INTRODUCTION
Cremated bone is found in a wide range of archaeological contexts but has, for a long time, been excluded from radiocarbon dating. Indeed, once fully calcined (i.e. cremated), bone is visibly very white and does not retain any organic matter, leaving no collagen for 14C dating. However, Lanting and Brindley (Reference Lanting and Brindley1998), and subsequently Lanting et al. (Reference Lanting, Aerts-Bijma and van der Plicht2001), demonstrated that reliable 14C dates could be obtained from the small carbonate fraction remaining within the bioapatite of cremated bone fragments, as long as the fragments are fully calcined and white (i.e. not gray or black). Charred bone (gray and black) is not a good candidate for dating as it still contains significant amounts of organic matter and is more prone to postburial alterations due to its lower crystallinity (Lebon et al. 2010; Snoeck et al. Reference Snoeck, Lee-Thorp and Schutling2014a).
As part of the Fifth International Radiocarbon Intercomparison (VIRI) exercise, six radiocarbon laboratories undertook dating of six cremated bones (including two duplicate pairs), with 14C ages spanning approximately 1500–2800 BP (Naysmith et al. Reference Naysmith, Scott, Cook, Heinemeier, van der Plicht, Van Strydonck, Bronk Ramsey, Grootes and Freeman2007). Five of the laboratories used pretreatments based on that proposed by Lanting et al. (Reference Lanting, Aerts-Bijma and van der Plicht2001), consisting of 1.5% sodium hypochlorite (NaOCl) to remove any remaining organic matter, followed by 1M acetic acid (CH3COOH) to remove any adsorbed or diagenetic carbonates. However, the Belgian Royal Institute for Cultural Heritage (KIK-IRPA) developed and applied another pretreatment protocol whereby the samples were treated with 1% HCl until 50% of the material is leached (Van Strydonck et al. Reference Van Strydonck, Boudin, Hoefkens and De Mulder2005, Reference Van Strydonck, Boudin and De Mulder2009). All six laboratories subsequently reacted the pretreated material with phosphoric acid in sealed vessels to liberate CO2 for accelerator mass spectrometry (AMS) 14C dating.
The rationale for the inclusion of the sodium hypochlorite bleach step is to remove any remaining organic matter. However, once fully calcined, no organic matter should remain in the sample, and such pretreatment is possibly superfluous. Furthermore, any soil organic matter deposited on the bone during burial should be physically removed prior to chemical pretreatment (e.g. by air-abrasion). Pretreating the sample with acetic acid only should be sufficient, and phosphoric acid should not react with any potential remaining organic matter anyway. Removing the initial bleach step would save time and money and potentially limit the amount of sample loss, as well as reducing the possibility of introducing additional contaminants.
Furthermore, at ORAU, the 1.5% NaOCl (pH ~10) step was replaced with acidified (pH 3) 1.5% sodium chlorite (NaO2Cl) (Brock et al. Reference Brock, Higham, Ditchfield and Bronk Ramsey2010) at some point after the VIRI intercomparison, although the reason for this change is not recorded. However, NaO2Cl is not as strong an oxidizing agent as NaOCl, and may not be as efficient at removing organic matter from cremated bones prior to dating.
The experiments designed here were aimed at answering the following three questions: (1) How efficiently do NaOCl and NaO2Cl remove organic matter from calcined bone? (2) Does the presence of organic matter impact on the isotope composition of the carbon dioxide emitted during the reaction of carbonates with phosphoric acid? (3) Does the bleach stage have any statistically significant impact upon the 14C dates produced (and therefore could the bleach step be removed from routine pretreatment of cremated bones for 14C dating)?
MATERIALS AND METHODS
Testing the Efficacy of Organic Matter Removal by NaOCl and Acidified NaO2Cl
To compare the efficacy of NaOCl and acidified NaO2Cl at removing organic matter, 2 aliquots of an unburned modern sheep bone were pretreated. Since cremated bone does not contain organic matter, testing the efficacy of NaOCl and NaO2Cl on such samples would have been difficult, hence the use of modern bone (containing significant amounts of collagen). The first bone aliquot was treated with 1% NaOCl and the second with 1.5% NaO2Cl at pH 3. Both samples were pretreated for 72 hr before thorough rinsing with ultrapure (Milli-Q™) water. (The standard ORAU pretreatment for cremated bones is only for 48 hr.) Both pretreated samples, as well as a fraction of untreated sheep bone, were then crushed using a pestle and mortar.
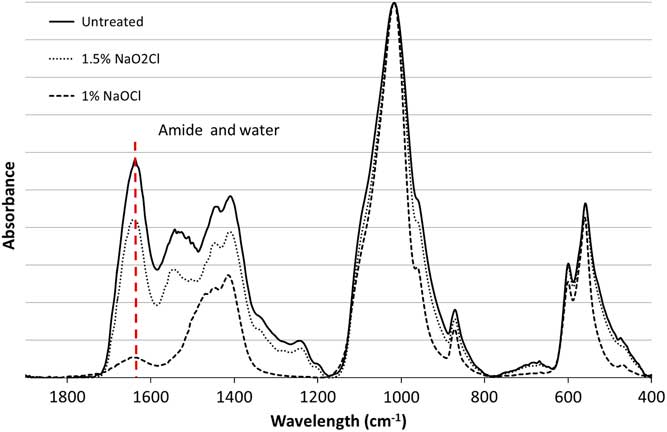
The pretreated samples and untreated material were analyzed by Fourier transform infrared spectroscopy with the attenuated total reflectance mode (FTIR-ATR; Agilent Technologies Cary 640 FTIR with GladiATRTM from Pike Technologies). Each sample was measured three times. The background was subtracted and a baseline correction was carried out using Agilent Resolution Pro software. The water-amide on phosphate index (WAMPI) can be calculated from the infrared spectra. This evaluates the amount of organic matter present in bone by comparing the amide I band of collagen at 1650 cm–1 and the phosphate band at 605 cm–1 (Roche et al. Reference Roche, Ségalen, Balan and Delattre2010). Structural water also absorbs around 1650 cm–1 (Praprotnik and Dušanka Janežič Reference Praprotnik and Janežič2005; Yoder et al. Reference Yoder, Pasteris, Worcester and Schermerhorn2012), making it difficult to detect small amounts of organic matter.
To measure the amount of nitrogen (i.e. protein, and therefore organic matter) present in the untreated and treated unburned modern bone samples, approximately 5 mg of powder was placed in a tin capsule and analyzed by mass spectrometry (Sercon Geo 2022 IRMS coupled to Sercon Europa EA–GSL running in continuous-flow mode with helium carrier gas at 80 mL/min). The limit of detection is 2 μg nitrogen.
Testing the Influence of Organic Matter on Carbon Isotopic Composition
To assess the impact of the presence of organic matter on the isotopic composition of the carbon dioxide released during the phosphoric acid treatment, 20 mg individual aliquots of the IAEA-C01 Carrara marble standard (δ13C=2.42±0.33‰ relative to PDBFootnote 1 ) were each contaminated with 10 and 50% of collagen (by weight) of known carbon isotope composition (δ13Ccoll = –21.8±0.3‰; n=28). These, as well as 2 aliquots of uncontaminated marble, were then reacted with 85% phosphoric acid under vacuum, without any additional pretreatment. The carbon dioxide emitted by the reaction was trapped cryogenically and subsequently reinjected into a coupled EA/IRMS system as above, and δ13C values measured. These experiments were carried out in duplicate.
Pretreatments of Calcined Bone for Radiocarbon Dating
Finally, to show that pretreatments with NaO2Cl are superfluous when dating fully calcined bone, two archaeological calcined bone fragments (one from the Neolithic court tomb of Annaghmare, Co. Armagh, Ireland and one from a Neolithic/Bronze Age house in Ness of Gruting 7, UK) were pretreated using both the routine pretreatment protocol at ORAU (Brock et al. Reference Brock, Higham, Ditchfield and Bronk Ramsey2010) and the same protocol without the initial NaO2Cl treatment. Furthermore, four of the six archaeological calcined samples used in the VIRI interlaboratory comparison study (Naysmith et al. Reference Naysmith, Scott, Cook, Heinemeier, van der Plicht, Van Strydonck, Bronk Ramsey, Grootes and Freeman2007) were prepared following the ORAU routine protocol but without NaO2Cl, having each been previously dated in duplicate at ORAU as part of the VIRI exercise. The calcined bones were reacted with phosphoric acid (85%) under vacuum, and the CO2 released was trapped cryogenically. As above, the CO2 collected was reinjected into a coupled EA/IRMS system, δ13C values were measured, and the CO2 was then converted into graphite and pressed into aluminium targets prior to AMS 14C dating on the ORAU HVEE tandem AMS system (as detailed by Bronk Ramsey et al. Reference Bronk Ramsey, Higham and Leach2004; Brock et al. Reference Brock, Higham, Ditchfield and Bronk Ramsey2010).
RESULTS AND DISCUSSION
Efficiency of NaO2Cl
The FTIR and nitrogen elemental analyses of the unburned modern sheep bone study (Table 1 and Figure 1) show that when NaO2Cl is used, almost no organic matter is removed. However, no detectable amount of organic matter remains after pretreatment with NaOCl. The small peak observed at 1650 cm–1 in the infrared spectrum of the sample pretreated with NaOCl is most likely due to structural water remaining in the sample after pretreatment. This confirms that NaO2Cl is much less efficient than NaOCl at removing organic components. However, the use of NaOCl induces the adsorption of atmospheric carbon dioxide (Zazzo et al. Reference Zazzo, Balasse and Patterson2006; Snoeck and Reference Snoeck and PellegriniPellegrini, 2015), which could have a significant impact on the 14C dates. (The switch to acidified NaO2Cl at ORAU may, at least in part, be linked to this observation.) The subsequent pretreatment with acetic acid should remove these adsorbed atmospheric carbon contributions. Nevertheless, each additional step in the pretreatment process could affect the 14C dates and limiting pretreatment to only what is necessary could improve the 14C dates obtained.
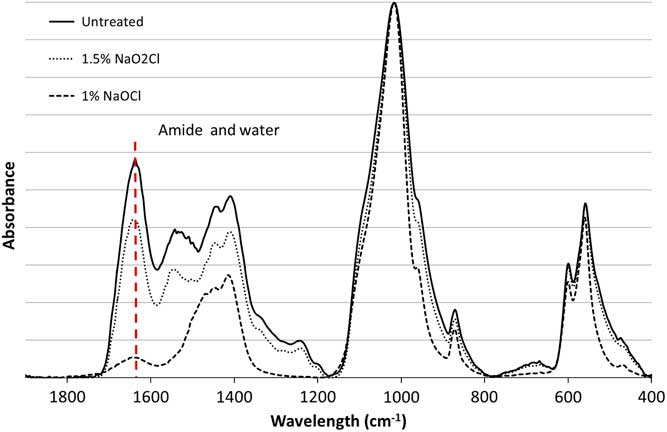
Figure 1 Infrared spectra of modern bone untreated (solid line), pretreated with 1.5% NaO2Cl at pH 3 (dotted line) and 1% NaOCl (dashed line); the band at 1650 cm–1 corresponding to amide (collagen) and water is highlighted.
Table 1 Infrared and nitrogen elemental analyses for the unburned modern bone samples, untreated and pretreated with NaO2Cl and NaOCl (WAMPI is the water-amide on phosphate index).

Reactivity of Organic Matter with Phosphoric Acid
The δ13C values of contaminated Carrara marble standard samples show limited variation (Table 2), within the accepted range of 2.42‰ with a standard deviation of 0.33‰. This suggests a very limited impact of organic matter on the δ13C values of the carbon dioxide emitted during the reaction of carbonates and phosphoric acid. The absence of variation in δ13C values strongly suggests that no variation would be observed in the 14C dates measured on calcined bone samples containing small amounts of remaining organic matter. This, once again, highlights that it is unnecessary to use a bleaching agent when dating properly calcined bone as the amount of organic matter remaining is well below 10%.
Table 2 δ13C values of carbonate standard samples contaminated with known amounts of collagen.

Furthermore, even if some organic carbon were to be released during the reaction, it could only have originated from endogenous carbon from the body, fuel, or atmospheric carbon (Hüls et al. Reference Hüls, Erlenkeuser, Nadeau, Grootes and Andersen2010; Van Strydonck et al. Reference Van Strydonck, Boudin and De Mulder2010; Zazzo et al. Reference Zazzo, Saliège, Lebon, Lepetz and Moreau2012; Snoeck et al. Reference Snoeck, Brock and Schulting2014b). At the time of cremation, if the body and fuel are contemporary (which is the underlying assumption for 14C dating of cremated bone, though there are some exceptions, e.g. Olsen et al. Reference Olsen, Heinemeier, Hornstrup, Bennike and Thrane2012; Van Strydonck et al. Reference Van Strydonck, Decq, Van den Brande, Boudin, Ramis, Borms and De Mulder2013), then all three carbon sources are identical from the 14C perspective.
Comparison of the Traditional and Updated Pretreatments
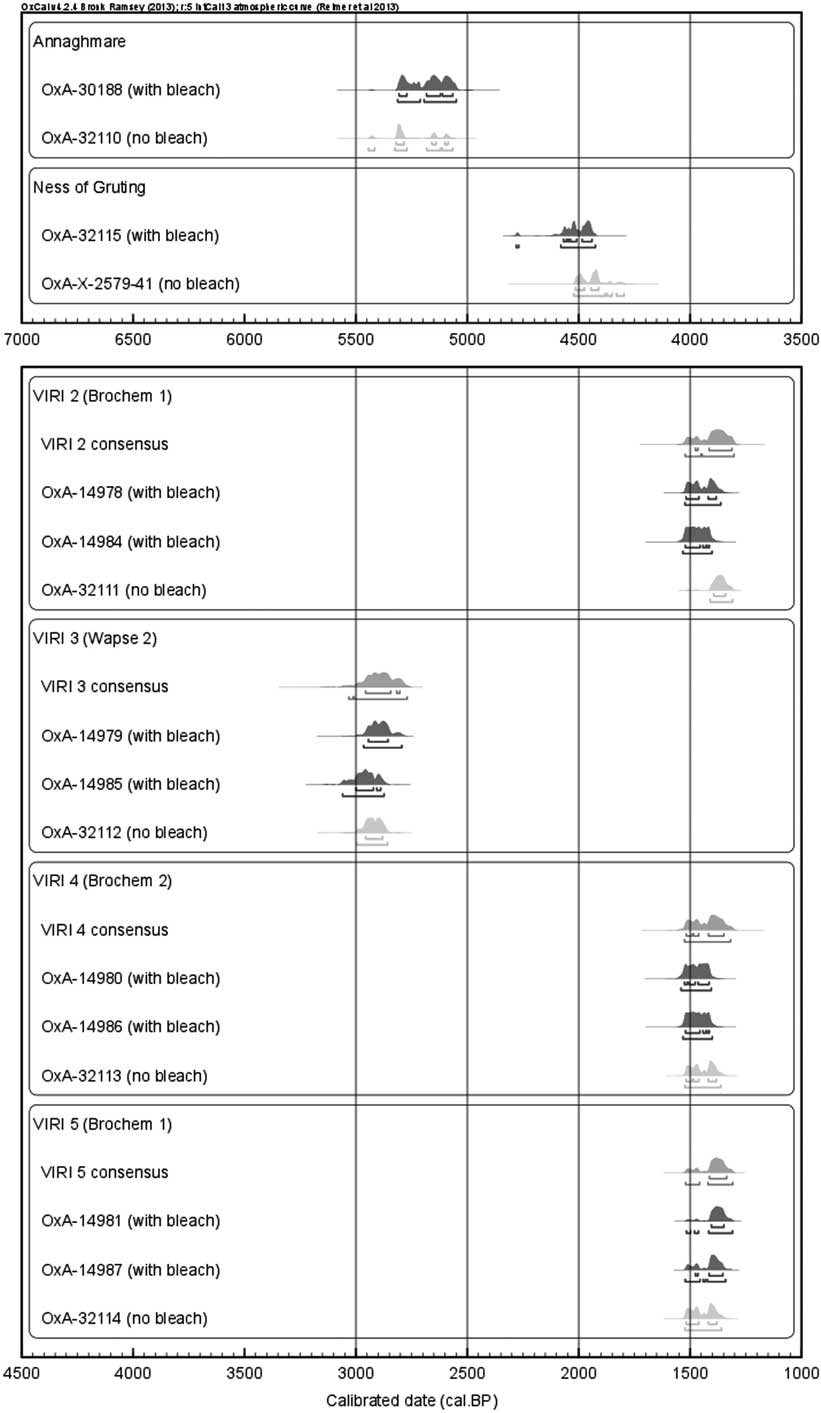
While the presented results emphasize that a pretreatment with only acetic acid should be sufficient, it is necessary to test this on archaeological samples. Here, two samples have been dated after having undergone both pretreatments with and without acidified sodium chlorite. Furthermore, four samples (out of the six) from the VIRI intercomparision exercise (Naysmith et al. Reference Naysmith, Scott, Cook, Heinemeier, van der Plicht, Van Strydonck, Bronk Ramsey, Grootes and Freeman2007) were redated using the updated pretreatment. The dates obtained are statistically identical (i.e. pass a χ2 test at 95% confidence) with both pretreatments (Figure 2, Table 3), confirming that the additional NaO2Cl pretreatment stage is unnecessary. Therefore, this step has now been removed from the ORAU standard pretreatment protocol for cremated bones (as of OxA-32109), and the pretreatment process will consist of only treatment with 1M acetic acid over 24 hr and subsequent water rinses with ultrapure Milli-Q water prior to reaction with phosphoric acid, as detailed by Brock et al. (Reference Brock, Higham, Ditchfield and Bronk Ramsey2010).

Figure 2 Calibrated ages of the six cremated bone samples, with and without the application of a bleach pretreatment stage. Additionally, the consensus values of the four VIRI samples (Naysmith et al. Reference Naysmith, Scott, Cook, Heinemeier, van der Plicht, Van Strydonck, Bronk Ramsey, Grootes and Freeman2007) are plotted in pink. The horizontal bars below each probability density function indicate the 68.2% and 95.4% highest probability density ranges, respectively. The IntCal13 calibration curve was applied (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffman, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013), using OxCal v 4.2.4 (Bronk Ramsey Reference Bronk Ramsey2013).
Table 3 Radiocarbon and stable isotopic results for archaeological samples.

* Samples dated at ORAU are routinely issued with OxA- laboratory codes. The Ness of Gruting sample with the revised pretreatment was issued an OxA-X- code due to low AMS target current; adata from Naysmith et al. (Reference Naysmith, Scott, Cook, Heinemeier, van der Plicht, Van Strydonck, Bronk Ramsey, Grootes and Freeman2007). Mean quoted error for consensus values is 32 yr. Note that Naysmith et al. (Reference Naysmith, Scott, Cook, Heinemeier, van der Plicht, Van Strydonck, Bronk Ramsey, Grootes and Freeman2007) reported considerable scatter in δ13C values for individual samples, with ranges from 4–10‰, attributed to variation in methods of measuring δ13C values and also uneven cremation temperatures; df(ORAU) compares all samples pretreated at ORAU with different pretreatments while df(VIRI) compares the date obtained after the updated pretreatment with the consensus values of the VIRI intercomparison exercise; df = degrees of freedom; T = the test statistic.
CONCLUSION
The results presented here highlight that (1) NaO2Cl is not very efficient at removing organic matter, (2) the presence of organic matter has a very limited impact (if any) on the isotopic composition of the carbon dioxide released during the reaction of carbonates with phosphoric acid, and (3) archaeological calcined bone samples pretreated with both the traditional and updated pretreatments return statistically identical 14C dates. The updated, simpler, and shorter pretreatment of several washes with 1M acetic acid over 24 hr proves to be sufficient prior to 14C dating of calcined bone. No pretreatment with NaO2Cl or NaOCl is required. Calcined bone will, from OxA-32109, be pretreated using the updated protocol when 14C dated at ORAU.
ACKNOWLEDGMENTS
This work was carried out while CS was a DPhil student at the University of Oxford, funded by the Philippe Wiener - Maurice Anspach (http://fwa.ulb.ac.be). The 14C dates of the two archaeological samples were funded by the British Academy grant (SG130690) ‘Coming to Knowth,’ while the VIRI redates were supported by the NERC National Radiocarbon Facility. We would like to thank G Ramsey and S McCartan on behalf of the Ulster Museum for the sample from Annaghmare as well as A Sheridan for the sample from Ness of Gruting. P Naysmith is acknowledged for providing material from the remaining four VIRI samples and M Pellegrini for the modern sheep bone. P Ditchfield, T Higham, and C Bronk Ramsey, and the staff at ORAU are thanked for their comments and help.