INTRODUCTION
Iodine-129 is a long-lived cosmogenic radionuclide, with a half-life of 15.7×106 yr. In nature, it is produced by cosmic-ray interactions with Xe in the upper atmosphere, and by spontaneous fission of 238U and neutron-induced fission of 235U in the lithosphere (Edwards Reference Edwards1962; Eisenbud and Gesell Reference Eisenbud and Gesell1997). The natural inventory has been estimated to be ~230 kg, most of which resides in the deep oceans (Rao and Fehn Reference Rao and Fehn1999). However, the primary source of 129I in the Earth has now been significantly altered by human nuclear activities, and the natural concentration of 129I has been affected by continuing additions from a variety of anthropogenic sources (Santschi and Schwehr Reference Santschi and Schwehr2004). Civil and military nuclear activities and nuclear accidents have released more than 6000 kg of 129I since 1945 (Aldahan et al. Reference Aldahan, Alfimov and Possnert2007; Jabbar et al. Reference Jabbar, Wallner and Steier2013).
Due in part to its long half-life, production, and distribution, 129I has been recognized as a valuable radionuclide for environmental tracing studies and in assessing short- and long-term consequences of radioactive contamination in the environment (Yiou et al. Reference Yiou, Raisbeck, Zhou and Kilius1994; Raisbeck et al. Reference Raisbeck, Yiou, Zhou and Kilius1995; Raisbeck and Yiou Reference Raisbeck and Yiou1999; Hou Reference Hou2004; Santschi and Schwehr Reference Santschi and Schwehr2004; Fan et al. Reference Fan, Hou and Zhou2013). Among several analytical techniques capable of quantifying 129I in environmental samples, accelerator mass spectrometry (AMS) is now recognized as the method of choice for its superior sensitivity and small sample size requirements. However, the conventional AMS method for 129I determination requires time-consuming chemical separation of iodine. Typically, iodine is released from solid samples and trapped in aqueous solutions, then extracted and precipitated using wet chemistry procedures to produce AgI or AgI-AgCl (Hou et al. Reference Hou, Hansen, Aldahan, Possnert, Lind and Lujaniene2009, 2010). These procedures normally take more than a day, and the time required limits applications requiring rapid analyses, especially in emergency situations when the evaluation of a large number of samples is needed (e.g. following nuclear accidents). In these cases, it is desirable to use a simple and rapid preparation method to identify samples that can then be selected to slower but more precise analytical protocols. We describe below how a direct AMS measurement method for screening samples meets this need.
Methodology
Standards, Blanks, and Target Materials
The 129I standards used in the experiment were prepared by coprecipitating AgI-AgCl, with a Cl/I mass ratio of 20 and 129I/127I atomic ratios of 1.14±0.03×10–10 (standard CST1) and 9.95±0.32×10–12 (standard CST2), using standardized 129I solutions and NaCl solutions. The 129I standard solutions were prepared by dilution of the NIST-SRM 4949c 129I standard (National Institute of Standards and Technology, USA) with a blank 127I solution, which is prepared by dissolution of crystalline iodine obtained from Woodward Iodine Corporation, USA, with an 129I/127I ratio of <2×10–13 (Hou et al. Reference Hou, Zhou, Chen, Zhang, Liu, Luo, Fan, Liang and Fu2010; Zhang et al. Reference Zhang, Hou, Zhou, Chen, Liu, Luo, Fan and Fu2013). All of the targets prepared in this study used 99.99% pure, 325-mesh niobium powder obtained from Alfa Aesar.
Samples
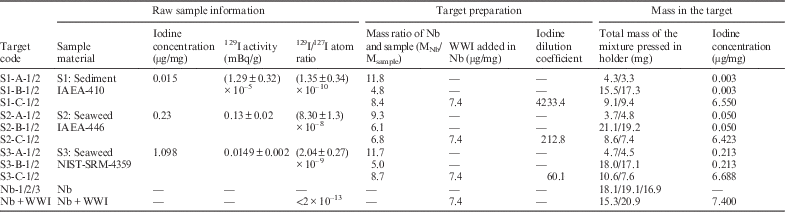
Three certified reference materials, including sediment (IAEA-410) and seaweeds (IAEA-446, and NIST-SRM-4359) (Outola et al. Reference Outola, Filliben, Inn, La Rosa, McMahon, Peck, Twining, Tims, Fifield, Smedley, Antón, Gascó, Povinec, Pham, Raaum, Wei, Krijger, Bouisset, Litherland, Kieser, Betti, Aldave de las Heras, Hong, Holm, Skipperud, Harms, Arinc, Youngman, Arnold, Wershofen, Sill, Bohrer, Dahlgaard, Croudace, Warwick, Ikäheimonen, Klemola, Vakulovsky and Sanchez-Cabeza2006; Pham et al. Reference Pham, Gastaud, La Rosa, Lee, Levy-Palomo, Oregioni and Povinec2006, Reference Pham, Benmansour, Carvalho, Chamizo, Degering, Engeler, Gascó, Gwynn, Harms, Hrnecek, Ibanez, Ilchmann, Ikaheimonen, Kanisch, Kloster, Llaurado, Mauring, MØller, Morimoto, Nielsen, Nies, Norrlid, Pettersson, Povinec, Rieth, Samuelsson, Schikowski, Šilobritiene, Smedley, Suplinska, Vartti, Vasileva, Wong, Zalewska and Zhou2014), were analyzed in this study. These materials are frequently used as quality control standard reference materials in 129I determinations. The 127I concentrations of the reference samples were analyzed by inductively coupled plasma mass spectrometry (ICP-MS) and their 129I activities were determined following standard procedures at the Xi’an AMS Laboratory (Zhang et al. Reference Zhang, Zhou, Hou, Chen, Liu, He, Fan, Luo, Wang and Fu2011) for comparison with the direct method. Table 1 lists the concentration of 129I and 127I, and 129I/127I atomic ratios in the samples.
Table 1 Information of sample and target preparation.

AMS Target Preparation and Measurement
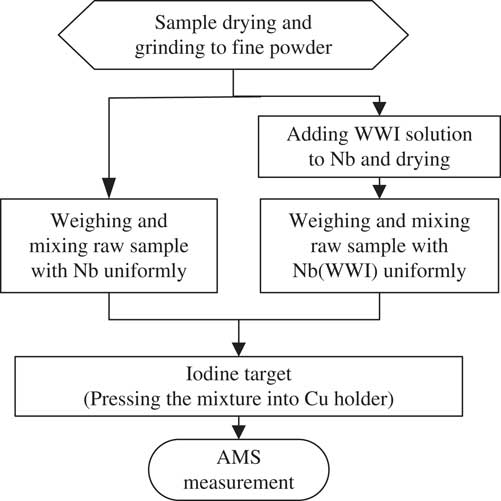
The schematic diagram of the rapid AMS 129I detection procedure is presented in Figure 1. In the experiments, three types of targets have been tested: A: directly mixed sample powder with Nb, pressed into ordinary sample holders; B: Same as A, but the mixture was pressed into sample holders with enlarged holes; C: Same as B, but stable iodine was first added into the Nb before mixing with the dried sample powder. Two identical targets were prepared for each sample used in the tests of all three methods. The total mass of the mixture pressed into the targets and their iodine concentrations are listed in Table 1. Targets pressed with Nb powder only were used as machine blanks.
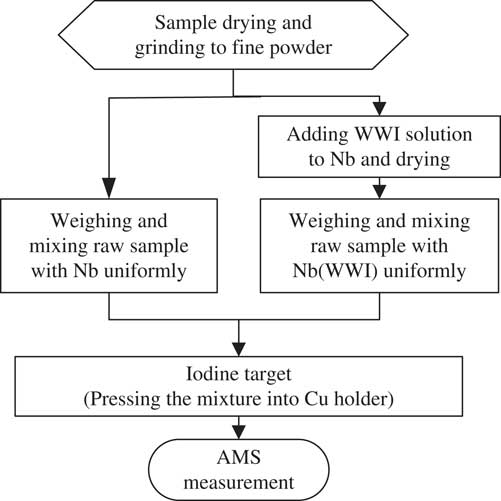
Figure 1 Schematic diagram of the analytical procedure for direct AMS measurement of 129I.
All targets were measured on the Xi’an 3.0MV AMS with the terminal voltage of 2.5MV and the charge state of +5, following the same steps as for the measurement of micro-iodine targets by the AgI-AgCl coprecipitation sample processing method, as described in Hou et al. (Reference Hou, Zhou, Chen, Zhang, Liu, Luo, Fan, Liang and Fu2010) and Liu et al. (Reference Liu, Hou, Zhou and Fu2015). The ion source parameters and status are given in Table 2.
Table 2 Parameters and status of cesium sputtering negative ion source (SO110).

RESULTS
Each target was measured sequentially, 10 min per cycle, for a total of six cycles. The machine blank level of 129I counts and digitized 127I ion charges collected were estimated using the average of corresponding Nb targets. The machine blank values were subtracted for each result to determine blank-corrected 129I/127I ratios for each cycle. The final ratio of 129I/127I was calculated as the average of multiple targets. The results are listed in Table 3.
Table 3 Analytical results of the direct AMS measurements for 129I.

* The 129I/127I ratio for the original sample was calculated based on the measured 129I/127I atomic ratio, the mass of the sample pressed in the holder and the amount of 127I added in the Nb powder.
DISCUSSION
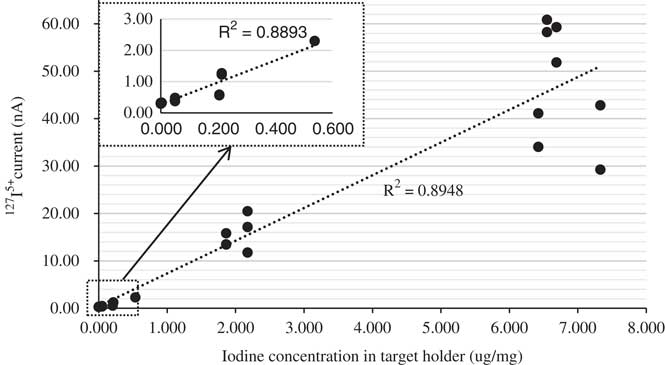
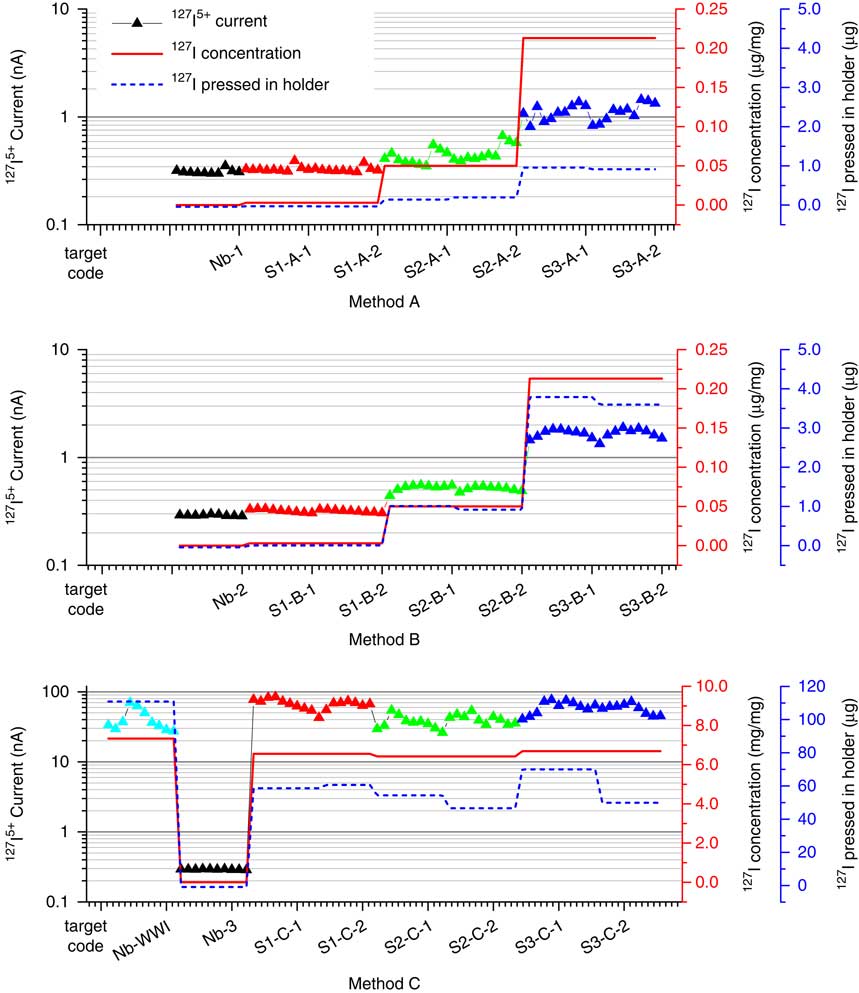
The performance of this rapid method can be evaluated according to two criteria: (1) signal stability and (2) measurement reproducibility. A stable but increased intensity of the 127I5+ current with iodine concentration was observed for the suite of reference samples (Figure 2), and multiple targets of the same reference material produced consistent 127I (Figure 3) and 129I results (Figure 4). Therefore, these criteria are satisfied. The 129I/127I ratios measured using the rapid method are also in general agreement with those of the conventional method including chemical pretreatment protocols, within their analytical uncertainties (Table 3).
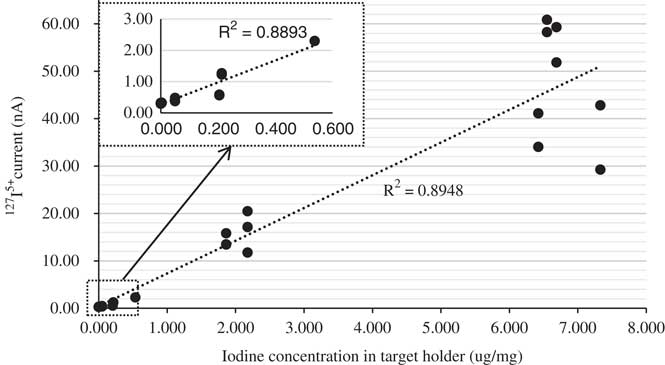
Figure 2 Correlation of the measured 127I5+ currents with iodine concentration in the targets.

Figure 3 Comparison of the measured 127I5+ current and the 127I concentration/content in different targets of different methods.

Figure 4 Comparison of the measured 129I count rate and the 129I concentration/content in different targets of different methods.
Sample Holder (Target) Design Improvement
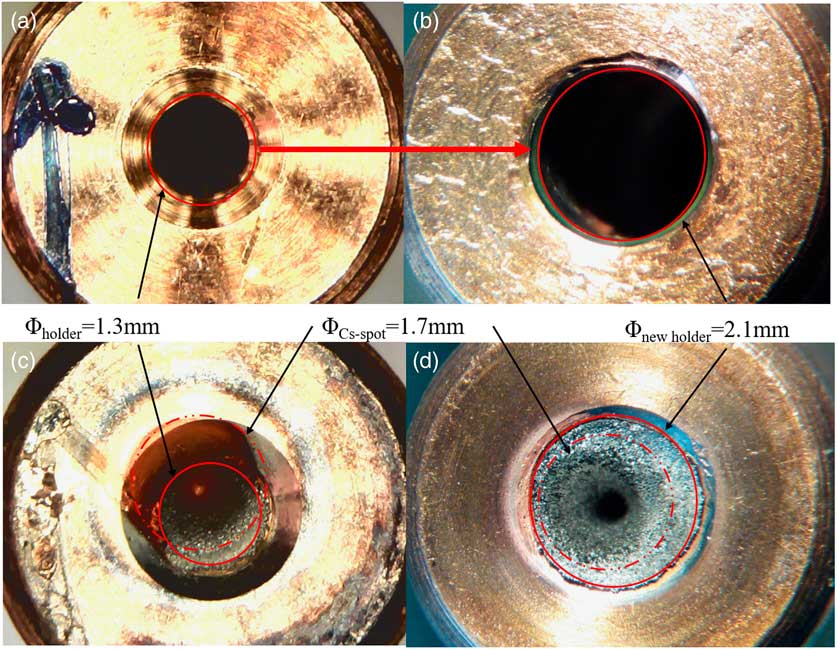
In the first measurements (Method A), the dried sample material with a known iodine content was directly mixed with Nb powder, and the mixture (3.3–4.7 mg) was pressed in the standard sample holders with a bore diameter of 1.3 mm. This batch of targets including S1-A-1/2, S2-A-1/2, and S3-A-1/2 was run to test the stability of the ion source. Preliminary data (Figures 3, 4) for the 129I count rates and 127I currents showed promise, but the 127I signal was weak. This is partly attributed to the low total amount of iodine that was loaded in each target (see Table 1, IAEA-410: 0.003 µg/mg; IAEA-446: 0.050 µg/mg; NIST-SRM-4359: 0.213 µg/mg). We also reasoned that incomplete coverage of the slightly off-centered Cs+ density distribution over the target area could limit the number of 127I ions emitted from the ion source. As shown in Figure 5c, the Cs+ density distribution appears slightly off-centered, part of which appears to miss the sample area of 1.3 mm in diameter. Hence, the bore was enlarged to ensure the sample area to be fully exposed to the Cs+ beam (Figure 2b). The diameter of the bore in the new holder was increased from 1.3 to 2.1 mm, and the amount of sample mixture pressed into the holder was increased from about 15 to 21 mg. The increased sample material need is not an issue for such a rapid method, and this led to a 50% to 100% increase in iodine current. The details of the modification of the target holder are shown in Figure 5.
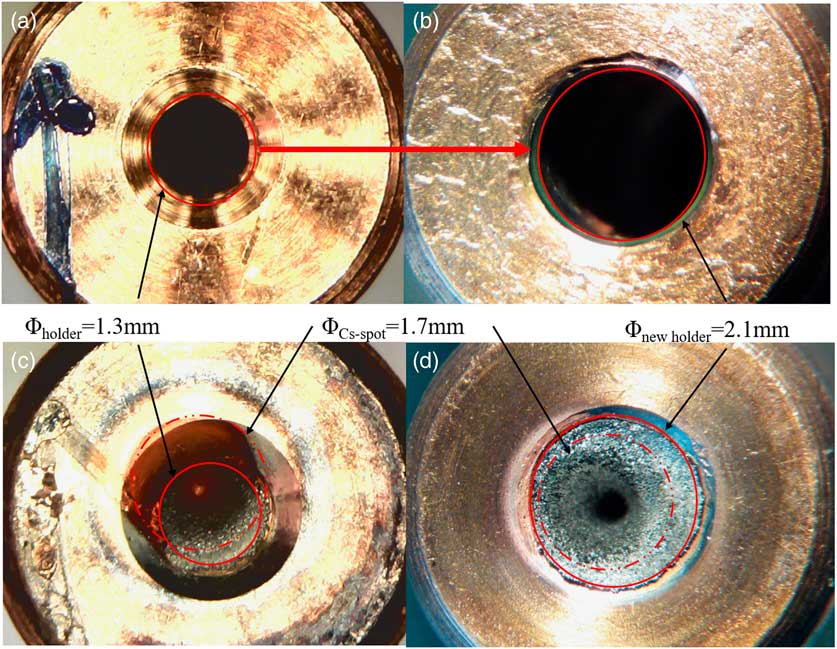
Figure 5 Sample holders (target): (a and b): original and improved target holders with different hole radii; (c and d): comparison of sputtered targets illustrating the efficient usage of sample material.
Analytical Precision and Direct 129I Activity Measurement by AMS
In Method A and B, the measured 129I/127I ratios differ from known values notably because of generally low 127I currents. To improve the analytical accuracy of 129I, the measurement accuracy of 127I current has to be improved. To this end, a carrier addition to the target (Method C) was proposed. In this approach, 127I carrier solution was added to the Nb powder, the mixture was then homogenized, dried, and mixed with the reference samples for measurement. According to our previous work on AMS measurement of 129I in microgram-level iodine targets (Liu et al. Reference Liu, Hou, Zhou and Fu2015), when the iodine concentration reaches 7 µg/mg in the target, an 127I5+ current of 30~80 nA can be measured at the high-energy end of the AMS; this 127I5+ current is sufficient to stabilize the 127I5+ beam position, and in turn to stabilize the terminal voltage by the slit stabilization function. In this work, the iodine concentration in the targets was increased by factors of 60, 213, and 4233 for NIST-SRM-4359, IAEA-446, and IAEA-410, respectively, with the addition of 127I carrier into the Nb powder. A precise 129I/127I ratio was measured, in which the 127I from the raw sample contributed a small faction (<1/60). In this case, we can neglect the iodine content in the raw sample material and directly calculate the 129I activity concentration by the formula:
 $$\eqalignno{ ({}^{{127}}I)_{{in\;{\rm }the\;{\rm }target}} {\rm \ corresponding\ to\ a\ unit\ mass\ sample}\,& \approx\,{{m_{{Nb}} {\rm {\asterisk}}{}^{{127}}I_{{concentration\ {\rm }\ added{\rm }\ in\ {\rm }the\ {\rm }Nb}} } \over {m_{{sample}} }}\cr & ={{{}^{{127}}I_{{concentration\ {\rm }added\ {\rm }in\ {\rm }the\ {\rm }Nb}} } \over {{\raise0.7ex\hbox{${m_{{sample}} }$} \!\mathord{\left/ {\vphantom {{m_{{sample}} } {m_{{nb}} }}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${m_{{Nb}} }$}}}}$$
$$\eqalignno{ ({}^{{127}}I)_{{in\;{\rm }the\;{\rm }target}} {\rm \ corresponding\ to\ a\ unit\ mass\ sample}\,& \approx\,{{m_{{Nb}} {\rm {\asterisk}}{}^{{127}}I_{{concentration\ {\rm }\ added{\rm }\ in\ {\rm }the\ {\rm }Nb}} } \over {m_{{sample}} }}\cr & ={{{}^{{127}}I_{{concentration\ {\rm }added\ {\rm }in\ {\rm }the\ {\rm }Nb}} } \over {{\raise0.7ex\hbox{${m_{{sample}} }$} \!\mathord{\left/ {\vphantom {{m_{{sample}} } {m_{{nb}} }}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${m_{{Nb}} }$}}}}$$
in which
![]() ${\rm \lambda }_{{{}^{{129}}I}} $
is the decay constant,
${\rm \lambda }_{{{}^{{129}}I}} $
is the decay constant,
![]() ${{{R =\,}^{{127}}I_{{concentration\ {\rm }added\ {\rm }in\ {\rm }the\ {\rm }Nb}} } = {{\raise0.7ex\hbox{${M_{{blank\ iodine}} }$} \!\mathord{\left/ {\vphantom {{m_{{sample}} } {m_{{nb}} }}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${M_{{Pure\ Nb}} }$}}}}$
is the iodine concentration added to Nb, and
${{{R =\,}^{{127}}I_{{concentration\ {\rm }added\ {\rm }in\ {\rm }the\ {\rm }Nb}} } = {{\raise0.7ex\hbox{${M_{{blank\ iodine}} }$} \!\mathord{\left/ {\vphantom {{m_{{sample}} } {m_{{nb}} }}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${M_{{Pure\ Nb}} }$}}}}$
is the iodine concentration added to Nb, and
![]() $r{\equals}{\raise0.7ex\hbox{${m_{{sample}} }$} \!\mathord{\left/ {\vphantom {{m_{{sample}} } {m_{{nb}} }}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${m_{{Nb}} }$}}$
is the mass ratio of sample and Nb (with blank iodine).
$r{\equals}{\raise0.7ex\hbox{${m_{{sample}} }$} \!\mathord{\left/ {\vphantom {{m_{{sample}} } {m_{{nb}} }}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{${m_{{Nb}} }$}}$
is the mass ratio of sample and Nb (with blank iodine).
The 129I activity concentration was then calculated by the measured 129I/127I ratio, combined with the mass of the raw sample, the mass of Nb powder added, and the concentration of iodine added to the Nb powder. The results (last column in Table 3) show that measured 129I concentrations are in excellent agreement with the reported values (fourth column in Table 1), within 3σ.
Reliable Lower Detection Limit of 129I
The 129I concentration in NIST-SRM-4359 (target S3-C-1/2) is about 0.0015 mBq/g in the mixture loaded in the target, which resulted in a stable 129I count rate of about 100 counts/min with a statistical error of 5% for a 5-min measurement. Considering that the count rate of 129I for the machine’s blank target (Nb) is about 1 count per minute (CPM), and we can set 10 times higher count rate (i.e. 10 CPM) for a reliable measurement of 129I, the lower detection limit can be set corresponding to the 129I concentration of 0.0015 mBq/g for the mixture. If the ratio of the raw sample to the Nb is maintained the same as the test samples, it can be concluded that the reliable detection limit of the method for accurate measurement of 129I activity is 0.0015 mBq/g in the raw sample materials. By this rapid method, assuming that all the conditions are kept the same as done in this experiment, if the count rate can get to 10 CPM or higher, we can directly report the 129I activity concentration; otherwise, we report that the 129I activity is lower than 0.0015 mBq/g in the raw sample. For the sample of IAEA-410 in this work, the 129I count rate is close to the machine background of Nb (about 1 CPM); we thus conclude that its 129I activity is lower than 0.0015 mBq/g, even though a value of (2.04±1.38)×10–5 (Table 3) is calculated after blank correction, which is close to the reference value.
Applicability
There were two types of samples tested in this work, seaweed and sediment. In this rapid measurement method, no particular anomaly was noted during Cs+ sputtering. The target integrity appeared quite normal and satisfied the need for direct AMS measurements. Based on this, it is expected that other environmental samples, such as soil, grass, and air particles, can be also analyzed using this method, as long they do not produce severe Cs+ sputter-induced adverse effects that prevent stable operation of the ion source. Because a small sample (1–3 mg) is used for analysis in this work, attention needs to be given to samples that might contain “hot” particles. Hot particles cause large variations in the analytical results, or errors in estimation of the average 129I level. Analyzing multiple sample subsets might be needed in this case.
It must be noted that because the stable iodine carrier added to the Nb powder and the 129I atoms bound by the raw samples are in different chemical states, isotope fractionation during Cs+ sputtering is possible. This is the fundamental reason why such a rapid method can only be used for screening or quick assessment purposes.
It is necessary to also note that such direct measurement was made using 129I+5 ions, for which the prime interfering molecular fragment is 103Rh+4 (Kilius et al. Reference Kilius, Zhao, Litherland and Purser1997). The abundance of Rh is extremely rare in Earth’s crust, which is perhaps an important reason why direct 129I+5 counting could succeed without chemical purification of the target material. The direct detection of 129I using lower charge states may require additional caution in order to contend with more common molecular fragment interferences.
CONCLUSION
This work developed a direct AMS method for sensitive measurement of 129I+5 in environmental samples without chemical separation of iodine. The technique includes the addition of 127I carrier into a matrix powder (Nb), which is then mixed with dried raw sample powder. The mixture is then loaded directly into a modified target holder with an area of material exposure to fully catch the Cs+ spot. Seaweeds and sediment were tested as typical materials, and the results show that this direct AMS measurement method worked reasonably well for these two types of sample materials. The 129I/127I ratios and 129I activities measured by the rapid method were found to be consistent with reported values within analytical uncertainties. It is therefore concluded that it is feasible to directly measure 129I/127I ratios and 129I activities using AMS without chemical separation. It is expected that such a rapid measurement method can be also applied to other environmental solid materials, as long as the ion source can function stably. This would be especially useful in emergency situations, where rapid screening analyses of large numbers of samples are necessary to evaluate potential contamination.
ACKNOWLEDGMENTS
This work was financially supported by the projects from National Science Foundation of China (No. 11405176 and 41271512) and the project of Innovation Methodology (No. 2012IM030200) from the Ministry of Science and Technology of China.