INTRODUCTION
Radiocarbon (14C) natural production takes place at high altitude in the atmosphere due to the nuclear reaction of thermal neutrons, generated by cosmic rays, with 14N nuclei. 14C thus formed oxidizes to carbon dioxide and results in a flux of 14CO2 in the troposphere where it is incorporated into plants through photosynthesis, as well as in the meteoric and ocean waters through CO2 exchange reactions. However, anthropogenic impacts on the environment are a major cause of significant change in the isotopic composition of carbon in the atmosphere. Over the last decades, various human activities have affected the 14C concentration (Povinec et al. Reference Povinec, Chudy and Sivo1986). The main contribution comes from the nuclear weapons tests that took place in the 1950s and 1960s (Baydoun et al. Reference Baydoun, El Samad, Nsouli and Younes2015). Bomb radiocarbon has been used as a tracer for the study of atmospheric transport, ocean circulation, exchanges between the carbon reservoirs, and the global carbon cycle (Levin and Hesshaimer Reference Levin and Hesshaimer2000; Randerson et al. Reference Randerson, Enting, Schuur, Caldeira and Fung2002; Levin et al. Reference Levin, Naegler, Kromer, Diehl, Francey, Gomez-Pelaez, Steele, Wagenbach, Weller and Worthy2010). These studies would not be possible without precise atmospheric 14CO2 observations. For mid-latitudes of the Northern Hemisphere radiocarbon measurement records are available for European background stations, such as Vermunt, Austrian Alps; Schauinsland, Black Forest, Germany; and Jungfraujoch, Swiss Alps, covering the period from the nuclear weapons tests to the present (Levin and Kromer Reference Levin and Kromer1997, Reference Levin and Kromer2004; Levin et al. Reference Levin, Kromer and Hammer2013; Hammer et al. Reference Hammer and Levin2017).
In the last few decades, discharges from nuclear facilities (nuclear fuel reprocessing and nuclear power plants) have also been a significant artificial source of radiocarbon. These releases have increased 14C concentration in the atmosphere, and several studies have been devoted to investigating local anthropogenic effects (Povinec et al. Reference Povinec, Sivo, Simon, Holy, Chudy, Richtáriková and Morávek2008, Reference Povinec, Chudy, Sivo, Simon, Holy and Richtáriková2009; Jeskovsky et al. Reference Ješkovský, Povinec, Steier, Šivo and Richtáriková2015; Xu et al. Reference Xu, Cook, Cresswell, Dunbar, Freeman, Hastie, Hou, Jacobsson, Naysmith and Sanderson2015). In the terrestrial environment, the specific activity is constant at equilibrium with the specific activity of atmospheric CO2 and in the absence of other sources. The specific activities of terrestrial biological compartments are currently around 238 Bq/kg (Roussel-Debet et al. Reference Roussel-Debet, Gontier, Siclet and Fournier2006). For 2015, the annual mean of Δ14C values measured for an undisturbed atmospheric (Jungfraujoch station, Swiss Alps) was 14‰ (Hammer et al. Reference Hammer and Levin2017).
In this paper, we describe our sample preparation procedures for radiocarbon measurements by direct absorption method and liquid scintillation counting (LSC) in order to establish the radiocarbon level in the atmosphere of Ramnicu Valcea, Romania. We also compare our specific results with 14CO2 observations at Jungfraujoch high altitude research station in the Swiss Alps conducted to monitor the background 14CO2 level over Europe and define the reference for regional estimates of fossil fuel CO2 (Hammer et al. Reference Hammer and Levin2017). Radiocarbon measurements on biological samples (control samples) were done in order to see if the 14C level in wild vegetation (WV), grapes and tree leaves was comparable with the 14C level in the atmosphere.
MATERIALS AND METHODS
Investigations of radiocarbon in the atmosphere were carried out in the vicinity of the Experimental Pilot Plant for Tritium and Deuterium Separation (PESTD) from the Institute of the Cryogenics and Isotopic Technologies (ICSI). The sampling location is placed about 10 km south from the center of Ramnicu Valcea city (Romania), in the Govora industrial area (Figure 1). This facility is an experimental project in the national nuclear energy research program. Considering the fact that one of the important releases of PESTD is gaseous radioactive effluents, the baseline of atmospheric 14C was vital for the environmental program. Until now, PESTD normal operation was with heavy water and tritiated water below the exemption level approved by Romanian legislation. Foreseen experiments will be done with tritiated heavy water moderator from Cernavoda NPP (CANDU reactor technology). It is well known that heavy water reactors (IAEA 2004) emit significant amounts of tritiated water and 14C. The 14C is a byproduct resulting primarily from neutron activation of 17O from heavy water molecules. The 14C enters the natural environment as CO2 and is found in water, air, soil, and sediments.
Figure 1 Ramnicu Valcea sampling location (adapted from Google Maps).
It should be noted that in the Govora industrial area operates a 315 MW coal-fired thermoelectric power plant. Due to the Suess effect (Suess Reference Suess1955), a relative decrease of the 14C activity on local scale is expected as a result of the dilution of the carbon isotopic mixture by fossil carbon.
Monthly sampling of atmospheric CO2 was carried out from period August 2012 to January 2018 in the Govora industrial area (45º2′N, 24º17′E) situated 10 km south from the Ramnicu Valcea city center. Samples of atmospheric CO2 were collected by static absorption of CO2 on the saturated carbonate-free NaOH solution (Kianpour et al. Reference Kianpour, Sobati and Shahhosseini2012). For that, initially, a thin layer of solid NaOH (around 80 g) was put in a plastic tray so it rapidly absorbs air humidity becoming saturated carbonate-free NaOH solution. The plastic tray was placed outside on the first floor of a three-floor building. The distance between the sampling location and the coal-fired thermoelectric power plant was approximately 400 meters. The tray was left outside for one month and after that, the sodium hydroxide was changed. We choose NaOH because it can easily react with CO2 as an alkali solution, and it has a high absorption efficiency (92–99%) (Peng et al. Reference Peng, Zhao and Li2012).
Biological samples were taken from the institute yard very close to the sampling location for atmospheric CO2. The samples were collected in 2013 as follows: wild vegetation (Agropyron repens) was sampled in spring and autumn, grapes (Vitis vinifera L.) in autumn and tree leaves (Populus nigra) in summer. In this way, we try to cover the entire growing season on different types of biological samples available nearby.
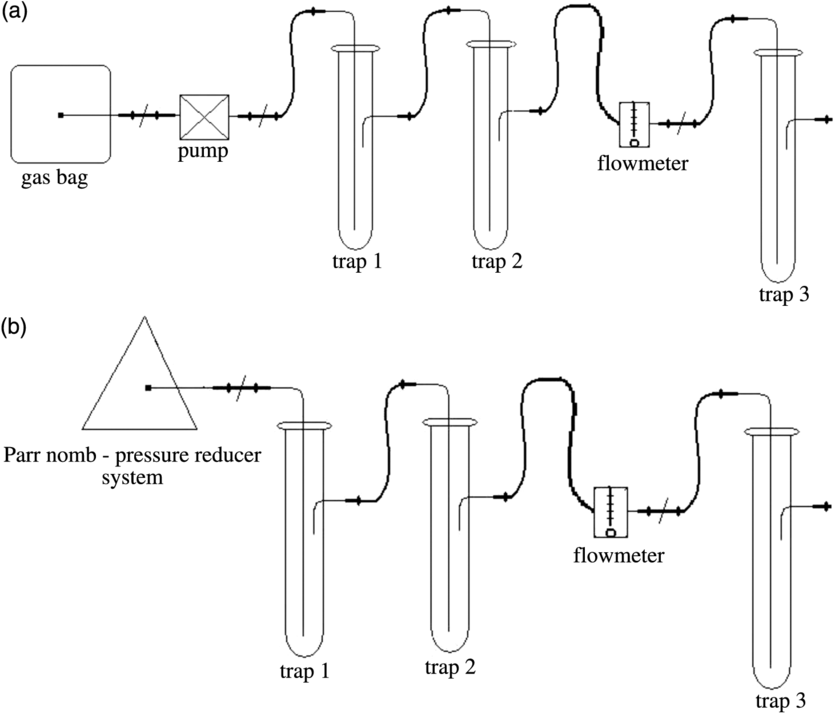
The method used for determining 14C concentration was the direct absorption method followed by LSC (Leaney et al. Reference Leaney, Herczeg and Dighton1994; Varlam et al. Reference Varlam, Stefanescu, Varlam, Faurescu, Popescu, Chalupnik, Schoenhofer and Noakes2006). This consists of measuring 14C contained in a known quantity of carbon, as carbon dioxide, obtained from a sample, standard and background material, counted in an ultra-low level liquid scintillation counter Quantulus™ 1220. The procedure applied in our laboratory has three steps. The first step is the preparation of the homemade liquid scintillation cocktail, which contains an amine (CarbonTrap, Meridian Biotechnologies Ltd), fluorescence substances (PPO and bis-MSB, PerkinElmer) and solvents (methanol and toluene). The second stage of the procedure is obtaining and purification of CO2. For air samples, the obtained Na2CO3 by static absorption of atmospheric CO2 has been acidified with HCl and in order to obtain pure CO2. The pure CO2 was collected in a gas bag, which then was connected to the bubbling system (Figure 2a) containing a pump, a purification system (trap 1 and trap 2—containing an aqueous solution of AgNO3), a flowmeter and the bubbler with scintillation cocktail (trap 3). The CO2 from the gas bag is then bubbled through the liquid scintillation solution with a flow rate of about 0.2 L/min for 10 min to ensure saturation of amine with the CO2 as carbamate.
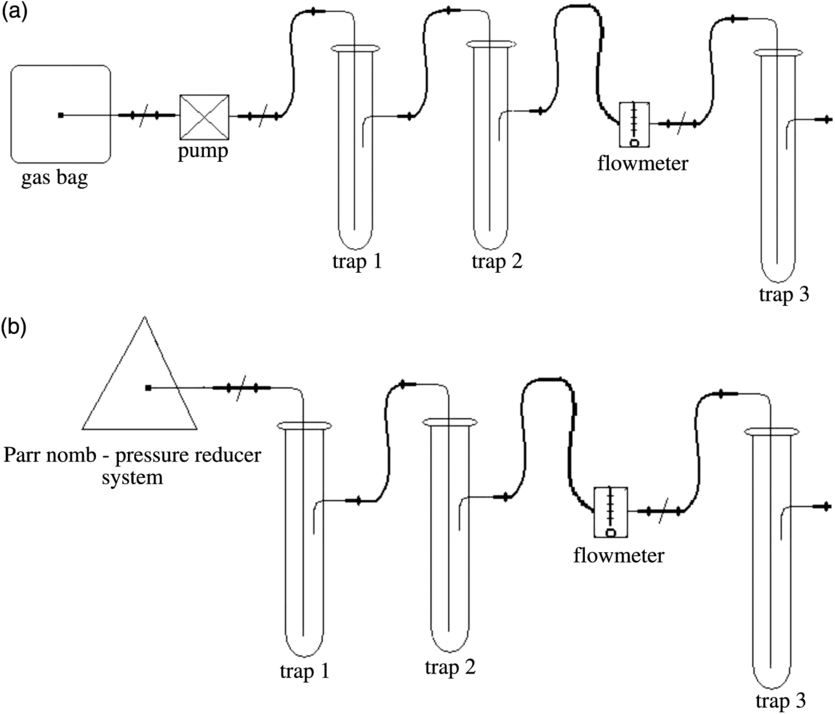
Figure 2 Bubbling line for CO2 absorption method: (a) bubbling of pure CO2 through the scintillation cocktail; (b) bubbling of the combustion gas mixture directly through the scintillation cocktail.
Biological samples were dried into the oven at 60ºC to constant weight and then ground and combusted in an oxygen atmosphere (17 atm.) in a Parr 1121 combustion vessel (Moghissi et al. Reference Moghissi, Bretthauer, Whittaker and McNelis1975) in order to obtain CO2. After combustion of the sample, the combustion vessel was cooled with cold water and then connected to the bubbling system (Figure 2b). Purification of the gas mixture was done using aqueous solutions of chromic acid (H2CrO4)—trap 1, and silver nitrate (AgNO3)—trap 2, respectively. Besides the purification system, the bubbling line contains also a pressure regulator, a flowmeter and the bubbler with scintillation cocktail (trap 3). The gas mixture from the combustion vessel was then bubbled through the liquid scintillation solution with a flow rate of about 0.2 L/min for 10 min.
To determine the 14C activity of the CO2 recovered, the quantity of carbon as CO2 was established. For that, a known quantity (by weight) of liquid scintillation cocktail was used in the bubbling line and after retaining the CO2, the liquid scintillation cocktail was weighed again. The difference between the initial and the final weight of the cocktail was determined as the mass of CO2 retained. After the bubbling step, the scintillation cocktail was transferred in 20-mL low-potassium glass vials (PerkinElmer, product no. 6000128) and then counted via conventional LSC. Due to the low-level radioactivity expected, we choose to use the internal standard method to establish counting efficiency. The background sample was prepared with CO2 obtained (by acidification with HCl) from marble. The standard sample was prepared in the same way as a background sample. In this one, a standard capsule, originating from an internal standard kit for liquid scintillation counting with known activity (PerkinElmer, product no. 1210-122) was dissolved. The labeled compound, [4 – 14C] – cholesterol produced by Amersham International, UK, was used. The absolute activity of the capsules is calibrated by comparison with reference standards of [1 – 14C]-n- hexadecane supplied by NIST (SRM No. 4222C). The vials were then counted via conventional 14C analysis using the following parameters: 1000 min counting time (10 × 100 min/cycle), counting efficiency at the best factor of merit was around 65% with a background around 2.2 CPM (counts per minute). For each batch the quench of prepared samples was checked, using the spectral quench parameter of the external standard, SQP(E). This was determined by using the internal gamma source of the Quantulus 1120 (152Eu, 37 kBq). The SQP(E) parameter also indicates the level of CO2 saturation of our scintillation cocktail. Data acquisition was performed by using WinQ Windows workstation software, and for spectra processing 1224-534 EASY View software was used.
The results of the radiocarbon measurements are reported in Δ14C (Stuiver and Polach Reference Stuiver and Polach1977). The effect of isotope fractionation has been taken into account in the data evaluation, and therefore the results of 14C activity are corrected for δ13C. The δ13C ratio was measured by isotope ratio mass spectrometry on a Delta V IRMS on small aliquots of sodium carbonate resulted from the absorption of CO2 into sodium hydroxide and dried control samples.
RESULTS AND DISCUSSION
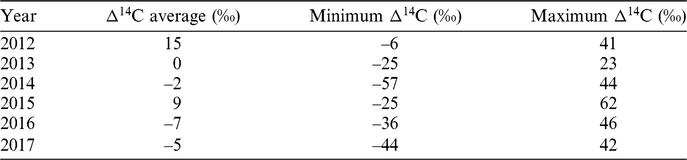
Figure 3 shows the atmospheric Δ14C data measured on the Ramnicu Valcea samples (grey line) during the period from August 2012 to January 2018 and Δ14C data set for Jungfraujoch location (black line) during the period August 2012 to February 2016. The long-term decrease of Δ14C in atmospheric CO2 observed since the 1960s has continued during the studied period. For the Ramnicu Valcea location, the measured Δ14C levels varied between –57‰ and 62‰, with a minimum value in October 2014 and a maximum value in November 2015. The mean of Δ14C for the studied period was around 0‰. The results have a decreasing trend, but due to local influence caused by the continuous production of fossil CO2, we cannot observe Δ14C seasonal variations. In Table 1, we present average, minimum and maximum values recorded year-by-year in the studied period.

Figure 3 Variation of the Δ14C for collected atmosphere samples in Ramnicu Valcea and Δ14C for Jungfraujoch (2012–2016). The dashed line represents the linear trend line for the Ramnicu Valcea values.
Table 1 Average, minimum and maximum Δ14C values recorded for Ramnicu Valcea in the period August 2012 to January 2018.

By comparison with Jungfraujoch, the Δ14C values for the Ramnicu Valcea location are lower due to the major impact of the coal-fired thermoelectric power plant in the immediate vicinity of the sampling location of the CO2 atmospheric samples. The recorded Δ14C values for the Ramnicu Valcea location were also lower compared to another undisturbed location, Hegyhátsál, Hungary (Major et al. Reference Major, Haszpra, Rinyu, Futo, Bihari, Hammer, Jull and Molnar2018). On the other hand, the Δ14C results observed for Ramnicu Valcea are similar to those observed in countries neighboring Romania, for example, the industrialized city of Zagreb, Croatia (Bronic et al. Reference Bronic, Horvatincic, Baresic and Obelic2009), or Debrecen, Hungary (Molnar et al. Reference Molnar, Haszpra, Svingor, Major and Svetlik2010).
Radiocarbon levels for the biological samples are shown in Figure 4. The mean of Δ14C values for control samples (wild vegetation [WV], grapes, and tree leaves) was 11‰. The maximum value was observed in grapes (42‰) while the minimum value was found in the wild vegetation in the autumn (–42‰). The observed values for our control samples were in the same range as those observed for the atmosphere. These kinds of samples do not necessarily reflect the radiocarbon level in the atmosphere at the time of collection but rather these are time-integrated samples that allow evaluating emissions over a longer period of time.

Figure 4 Variation of the Δ14C for collected control samples.
In the context of climate change, the assessment of the fossil-fuel-derived CO2 component in the atmosphere is very important. This study will be continued not only for monitoring but also to use time-integrated observations of the radiocarbon content of CO2 to make some correlations between 14C activity in the air and the vegetation. Both fast-growing plant material (e.g. wild vegetation, tree leaves) and CO2 collected by absorption into sodium hydroxide solution provide excellent time-integrated records of atmospheric 14CO2. These time-integrated samples allow the evaluation of emissions over a longer period of time with only a modest number of measurements.
SUMMARY AND CONCLUSION
In this study, we have presented results obtained in determining the level of radiocarbon in the atmosphere of Ramnicu Valcea, Romania, in the period August 2012 to January 2018. Due to the necessity of establishing a baseline of atmospheric 14C, the sampling was done near a nuclear facility from which one of the important releases is gaseous radioactive effluents. The sampling and preparation procedures were also presented. These have proven to be simple procedures both for 14CO2 sampling by static absorption into a sodium hydroxide solution and for sample preparation. It must also be noted that LSC 14C measurement is less expensive than other techniques, but with the disadvantage of higher uncertainties.
The monitored site is a particular one due to the Suess effect caused by the continuous production of fossil CO2 by a coal-fired thermoelectric power plant. By comparison with Δ14C values recorded at Jungfraujoch and other undisturbed locations, our values were smaller. Also, seasonal variations could not be highlighted. Δ14C values for wild vegetation, grapes, and tree leaves were in the same range as those observed for the atmosphere.
In the future, we want the development of an automatic CO2 sampling device to allow CO2 capture by bubbling into a sodium hydroxide solution. This will allow both monitoring locations similar to those studied, but also undisturbed locations or close to nuclear facilities.
ACKNOWLEDGMENTS
This work was supported by project PN 18 12 03 04, part of Core Program ICSI 4E supported by the Romanian Ministry of Research and Innovation, by a grant of Ministry of Research and Innovation, CNCS - UEFISCDI, project number PN-III-P1-1.1-PD-2016-0532, within PNCDI III and monitoring program of Tritium Removal facility PESTD.