization of food (Cohen Reference Cohen2008; Hershkovitz and Gopher Reference Hershkovitz and Gopher2008; Wittwer-Backofen and Tomo Reference Wittwer-Backofen and Tomo2008), the appearance of diseases that accompany the farming reality, the development of social inequalities (access or not in production structures), and competition between groups within and outside the community (location/relocation/expansion) are all mentioned as possible causes for the spread of rural populations (Ammerman and Biagi Reference Ammerman and Biagi2003).
Archaeological Excavation of Dispilio Settlement
Dispilio belongs to the Kastoria municipality in western Macedonia and is 8 km from the city of Kastoria. The site is located in an area that developed during the Holocene as the result of alluvial and deltaic deposits of closed basins, such as Kastoria Lake and the terraces of the Aliakmonas River (Melfos and Stratoulis Reference Melfos and Stratoulis2002). The territorial installation of Dispilio is on the southwest side of Kastoria Lake, while the other three sides are characterized by the two mountains: the eastern slopes of Aghios Athanasios mountain and the mountain of the Taxiarchis. The Aliakmonas River, which springs from the mountains of Pindos and Grammos and crosses Kastoria basin in the west–east direction, is located 5 km to the west of Dispilio (Chatzitoulousis Reference Chatzitoulousis2008). The archaeological settlement was discovered in 1932 (Keramopoulos Reference Keramopoulos1932) as the lake level decreased, highlighting the existence of wooden post remains. From 1992 to 2013, systematic excavations were conducted by the Aristotle University of Thessaloniki, under the direction of the late Prof. G. Chourmouziadis (Chourmouziadis Reference Chourmouziadis2002; Chourmouziadis and Sofronidou Reference Chourmouziadis and Sofronidou2007; Sofronidou Reference Sofronidou2008), whereas since 2015, the director of the excavation has been Prof. K. Kotsakis. This archaeological site presents the first signs of habitation in the Middle Neolithic to the early Chalcolithic era. The archaeological findings demonstrated a typical Neolithic settlement with an organized food production. Many ceramic, bone, and stone materials responded to food production, processing, and storage. Mixed agriculture and livestock activities with significant, but not specialized, fishing activities as well as seasonal hunting and foraging were the economy model in Dispilio settlement (Touloumis Reference Touloumis2002). The archaeobotanical material from the Dispilio excavation consists of cereals (Triticum monococcum, Triticum dicoccum, Triticum spelta, Triticum aestivum), legumes (Lens culinaris Medic., Pisum sativum., Lathyrus sativus, Vicia ervilia), fruits (Ficus carica, Pyrus amygdaliformis, Rubus fruticosus aggregate, Cornus mas, Sambucus ebulus), nuts (Corylus avellana), and other native plants, but there are not enough findings to assert their systematic cultivation (Magafa Reference Magafa2002). Ntinou (Reference Ntinou2002) reported the periodic competitive variations between oak woods and conifers. These pressures are accompanied by intense construction activities, animal husbandry, and agriculture development in different periods. The cultivation spread was placed in the VI phase at 7500–5500 BP while the spread of grassland was placed earlier in the IV stage. The spatial distribution of crops extended in or around the limit of the settlement (Kouli Reference Kouli2002). Phoca-Cosmetatou (Reference Phoca-Cosmetatou2008) studied the bone material from V and VI phases whereas Samartzidou (Reference Samartzidou2012, Reference Samartzidou2014) studied 27.512 specimens and identified them from surface layer to phase IV. She noted the importance of domestic fauna in all phases and the gradual increase in the percentage of wild fauna from the earlier to the later phases.
Among domesticated animals, sheep (Ovis aries) is the commonest species, followed by pig (Sus domesticus), cattle (Bos taurus), goat (Capra hircus), and dog (Canis familiaris). Among the wild taxa, red deer (Cervus elaphus) and roe deer (Capreolus capreolus) numerically prevail, followed by wild boar (Sus scrofa) and hare (Lepus capensis). The least common species are aurochs (Bos primigenius), red fox (Vulpes vulpes), brown bear (Ursus arctos), badger (Meles meles), otter (Lutra lutra), marten (Martes foina), squirrel (Sciurus vulgaris), and hedgehog (Erinaceus europaeus). Bones of Ranidae, Bufonidae and Testudo were also identified. The bone material studied here comes from phases I to IV (Late Neolithic I). During the Late Neolithic, the southern part in Kastoria Lake presented intense human activity with respect to previous Neolithic periods. It is believed that this activity was prompted by the development of a communication network through the ridge of Siatista and generally with the southern part of Ptolemaida basin (Kokkinidou and Trantalidou Reference Kokkinidou and Trantalidou1991). Chourmouziadis (Reference Chourmouziadis2002) reported that there are no obvious signs of abrupt abandonment and it seems that prehistoric people left the area, taking with them their livestock structure. This observation lies on the general trend in the Final Neolithic in Greece where the centroid of the settlements shifted to the south with the northern settlements to draw back (Tselika Reference Tselika2006). Although there are many studies based on excavation materials from Dispilio trying to highlight the culture, economy, the social or environmental frame of the Neolithic settlement, the absence of isotopic analysis that could contribute significantly to the information database is evident. Indeed, in recent years, the role of stable isotopes in archaeological research has shifted from a specialized approach to a key application in both archaeological materials and excavation environment (Szpak Reference Szpak2014; Canti and Huisman Reference Canti and Huisman2015; Rehren et al. Reference Rehren, Connolly, Schibille and Schwarzer2015) addressing successfully many questions related to nutrition, living, mobility and social practices of ancient populations. The pioneer study of DeNiro and Epstein (Reference DeNiro and Epstein1978, Reference DeNiro and Epstein1981) based on nutritional experiments on small animals, concluded that carbon and nitrogen isotopic composition in most tissues directly reflects the nutrition isotopic composition. Therefore, in order to highlight gaps in our knowledge about the paleodietary regime in Neolithic settlement of Dispilio, bone collagen samples from wild animals were subjected to carbon and nitrogen isotope analysis.
MATERIAL AND METHODS
Isotopic analysis and protein model reconstruction follow the application of protocols for animal bone collagen extraction methods. First, it was attempted to remove lipids as they are isotopically depleted regarding δ13C values and could result in more negative carbon isotope values of bulk collagen. However, lipids had not been preserved, therefore the measured samples refer to bulk collagen. Animal bone samples from Dispilio excavation were demineralized using 0.5 M HCl at 5ºC over a period of 3–10 days and subsequently washed three times with distilled water. Next, they were soaked in 0.1 M NaOH for 24 hr and rinsed as many times as it took to wash out the brown humic substances (up to 9 rinses) The residue was gelatinized in deionized water solution for 20 hr at 80ºC and subsequently freeze dried. The isotope analysis in the current study was undertaken at the Laboratory of Stable Isotopes and Radiocarbon, Institute of Nanoscience and Nanotechnology, NCSR Demokritos, Greece. The samples were analyzed using an automated carbon and nitrogen analyzer and a continuous-flow isotope-ratio monitoring mass-spectrometer (Flash elemental analyzer coupled with Delta V Plus isotope ratio mass spectrometer). Typical analytical errors were about ±0.2‰ for δ13C and δ15N, and alanine standards were used. Only samples that produced collagen yields with a C:N ratio between 2.9 and 3.6 (DeNiro Reference DeNiro1985) were used in the analysis of paleodiet (Ambrose Reference Ambrose1991) (Table 1).
RESULTS AND DISCUSSION
Radiocarbon Setting
Collagen is a reliable material for radiocarbon (14C) dating, which generally resists contaminants, e.g. burial environment, retaining its authentic signal. Therefore, once the diagenesis tests (C:N ratio) in extracted collagen recommend its preservation, the biological material refers to the diet of the period that the organism lived. There is a database of 14C measurements for Dispilio. Karkanas (Reference Karkanas2002) presented 14C dates on charcoal material while Kouli (Reference Kouli2002) on pollen material. Within this study, 14C analysis was performed on six collagen samples of wild animals and two sediment samples from two different sediment cores (Table 1). In Figure 1, all the published 14C dates are listed, including those of this study, versus their depth. However, the weak coefficient factor (r2=0.38) follows the fact that 14C dates of charcoal material do not present any strong variation in stratigraphic development. Isolating the charcoal material first and sediments afterwards, the coefficient factor is strengthened to r2=0.75 and r2=0.89, respectively. A first comment that arises is that charcoal material does not correspond to the stratigraphic order. Moreover, the sedimentation ratio derived from collagen and pollen samples is higher than that with the sediment material. This could suggest the activation of mechanisms close to the settlement sub-basin that allowed a more intense sedimentation rate. Such mechanisms could be pronounced wet periods, local deforestation and/or human-transporting material for engineering works (foundations, siltation, etc.). Regarding the local deforestation, Kouli (Reference Kouli2002) based on palynology data reported a reduction in natural forest vegetation under ecological human pressure, basically on intermediate altitudes around the lake. This reduction is connected to the intense construction activity of the lakeside settlement but also the development of the agro-pastoral sector. This observation is confirmed by the sedimentological analysis of Karkanas (Reference Karkanas2002), however without excluding human-transporting material for engineering works. Figure 2 shows the elevation contours of the settlement sub-basin that could justify higher sedimentation ratio under the destruction (e.g. local deforestation) of natural control mechanisms. Finally, collagen and pollen samples seem to present the best correspondence to stratigraphic order, making them valuable materials for paleoecological studies.
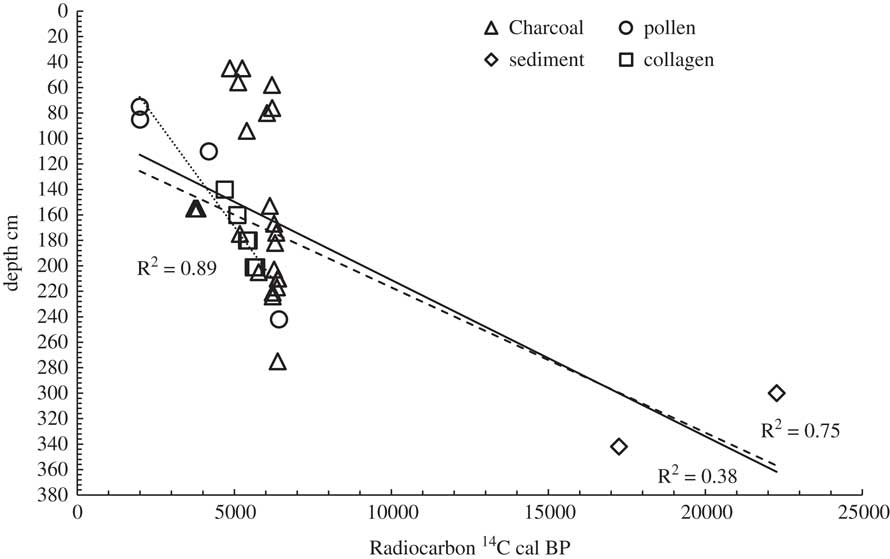
Figure 1 Measured and published 14C cal BP versus their stratigraphic reference in Dispilio area [charcoal: Karkanas Reference Karkanas2002; pollen: Kouli Reference Kouli2002; collagen: this study; —: all 14C dates; - - -: all 14C dates without charcoal material; ……: collagen and pollen 14C dates].
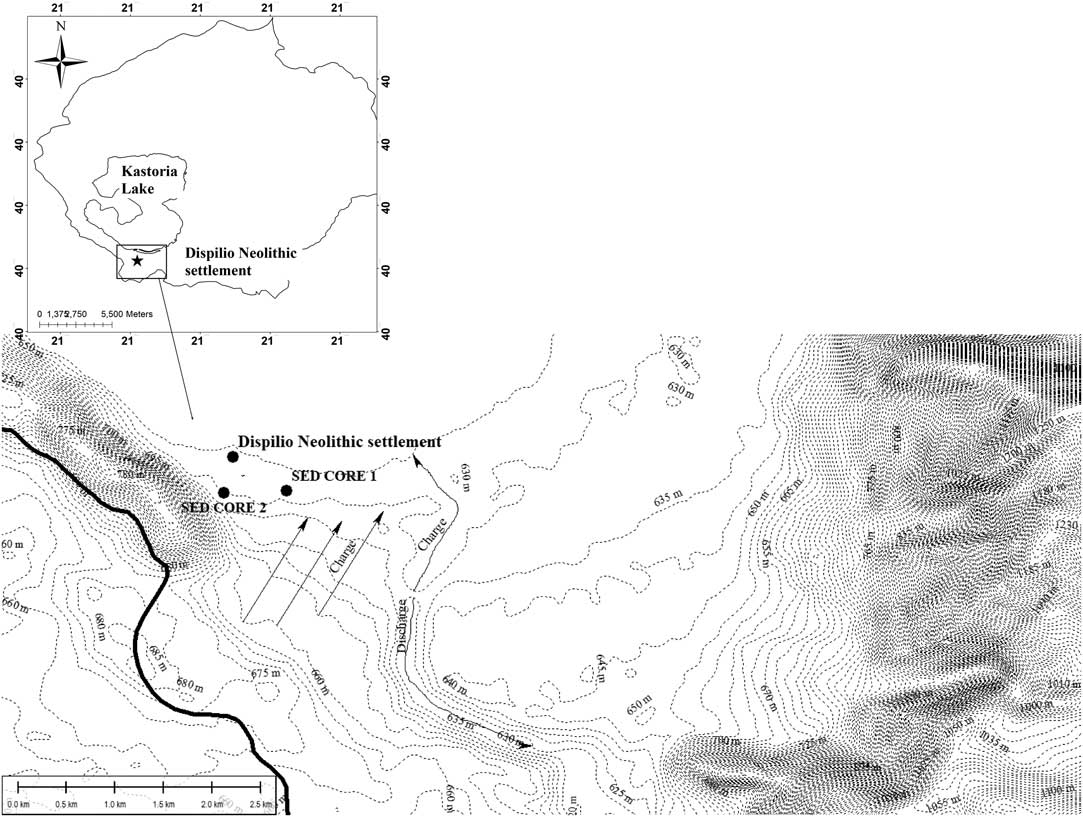
Figure 2 Map of elevation contours in Kastoria Lake basin with the detailed Dispilio sub-basin settlement.
Table 2 14C measurements on sediments and animal bone collagen from Dispilio excavation.

* A = accelerator mass spectrometry (AMS); B = liquid scintillation counting (LSC).
a Calibration was calculated using the one of the databases associated with the IntCal13 program.
b The calibration curve IntCal13 and the software OxCal4.2.4 were used.
Isotope Variation in the Food Chain
Collagen is continuously re-formed and incorporated in bone, replacing the old part. However, each bone type is differentiated with respect to the frequency of collagen renewal. For example, rib bones need approximately 5 yr, while femur bone incorporates new collagen after 40 or 50 yr (Lidén and Angerbjörn Reference Lidén and Angerbjörn1999; Hedges et al. Reference Hedges, Clement, Thomas and O’ Connel2007a). Bone collagen records the diet, which is reconstructed by carbon and nitrogen isotopes (Bocherens et al. Reference Bocherens, Drucker, Billiou, Patou-Mathis and Vandermeersch2005). Those parts of bones that present a slow collagen recovery are usually used in paleodietary studies while those with rapid recovery are usually used to detect climate micro-variations.
Carbon Isotopes of Collagen
The carbon isotope values measured in collagen reflect the protein part and not all the carbon that has been consumed. The amino acids are absorbed through diet and after digestion new amino acids are formed and incorporated to collagen. This metabolic process leads to fractionation between food protein and collagen about 5‰. Moreover, atmospheric CO2 presents a mean δ13C between –7 and –8‰, however, local variations have been recorded. During photosynthesis, plants absorb more 12C than 13C of CO2 resulting in depleted organic δ13C values with respect to CO2. Plants are grouped in three different photosynthetic pathways where each type results in a different fractionation factor with CO2. C3 type is more diversified with the atmospheric CO2 presenting more negative δ13C values, e.g. –27‰ while C4 type exhibits less diversified values of about –13‰. CAM type presents intermediate values between the other two types and depends on plant development conditions. Most of the plants in temperate conditions exhibit the C3 photosynthetic type (trees and cereals) while in arid environments C4 type is referred. CAM is a rare type and it usually occurs in succulents like a cactus. In the Dispilio area carbon isotopes in collagen samples from fauna that referred to Middle/Late Neolithic period were measured. Wild boar samples (n=12) presented δ13C values ranging from –20.8‰ to –18.9‰ (mean –19.9‰) and roe deer samples (n=16) from –21.9‰ to –17.5‰ (mean –20.6‰) (Table 1). Considering the fractionation between food protein and collagen (about 5‰) it is concluded that both species present carbon isotope values that reflect a diet based on C3 plant type. Moreover, a slight differentiation between their mean values is noticed. This could be attributable to food preferences, e.g. roe deer are herbivores whereas wild boar are considered omnivores, and/or to differentiations in their metabolic system. The last has been reported regarding oxygen isotope of hydroxyapatite where the drinking-water carbonate equation is different for wild boar and roe deer: (1) δ18Oc=1.07 × δ18Ow+28.83 for wild boar [Longinelli et al. Reference Longinelli and Selmo2011 (modified with δ18Op=0.98 × δ18Oc – 8.5; Iacumin et al. Reference Iacumin, Bocherens, Mariotti and Longinelli1996)] and (2) δ18Oc = 0.998×δ18Ow+33.63 for modern deer (Iacumin et al. Reference Iacumin, Bocherens, Mariotti and Longinelli1996).
Nitrogen Isotopes of Collagen
Nitrogen isotopes constitute a reliable dietary marker regarding protein intake of terrestrial or aquatic origin. Considering that in food chain nitrogen isotopes are enriched higher in diet pyramid (Minagawa and Wada Reference Minagawa and Wada1984; Schoeninger and DeNiro Reference Schoeninger and DeNiro1984) a first approach could be based on the comparison between herbivores with respect to human values, in the same ecosystem (Bocherens et al. Reference Bocherens, Drucker, Billiou, Patou-Mathis and Vandermeersch2005; Richards and Trinkaus Reference Richards and Trinkaus2009). Although domesticated animals better reflect the human diet, their δ15N values usually present a higher variation reflecting conscious agriculture choices with exogenous nitrogen sources (Makarewicz and Tuross Reference Makarewicz and Tuross2006; Bogaard et al. Reference Bogaard, Heaton, Poulton and Merbach2007; Fraser et al. Reference Fraser, Bogaard, Heaton, Charles, Jones, Christensen, Halstead, Merbach, Poulton, Sparkes and Styring2011; Makarewicz Reference Makarewicz2014). It is important to note that δ15N values in different species, wild or domesticated, depend on dietary habits and seasonality of available food sources (Balasse et al. Reference Balasse, Bocherens, Mariotti and Ambrose2001; Darimont and Reimchen Reference Darimont and Reimchen2002; Sponheimer et al. Reference Sponheimer, Robinson, Ayliffe, Roeder, Hammer, Passey, West, Cerling, Dearing and Ehleringer2003a, Reference Sponheimer, Robinson, Ayliffe, Passey, Roeder, Shipley, Lopez, Cerling, Dearing and Ehleringer2003b; Makarewicz Reference Makarewicz2014). For example, variations about 3‰ in δ15N values have been reported for herbivores from the same basin (Codron et al. Reference Codron, Codron, Lee-Thorp, Sponheimer, Bond, de Ruiter and Grant2005). Moreover, physiology and metabolism are important factors that determine the isotopic record of nitrogen in consumers (Reitsema Reference Reitsema2013).
Recent studies of controlled diets have detected that different species of herbivores that consume the same food have different values in the range up to 4.5‰ (in hair keratin), suggesting that differences in digestion between species could result in different nitrogen isotope values (Sponheimer et al. Reference Sponheimer, Robinson, Ayliffe, Roeder, Hammer, Passey, West, Cerling, Dearing and Ehleringer2003a, Reference Sponheimer, Robinson, Ayliffe, Passey, Roeder, Shipley, Lopez, Cerling, Dearing and Ehleringer2003b). Within this study, as the first approach on nitrogen isotopes to the area, the analysis was applied on collagen samples extracted from wild animals trying to isolate possible strong variations of δ15N values due to agriculture techniques. The results ranged from 3.7‰ to 7.6‰ (mean 5.3‰) for wild boar samples (n=12) and from 2.8‰ to 5.3‰ (mean 3.8‰) for roe deer samples (n=16) (Table 1). It is believed that the slight enrichment in wild boar δ15N values, as in δ13C values, reflects rather the dietary habits of these two species. Wild boar, in contrast to roe deer, could have consumed eggs, carrion, or small rodents, except flora (Chapman and Trani Reference Chapman and Trani2007) resulting in the observed positive “shift”. However, further interpretation is needed as nitrogen isotopes are strongly influenced by many factors.
Carbon and Nitrogen Sediment Isotopes
The factors that control the isotopic values in lacustrine sediments are bulk carbonate material with particles of different origin, the water cycle, and water temperature at which the carbonate material is precipitated, and finally the biological activity, which affects the chemistry. The sediment δ15N and δ13C values ranged from 5.3‰ to 9.5‰ (mean 7.4‰) and from –21.7‰ to –9.1‰ PDB (mean –13‰), respectively, with a weak correlation (r2=0.04) (Table 1). The elevated NO3 – values from 2.3 mg/L to 20.7 mg/L (unpublished data from Laboratory of Stable Isotopes and Radiocarbon) in the entire shoreline of Kastoria Lake conclude to a recent contamination, which agrees with the enriched δ15N values of the first layer 0.20 m; therefore, this sediment layer is excluded. The sediment layer of 0.80 m (sample Sc_4) presents a strong negative δ13C value (–12.5‰), however, δ15N values do not follow this trend. Excluding the 0.80-m sediment layer as well, the correlation between δ15N and δ13C is very good (r2=0.63), suggesting that the 0.80-m layer reflects a different mechanism. If this remarkable negative shift of δ13C was the result of a eutrophication episode (Whiticar Reference Whiticar1999), a shift to negative δ15N values should also be present (Hoefs Reference Hoefs2009). On the contrary, a minor fluctuation to more positive values is observed, implying a higher lake level or excessive runoff charging the lake with more positive δ15N sediments (Talbot and Lærdal Reference Talbot and Lærdal2000). This observation agrees with the conclusions for higher sedimentation rate due to a local deforestation episode based on 14C dates.
Table 1 Carbon and nitrogen isotope on collagen material from animal bones from Dispilio excavation.

Protein Model Reconstruction
For interpretation of the protein model, literature data were used to compare δ13C and δ15N values that correspond to the Dispilio excavation with those from archaeological excavations of the same period in Europe. Focusing on Figure 3, it is noticed that northern sites behave differently than southern sites. Regarding mean δ13C values, northern areas (Sweden, Germany, northern France) are pictured on the left side of the diagram with more depleted values, whereas southern areas (Greece, southern France) are shifted to the right, presenting more enriched carbon isotope values. This observed north–south enrichment on δ13C values has been previously documented in food samples (Bréas et al. Reference Bréas, Guillou, Reniero, Sada and Angerosa1998; Angerosa et al. Reference Angerosa, Bréas, Contento, Guillou, Reniero and Sada1999; Chantzi et al. Reference Chantzi, Poutouki and Dotsika2016). It is worth noting that roe deer from Dispilio present a higher variation of δ13C values in contrast to wild boar, which are closely grouped. As previously discussed this could be attributable to diet habits and/or to differentiations in their metabolic system. Roe deer are mainly foraging consumers with the C3 type of leafy forbs, shrubs or wheat, rejecting (unless there is no other type of food) generally C4 plant types (Noe-Nygaard et al. Reference Noe-Nygaard, Price and Ulfeldt Hede2005). This animal is a selective consumer, covering its nutritional needs in forests and/or open landscapes and traveling long distances (Ecker et al. Reference Ecker, Bocherens, Julien, Rivals, Raynal and Moncel2013), presenting a higher variation on isotope values. On the other hand, wild boar diet is based on plant matter while also preferring agricultural crops to natural plants (Leránoz Reference Leránoz1983). However, their typical diet also contains a limited but consistent fraction of animal matter (Schley and Roper Reference Schley and Roper.2003). This is evident in the Dispilio samples. The fact that wild boar from Dispilio exhibit higher δ15N values than roe deer without strong variation implies that these samples probably reflects the involvement of a consistent animal protein fraction in their diet.
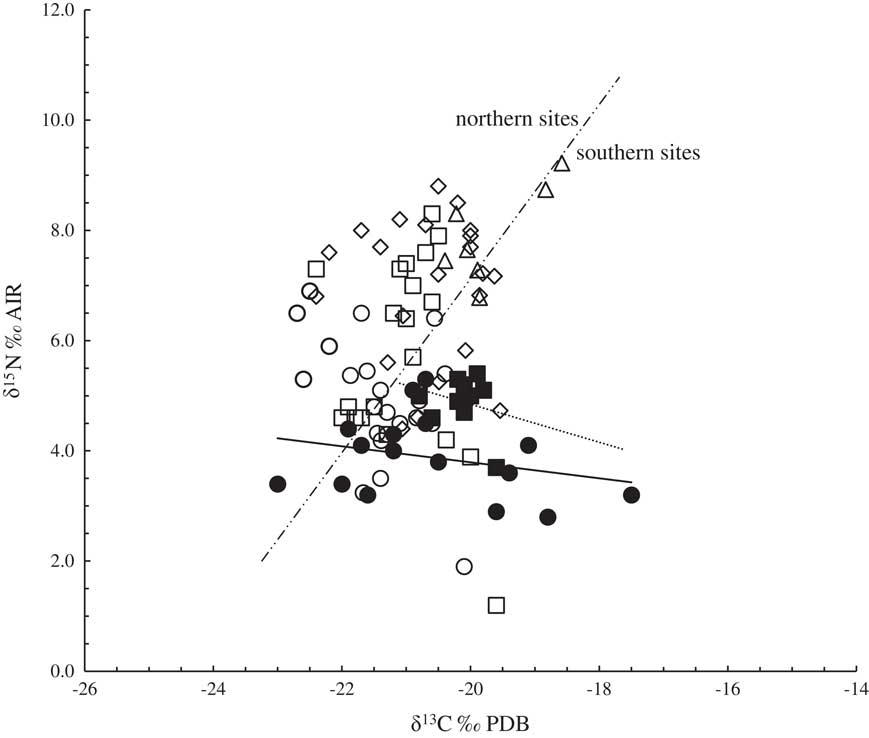
Figure 3 δ13C‰ PDB versus 15N‰ AIR on collagen material from Dispilio Neolithic settlements. Published Neolithic data are displayed for further interpretation. Square: wild boar; circle: roe deer; diamond: domesticated pig; triangle: human; filled marks: Dispilio (this study); open marks: literature data [Greece (Papathanasiou and Richards Reference Papathanasiou and Richards2015), Romania (Balasse et. al. Reference Balasse, Evin, Tornero, Radu, Fiorillo, Popovici, Andreescu, Dobney, Cucchi and Bălăşescu2016), Germany (Oelze et al. Reference Oelze, Siebert, Nicklisch, Meller, Dresely and Alt2011), Sweden (Fornander et. al. Reference Fornander, Eriksson and Lidén2008), France (Goude and Fontugne Reference Goude and Fontugne2016)].
Dispilio wild boar is placed between wild and domesticated pig literature data from Greece (Papathanasiou and Richards Reference Papathanasiou and Richards2015), but closer to the second. Furthermore, most of the pig samples from Greece discriminate from Romanian and German (north) those whose diet has been documented to include human food waste with animal origin protein (Oelze et al. Reference Oelze, Siebert, Nicklisch, Meller, Dresely and Alt2011; Balasse et al. Reference Balasse, Evin, Tornero, Radu, Fiorillo, Popovici, Andreescu, Dobney, Cucchi and Bălăşescu2016). In this group (Romanian and German domesticated pigs) some Greek Neolithic human values are also noticed, confirming the upgraded diet record. The distance between the values of domesticated fauna and human diet Δδ15Nfauna-human for Greek Neolithic settlements is similar to that of European settlements (Hedges et al. Reference Hedges and Reynard2007b). Furthermore, domesticated pig samples from Greek Neolithic settlements are placed between wild boar and human samples, implying that their diet could include human food waste as part of their domestication (Figure 3). As Dispilio wild boar is better grouped with domesticated Greek Neolithic pigs, this leads to the assumption that in Dispilio settlement wild boar either lived in the wider area of Kastoria Lake basin close to the settlement or were on a domestication phase. The fact that two wild boar samples from Dispilio area exhibit more positive δ15N isotope values of about 7.3‰ and 7.6‰, close to human samples from Greek literature data, could imply an upgraded diet. Thus, based on the above discussion, the question arises whether wild boar collagen is actually representative of animal protein and what is the ratio of this protein fraction.
Plants’ δ15N values are linked to those of soil. For environments with limited exogenous nitrogen source, δ15N values are close to the atmospheric about 0‰ (Ambrose Reference Ambrose1991). A typical enrichment of δ15N values between the diet and the consumer, along the food chain, is about 3.0‰ (Post Reference Post2002; Vanderklift and Ponsard Reference Vanderklift and Ponsard2003; Caut et al. Reference Caut, Angulo and Courchamp2009). If freshwater fish is part of the diet, Δδ15Ndiet-body is higher, about 5‰, because freshwater fish present more positive δ15N values, about 9–10‰ (Keaveney and Reimer Reference Keaveney and Reimer2012). Following the isotopic enrichment in food chain, collagen of herbivores should present δ15N values between 2.5‰ AIR and 3.4‰ AIR (mean 3.0‰ AIR). As pointed out earlier, the two samples with higher δ15N values (7.3‰ and 7.6‰) considered to be representative of an upgraded diet. Therefore, the data were divided in two homogeneous sets: (1) all but the two higher values and (2) the two higher values. The isotopic equilibrium calculations were applied (Table 4a) and then the diet with the best fit to both sets was selected. The model concluded to 35% plant and 65% animal protein, suggesting a predominantly animal protein diet. However, the conclusions are unsafe, as the measured δ15N values of roe deer in Dispilio (2.8–5.3‰ AIR) are far from the estimated δ15N values of herbivores (2.5–3.4‰ AIR) based on the above model. This inconsistency could be attributed to climatic factors as there has been reported a correlation between δ15N values and rainfall (Heaton et al. Reference Heaton, Vogel, von la Chevallerie and Collett1986; Sealy et al. 1987; Cormie and Schwarcz Reference Cormie and Schwarcz1995; Gröcke et al. Reference Gröcke, Bocherens and Mariotti1998). Specifically, Heaton (Reference Heaton1987) reported a negative relationship between rainfall and plant δ15N values with a ratio about –0.39 (±0.13) ‰ δ15N/100 mm of annual precipitation for a set of non-coastal stations in South Africa. This ratio, however, respond to terrestrial stations with very low rainfall, even less than 100 mm.
In contrast, Kastoria Lake basin exhibits a much more dynamic regime (Table 3) where the smallest average annual rainfall is 592 mm in Argos Orestiko station at 660-m altitude. Therefore, considering only the stations with annual rainfall >500 mm, the slope between plant δ15N values and annual precipitation become –0.63‰ δ15N/100 mm. Moreover, for Dispilio, the soil δ15N value about 0‰ is considered to represent higher altitudes, in the forest, about 1200 m based on previous studies (Kouli Reference Kouli2002). The altitude difference between this site and the lake (630 m) corresponds to ΔH=600 m. Given that the precipitation/altitude ratio, based on most recent available data, is 57.41 mm/100 m this altitude difference corresponds to a precipitation difference of about 345 mm. Therefore, the precipitation difference that is due to the altitude difference of Dispilio basin corresponds to an isotopic difference Δδ15N= –2.1‰. Thus, plant δ15N values in the wider area of Kastoria Lake basin reach 2.1‰ AIR with mean value 1.05‰ AIR. Following the isotopic enrichment in food chain, herbivore’s collagen δ15N values should be between 2.5‰ AIR and 5.5‰ AIR with mean value 4‰ AIR. This estimation agrees with the measured δ15N values in roe deer samples ranging between 2.8‰ AIR and 5.3‰ AIR with mean value 3.8‰ AIR. Applying isotopic equilibrium equations (Table 4b) it is concluded that wild boar collagen reflects predominantly plant protein. More specifically, the model concluded to 70% plant and 30% animal protein, demonstrating a predominantly plant protein diet.
Table 3 Characteristics of rainfall stations in Kastoria Lake basin (data from Public Power Corporation SA-Hellas for the decade 1990–2000).

* Geodetic system: GGRS 87.
Summarizing the above it is concluded that rainfall is an important factor that affects plant, and consequently animal, δ15N values. Generally, Hedges et al. (Reference Hedges and Reynard2007b) reported the environmental impact on increased δ15N values of human samples from the archaeological excavations of Kefala in Skiathos and Franchthi, in southern Greece. Under the assumption that modern precipitation/altitude ratio corresponds to that of Middle/Late Neolithic period in Dispilio, it was concluded that wild boar collagen reflects predominantly plant protein (70%). Moreover Heaton (Reference Heaton1987) reported that the ratio of herbivores δ15N values and annual precipitation is between –1.1‰ and –1.3‰/100 mm × yr–1. This correlation was updated by Schwarcz et al. (Reference Schwarcz, Dupras and Fairgrieve1999) resulting in the characteristic equation δ15Ν = [–0·0094 ± 0·00106] x + [12.33 ± 2.3], where x is the precipitation height mm. Appling this equation on wild boar and roe deer samples from Dispilio resulted to rainfall 730 mm and 880 mm, respectively, where based on the observed precipitation/altitude ratio reflect 890-m and 1150-m altitudes, respectively. These conclusions are consistent with the previous reports where wild boar prefer lower altitudes close to the settlement in contrast to roe deer, which cover greater distances in wooded areas at higher altitudes, as selective consumers.
CONCLUSION
The Neolithic period in Greece enumerates many settlements. Dispilio Neolithic settlement in Kastoria lake is the unique lakeside settlement excavation in Greece. The Late Neolithic wild boar and roe deer samples were subjected to collagen extraction protocols to reconstruct the flora regime and dietary habits. Furthermore, the 14C database regarding Dispilio excavation was further enriched including analysis on collagen material from fauna. The 14C model concluded to a possible local deforestation effect in the settlement sub-basin, which was confirmed by sediment δ13C and δ15N values. Carbon isotopes in collagen samples ranged from –20.8‰ to –18.9‰ (mean –19.9‰) for wild boar and from –21.9‰ to –17.5‰ (mean –20.6‰) for roe deer suggesting a C3 plant type diet. The higher variation of δ13C values indicated the different nutritional habits and/or metabolic system of the two species. This differentiation between two species is further supported by nitrogen isotope analyses where the values ranged from 3.7‰ to 7.6‰ (mean 5.3‰) for wild boar and from 2.8‰ to 5.3‰ (mean 3.8‰) for roe deer. Compared to literature data it is assumed that wild boar in Dispilio settlement rather lived in the wider area of Kastoria Lake basin close to the settlement presenting a consistent animal protein fraction. The constructed protein model resulted in a mixed diet of 70% plant and 30% animal protein. Summarizing the above it is concluded that rainfall is an important factor that affects plant, and consequently animal, δ15N values. Therefore, the rainfall regime should always be considered in paleodietary studies.
Table 4a Protein model for wild boar, without considering the effect of the rainfall regime based on terrestrial and non-terrestrial protein and δ15Nbody-diet.

Table 4b Protein model considering the effect of the rainfall regime based on terrestrial and non-terrestrial protein and δ15Nbody-diet.

ACKNOWLEDGMENTS
The authors would like to thank the late Prof. G. Chourmouziadis, who kindly supported the concept of this study.










