INTRODUCTION
Radiocarbon (14C) dating of mollusk shells is commonly employed to constrain the evolution of marine environments throughout the Holocene. Owing to their macroscopic size, marine mollusk shells are easy to sample and handle, and can usually provide amounts of datable material far above the limits needed for typical 14C age determination with AMS method. Marine mollusk shells may however lack in coastal strata, depending on biological productivity, depositional environment, and post-mortem degradation of skeletal remains prior to definitive burial (Kidwell and Bosence Reference Kidwell, Bosence, Allison and Briggs1991). As an alternative, calcareous tests produced by foraminifera can be recovered from sediments for 14C age determination, at the expense of a greater sampling effort through “picking.” Several studies have reported anomalous 14C ages obtained on open sea benthic foraminifera, with samples appearing several thousands of years older than their expected time of deposition (Berger and Heath Reference Berger and Heath1968; Peng et al. Reference Peng, Broecker and Berger1979). Biases have been related to the effects of reworking of calcareous tests (Heier-Nielsen et al. Reference Heier-Nielsen, Conradsen, Heinemeier, Knudsen, Nielsen, Rud and Sveinbjörnsdóttir1995; Cearreta and Murray Reference Cearreta and Murray2000; Callard et al. Reference Callard, Long, Plets, Cooper, Belknap, Edwards, Jackson, Kelley, Long, Milne, Monteys and Quinn2013), bioturbation (Broecker et al. Reference Broecker, Matsumoto, Clark, Hajdas and Bonani1999), incorporation of secondary calcite in opal-rich sediments (Broecker et al. Reference Broecker, Barker, Clark, Hajdas and Bonani2006), differential dissolution and fragmentation at the sediment/water interface (Barker et al. Reference Barker, Broecker, Clark and Hajdas2007), among others. Similar age discrepancies have been identified on coastal and estuarine foraminifera, but studies are less common in comparison, documenting the effects of reworking (Cearreta and Murray Reference Cearreta and Murray2000) and of time-averaging (Martin et al. Reference Martin, Harris and Liddel1995).
14C dating of coastal carbonates can be challenged by several issues that include regional variations in the reservoir age (Lougheed et al. Reference Lougheed, Filipsson and Snowball2013) or contamination by organic (Vonk et al. Reference Vonk, Drenzek, Hughen, Stanley, McIntyre, Montluçon, Giosan, Southon, Santos, Druffel, Andersson, Sköld and Eglinton2019) and inorganic (Liu et al. Reference Liu, Zhao, Sun, Yang, Chen, Yang, Zeng and Zeng2017) carbon supplied by rivers, to name but a few. Little is known therefore about the potential complications that may arise from the use of 14C-dated foraminifera in coastal settings. This study reports the fortuitous finding of anomalous 14C ages obtained on two estuarine foraminifera samples, for which age offsets cannot be attributed neither to reworking (Cearreta and Murray Reference Cearreta and Murray2000) nor to time-averaging (Martin et al. Reference Martin, Harris and Liddel1995). These data were not initially acquired for the purpose of this article, as they come from two previously published multi-proxy core analyses (Poirier et al. Reference Poirier, Chaumillon and Arnaud2011; Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). Despite the very small size of the dataset, a hypothetical explanation based on the available literature is proposed to explain the unexpected 14C age discrepancies. The aim of the study is to raise awareness about a potential problem of in-situ, syndepositional contamination of living foraminifera with 14C-depleted carbon of continental origin. This article is expected to serve as a call for future studies that would help to address this issue with more robust, direct evidence acquired on purpose.
METHODS
Study Area
The study area is located along the Atlantic Coast of France, in Charente Maritime (46°N, 01°W), and corresponds to the northern boundary of the Aquitaine Basin, at the contact with the Armorican Massif (Figure 1; Biteau et al. Reference Biteau, Marrec, Vot and Masset2006). Anticlines and synclines formed in the northern part of the Aquitaine basin during the late Cretaceous and Paleocene, in response to southward compressive deformation of the crystalline basement related to Pyrenean orogeny (Sibuet et al. Reference Sibuet, Srivastava and Spakman2004). Differential erosion of Mesozoic to Cenozoic loose marls and sandstones in synclines during the Pleistocene contributed to the formation of NW–SE incised valleys, which provided a large accommodation space for the deposition of Holocene marine and continental sediments (Weber et al. Reference Weber, Chaumillon and Tesson2004a, Reference Weber, Chaumillon, Tesson and Garlan2004b; Chaumillon and Weber Reference Chaumillon, Weber, Dalrymple, Leckie and Tillman2006; Chaumillon et al. Reference Chaumillon, Proust, Menier and Weber2008).
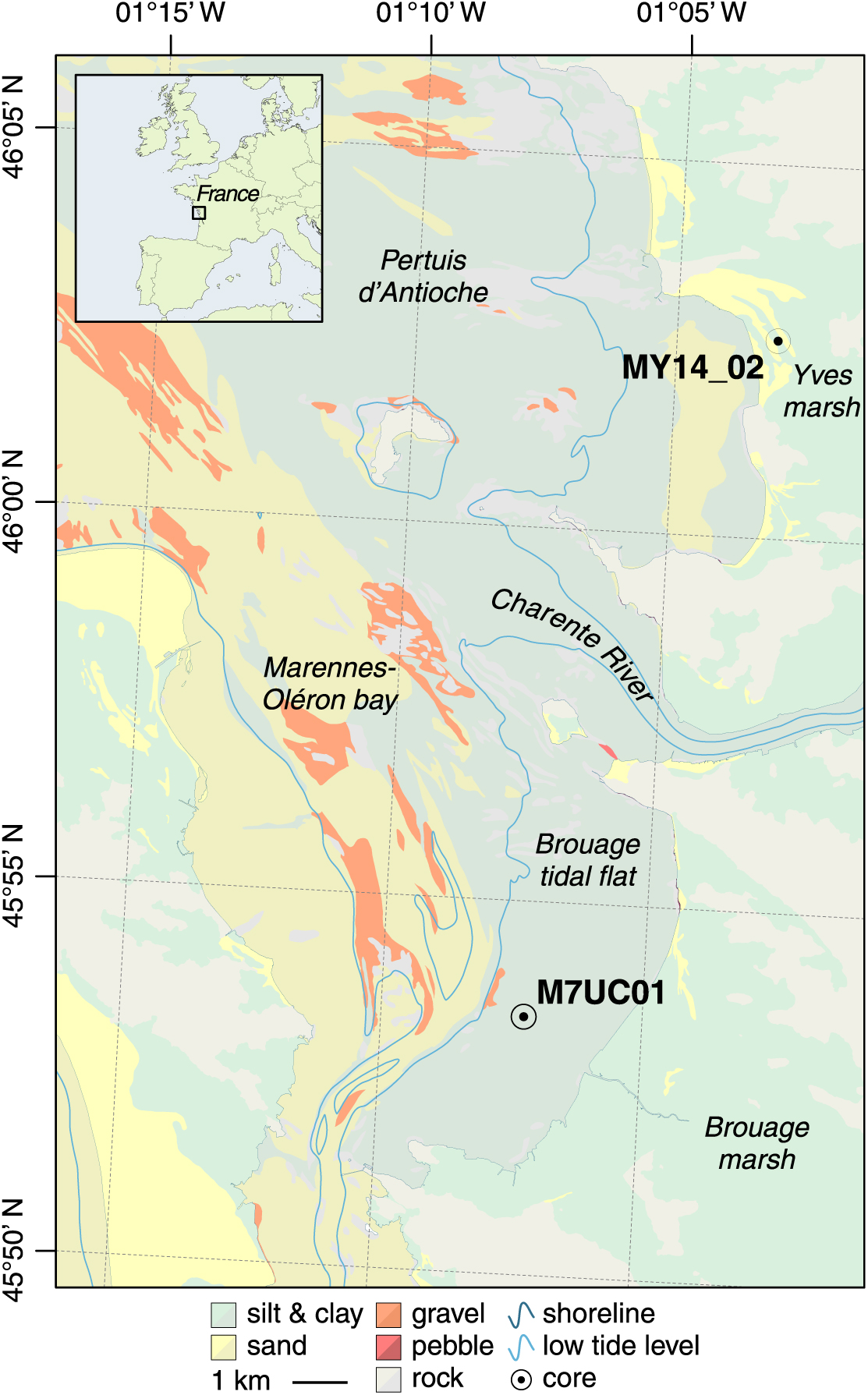
Figure 1 Map of the study area. Upper left inset: map of Western Europe showing the location of the study area in France.
These incised valleys are locally referred to as the “Pertuis Charentais.” The word pertuis locally designates the outer marine segment of three main incised valleys, which extend inland into large low-lying coastal marshes (Breilh et al. Reference Breilh, Chaumillon, Bertin and Gravelle2013). The Pertuis Charentais seafloor is shallow, with more than 90% of its total area between 0 and 20 m NGF (French reference vertical datum, zero being the mean sea level at Marseille). The area is affected by semi-diurnal tides ranging from less than 2 to more than 6 m, and by waves originating from the North Atlantic Ocean. Wave climate is highly variable within a given year and from one year to another, but low to moderate wave conditions prevail (Hs1/4 = 0.8–2.5 m, Tp1/4 = 6–12 s, Dirp1/4 = 274–292°; Bertin et al. Reference Bertin, Castelle, Chaumillon, Butel and Quique2008).
Internal architecture, sedimentology and timing of deposition of the Holocene sediment-fill of the Pertuis Charentais have been extensively investigated with very high resolution seismic surveys ground-truthed by sedimentary cores and 14C dating of mollusk shells (Chaumillon et al. Reference Chaumillon, Tessier, Weber, Tesson and Bertin2004; Weber et al. Reference Weber, Chaumillon and Tesson2004a, Reference Weber, Chaumillon, Tesson and Garlan2004b; Chaumillon and Weber Reference Chaumillon, Weber, Dalrymple, Leckie and Tillman2006), with particular emphasis on the Marennes-Oléron Bay (Billeaud et al. Reference Billeaud, Chaumillon and Weber2005; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010; Poirier et al. Reference Poirier, Chaumillon and Arnaud2011). Within historical times, morphological evolution of the shoreline and seafloor is also constrained by several marine charts and lead line bathymetric surveys (Bertin et al. Reference Bertin, Chaumillon, Weber and Tesson2004; Bertin and Chaumillon Reference Bertin and Chaumillon2005; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010).
The study focuses on the Marennes-Oléron bay and Yves marsh, which are connected to the Pertuis d’Antioche, which corresponds to the incised valley of the Charente River (Figure 1; Weber et al. Reference Weber, Chaumillon, Tesson and Garlan2004b; Chaumillon and Weber Reference Chaumillon, Weber, Dalrymple, Leckie and Tillman2006). Sediment-fill of the Marennes-Oléron bay, Yves Marsh and Pertuis d’Antioche (Figure 1) mainly consists (1) of marine sands supplied by wave processes and reworked by tidal currents (Chaumillon et al. Reference Chaumillon, Gillet, Weber and Tesson2002; Guérin et al. Reference Guérin, Bertin and Chaumillon2016), and (2) of silts and clays supplied by rivers (Parra et al. Reference Parra, Trouky, Jouanneau, Grousset, Latouche and Castaing1998; Dabrin et al. Reference Dabrin, Schäfer, Bertrand, Masson and Blanc2014) and reworked by tidal currents and/or wind waves (Bassoullet et al. Reference Bassoullet, Le Hir, Gouleau and Robert2000). Therefore, sand accumulates in tidal channels, inlets and banks (Chaumillon et al. Reference Chaumillon, Gillet, Weber and Tesson2002; Bertin et al. Reference Bertin, Chaumillon, Weber and Tesson2004; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010) and along coastal barriers (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017), while fine-grained sediments concentrate on the sheltered, eastern tidal flats (Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010; Poirier et al. Reference Poirier, Chaumillon and Arnaud2011; Figure 1).
Small coastal rivers flow directly into the area, the largest being the Charente river (Figure 1). The Gironde estuary, which is located 35 km southward, indirectly contributes to as much as 84% of the suspended matter (both organic and mineral) inputs on average (Dabrin et al. Reference Dabrin, Schäfer, Bertrand, Masson and Blanc2014), owing to northeastward deviation of the Gironde turbid plume under flood tide and high river discharge condition (Froidefond et al. Reference Froidefond, Jegou, Hermida, Lazure and Castaing1998). Annual inputs of suspended matter from the Gironde to the Marennes-Oléron bay are estimated between 430,000 and 520,000 t.yr−1 (Dabrin et al. Reference Dabrin, Schäfer, Bertrand, Masson and Blanc2014), which is about one order of magnitude greater than those from the Charente River (Poirier et al. Reference Poirier, Chaumillon and Poitevin2016). Suspended particulate organic matter (SPOM) delivered to the Pertuis d’Antioche and Marennes-Oléron bay is supplied by several sources (Malet et al. Reference Malet, Sauriau, Ryckaert, Malestroit and Guillou2008). Allochthonous SPOM is mostly provided by the Charente River (Riera and Richard Reference Riera and Richard1996) and Gironde estuary (Fontugne and Jouanneau Reference Fontugne and Jouanneau1987), while the contribution of oceanic phytoplankton is low. Terrestrial SPOM has a δ13C ranging between –32 and –28‰ (Richard et al. Reference Richard, Riera and Galois1997), reflecting a large contribution of freshwater phytoplankton relative to upper plants. Autochthonous SPOM is mostly provided by benthic diatoms (microphytobenthos), while the contribution of salt marsh upper plants and Zostera sp. seagrass meadows is very low (Malet et al. Reference Malet, Sauriau, Ryckaert, Malestroit and Guillou2008). Overall, the study area is therefore under a marked fluvial influence.
Resuspension of fine sediments in shallow waters of the Pertuis Charentais is mostly driven by wind waves, contributing to high turbidity of the water column during storms. On the Brouage tidal flat (Figure 1), short-lived isotope profiles of sediments (7Be, 210Pbxs) indicate that resuspension is restricted to the first 15–20 cm of sub-surface fluid muds, which lie above a basement of older (>100 years), cohesive sediments (Gouleau et al. Reference Gouleau, Jouanneau, Weber and Sauriau2000). In this area, waves contribute to an overall offshore flux of sediments and tides to an onshore flux, these two contradicting hydrodynamic processes leading to a quasi-equilibrium state over annual timescales (Bassoullet et al. Reference Bassoullet, Le Hir, Gouleau and Robert2000).
Sediment Cores
The present article is based on two previously published sediment cores (Figure 1). M7UC01 is an 840-cm-long core sampled and described by Poirier et al. (Reference Poirier, Chaumillon and Arnaud2011), with a focus on the first 500 cm. The core was recovered on the Brouage intertidal mudflat, in the eastern part of the Marennes-Oléron bay (Figure 1), with a 3-m-long UWITEC stationary piston coring device mounted with a motor hammer. MY14_02 is a 150-cm-long core sampled and described by Baumann et al. (Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). The core was recovered in the Yves marsh, which is isolated from the sea by a sandy coastal barrier built by attenuated swell and wind waves, with a stationary piston coring device mounted with a motor hammer (de Resseguier Reference de Resseguier1983).
The succession of depositional environments along the two sediment records has been interpreted from multi-proxy sedimentological, geochemical and micropaleontological analyses. Geochronological data acquired on the M7UC01 and MY14_02 cores have not been entirely published so far (Poirier et al. Reference Poirier, Chaumillon and Arnaud2011; Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). The present study provides a synthesis of these published and unpublished geochronological data, as well as other chronological markers derived from other works for the M7UC01 core (Bertin and Chaumillon Reference Bertin and Chaumillon2005; Poirier et al. Reference Poirier, Chaumillon and Poitevin2016).
Sample Processing and Dating
14C dating was carried out for age determination of mollusks, foraminifera and organic matter in the two cores, without replication at a given sediment layer. Age determination was carried out by the Poznań Radiocarbon Laboratory (Poland) for the M7UC01 core, and by Beta-Analytics (USA) for the MY14_02 core, both using the AMS method.
Marine mollusk shells and foraminifera tests were sampled in both the M7UC01 and MY14_02 cores. Samples were carefully chosen in order to prevent dating of reworked material. When possible, unbroken shells or tests in a good taphonomical state and belonging to species ecologically compatible with the depositional environment were chosen (Fujiwara et al. Reference Fujiwara, Kamataki and Masuda2004). Thin-shelled infaunal mollusks were preferred, because they are indicative of a short post-mortem residence at the water-sediment interface and are thus expected to record more accurately the true timing of sediment deposition (Poirier et al. Reference Poirier, Sauriau, Chaumillon and Bertin2010). Chemical pre-processing of mollusk shells involved removal of the organic coating with H2O2, and removal of the outer carbonate layer with HCl. 14C ages determined on marine mollusk shells and foraminifera were calibrated with the Marine13 calibration curve (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) using Bchron 4.2.6 (Haslett and Parnell Reference Haslett and Parnell2008; Parnell et al. Reference Parnell, Haslett, Allen, Buck and Huntley2008) implemented in open-source software R 3.4.2 (R Core Team 2017). The regional reservoir offset value ΔR, which is –28 ± 23 years, is the inverse-error weighted average of 4 measurements carried out on mollusk shells of the Pertuis Charentais (–36 ± 40, –34 ± 60, –32 ± 39, +7 ± 60 yr; Tisnérat-Laborde et al. Reference Tisnérat-Laborde, Paterne, Métivier, Arnold, Yiou, Blamart and Raynaud2010). Calibrated ages are reported with their 2σ (95%) interval.
Organic matter was sampled in the M7UC01 core. Sediment was sieved on a 500-µm mesh with tap water. The >500-µm fraction contained abundant dark brown organic debris that were separated from the sediment particles and examined under a stereomicroscope. Debris showed a fibrous, woody appearance but could not be taxonomically identified. Previous Rock-Eval analysis of this organic material (Poirier et al. Reference Poirier, Chaumillon and Arnaud2011) yielded a Hydrogen Index smaller than 200 mg HC.g−1 TOC, indicative of organic matter derived from terrestrial vascular plants (Espitalié et al. Reference Espitalié, Deroo and Marquis1985). Combined to the presence of beetle and chironomid remains in the >500 µm fraction, which were not sent for 14C age determination, it is assumed that these organic debris are composed of lignocellulose with a continental origin. This assumption is also supported by (1) the large absolute (Dabrin et al. Reference Dabrin, Schäfer, Bertrand, Masson and Blanc2014) and relative (Fontugne and Jouanneau Reference Fontugne and Jouanneau1987; Riera and Richard Reference Riera and Richard1996) contribution of fluvial sources to the pool of SPOM, and (2) the widespread occurrence of the foraminifera Nonionella turgida in fine-grained sediments of the study area (Poirier Reference Poirier2010), the species being typical of prodeltaic environments subjected to high concentrations of continental organic matter (e.g. Mojtahid et al. Reference Mojtahid, Jorissen, Lansard, Fontanier, Bombled and Rabouille2009). Chemical pre-processing of these organic samples involved a standard acid-alkali-acid treatment, as described by Brock et al. (Reference Brock, Higham, Ditchfield and Bronk Ramsey2010). 14C ages determined on organic matter in the M7UC01 core were therefore calibrated with the IntCal13 atmospheric calibration curve (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013) using Bchron.
In the MY14_02 core, short-lived isotope dating (210Pbxs, 137Cs) was also carried out for age determination of sub-surface sediments following standard analytical procedures. The reader is referred to Baumann et al. (Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017) for methodological details.
M7UC01 Chronological Markers
Published Data
In the M7UC01 core, mollusk shells were sampled at core depths of 61, 160.5, 227.5, 299, 383, and 502.5 cm (Table 1). They were single articulated bivalves (Mytilus edulis, Cerastoderma edule, Nucula nitidosa) or single disarticulated valves (Nucula nitidosa) that were in an excellent state of preservation, without any sign of abrasion and corrosion.
Table 1 Chronological markers (M7UC01 core above, MY14_02 core below). Anomalous 14C ages are in bold.

Two other independent chronological markers were used for the M7UC01 core. First marker is derived from a lead-line bathymetric survey of the Marennes-Oléron bay that was carried out in AD 1882. This dataset has already been used to quantify morphological changes in the southern part of the bay (Bertin et al. Reference Bertin, Chaumillon, Weber and Tesson2004). In the vicinity of the M7UC01 core, the AD 1882 bathymetric data indicates that seafloor was 108 ± 35 cm below the present-day surface at that time. The reader is referred to Bertin et al. (Reference Bertin, Chaumillon, Weber and Tesson2004) for methodological details.
Second marker is derived from sedimentological data. From about 150–210 cm (core depth), the M7UC01 displays a facies of millimeter- to centimeter-thick heterolithic, sand silt alternations. High-resolution grain size data obtained on the core has been matched with a paleoclimate reconstruction of rainfall intensity over the Charente catchment, using a peak-matching algorithm (Poirier et al. Reference Poirier, Chaumillon and Poitevin2016). Based on these results, a conspicuous 4 cm thick silt layer found at 194 ± 2 cm has been attributed to above-average precipitation rates that occurred between AD 1718 and AD 1722, according to the paleoclimate reconstruction.
Unpublished Data
In the M7UC01 core, 14C dating was carried out on three additional samples that were not reported in the initial core description (Poirier et al. Reference Poirier, Chaumillon and Arnaud2011). Two 14C ages were obtained on lignocellulose debris recovered at depths of 130 and 137.5 cm. The third one was obtained on Haynesina germanica (Ehrenberg 1840) individuals recovered from the total foraminifera assemblage (125–500 µm fraction) at 172.5 cm. The assemblage was in an excellent state of preservation, without any sign of test abrasion. About 3000 translucent, unbroken tests were picked to reach a sufficient sample mass.
MY14_02 Chronological Markers
Published Data
In the MY14_02 core, geochronological data included (1) short-lived isotope dating (210Pbxs, 137Cs) of the top 10 cm, and (2) 14C age determination of two marine mollusk shells, including a single articulated bivalve (Mytilus edulis) at core depth of 53 cm and several micro-gastropods (Peringia ulvae) at 63.5 cm. Shells were in an excellent state of preservation, without any sign of abrasion and corrosion.
Unpublished Data
In the MY14_02 core, 14C dating was carried out on one additional sample that was not reported in the initial core description (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). The total foraminifera assemblage was picked from the 150–500 µm fraction at 63 cm. Individuals were in a variable state of preservation, ranging from translucent, unbroken tests to opaque and/or broken tests. About 500 translucent, unbroken tests were picked to reach a sufficient sample mass.
Age-Depth Models
Age-depth models were built from the combined published and unpublished data using Bchron 4.2.6 (Haslett and Parnell Reference Haslett and Parnell2008; Parnell et al. Reference Parnell, Haslett, Allen, Buck and Huntley2008) and Bacon 2.3.4 (Blaauw and Christen Reference Blaauw and Christen2011) implemented in open-source software R 3.4.2 (R Core Team 2017). Bchron and Bacon rely on Bayesian statistics to infer age-depth relationship along a core, under the simple assumption that sediment accumulation through time is monotone, i.e. without chronological inversions. The two methods were tested and predicted similar median ages, in accordance with Trachsel and Telford (Reference Trachsel and Telford2016) findings. However, Bchron was preferred to Bacon because the former could handle the uncertainty in vertical positioning of the 1882 historical bathymetry marker in the M7UC01 core.
Both the M7UC01 and MY14_02 age-depth models included core top (0 cm) as the age of core extraction, which are AD 2008 and 2014, respectively.
RESULTS
Sediment Facies and Depositional Environments
In the following section, a short description of sediment facies and interpreted depositional environments is provided for each core. More detailed information is available in the original articles (Poirier et al. Reference Poirier, Chaumillon and Arnaud2011; Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017).
In the M7UC01 core, deposits consist of a 500 cm-long fining upward sequence split into three main sedimentary units. From base to 370 cm, sediment is exclusively composed of well-sorted fine sands (160 µm) interpreted as an open, mixed tide- and wave-dominated, shallow subtidal bay environment (unit UT3 in Poirier et al. Reference Poirier, Chaumillon and Arnaud2011). Millimeter to centimeter thick silt-sand alternations appear as the contribution of poorly-sorted fine silts (5–9 µm) to the sediment increases gradually from 370 to 130 cm (unit UT4.1), indicative of greater tidal and/or fluvial influence. The top 130 cm are exclusively composed of fine silts, and correspond to the present-day Brouage intertidal mudflat (unit UT4.2). In this latter unit, the foraminifera assemblage includes Haynesina germanica that accounts for about 70% of the relative abundance, along with Ammonia tepida (10%), Elphidium excavatum (8.5%) and E. gunteri (4.5%). Abundance ranges between 500 and 1000 individuals per 10 g of sediment (unpublished data; Poirier Reference Poirier2010).
In the MY14_02 core, deposits consist of homogeneous brownish fine silts and clays. Average grain size is not greater than 10 µm, coarsening upward to 70 µm in the top 10 cm. Four sedimentary units are recognized, and correspond to the transition from an open tide/wave-dominated intertidal mudflat to a sheltered back barrier environment, ultimately colonized in the top 10 cm by terrestrial marsh vegetation. The mudflat to back barrier transition is identified at 47 cm from micropaleontological data. It is characterized by a sharp drop in foraminifera abundance together with the rapid decline of open-marine species (Elphidium sp., Lobatula lobatula, Quinqueloculina sp.). Haynesina germanica accounts for 20 up to 95% of the total foraminifera assemblage along the whole sedimentary succession. Abundance ranges between 100 and 1000 individuals per 10 g of sediment in the lower part of the core (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017).
Chronological Markers and Age-Depth Models
Published and unpublished chronological markers are summarized in Table 1, and resulting age-depth Bchron models are shown in Figure 2.
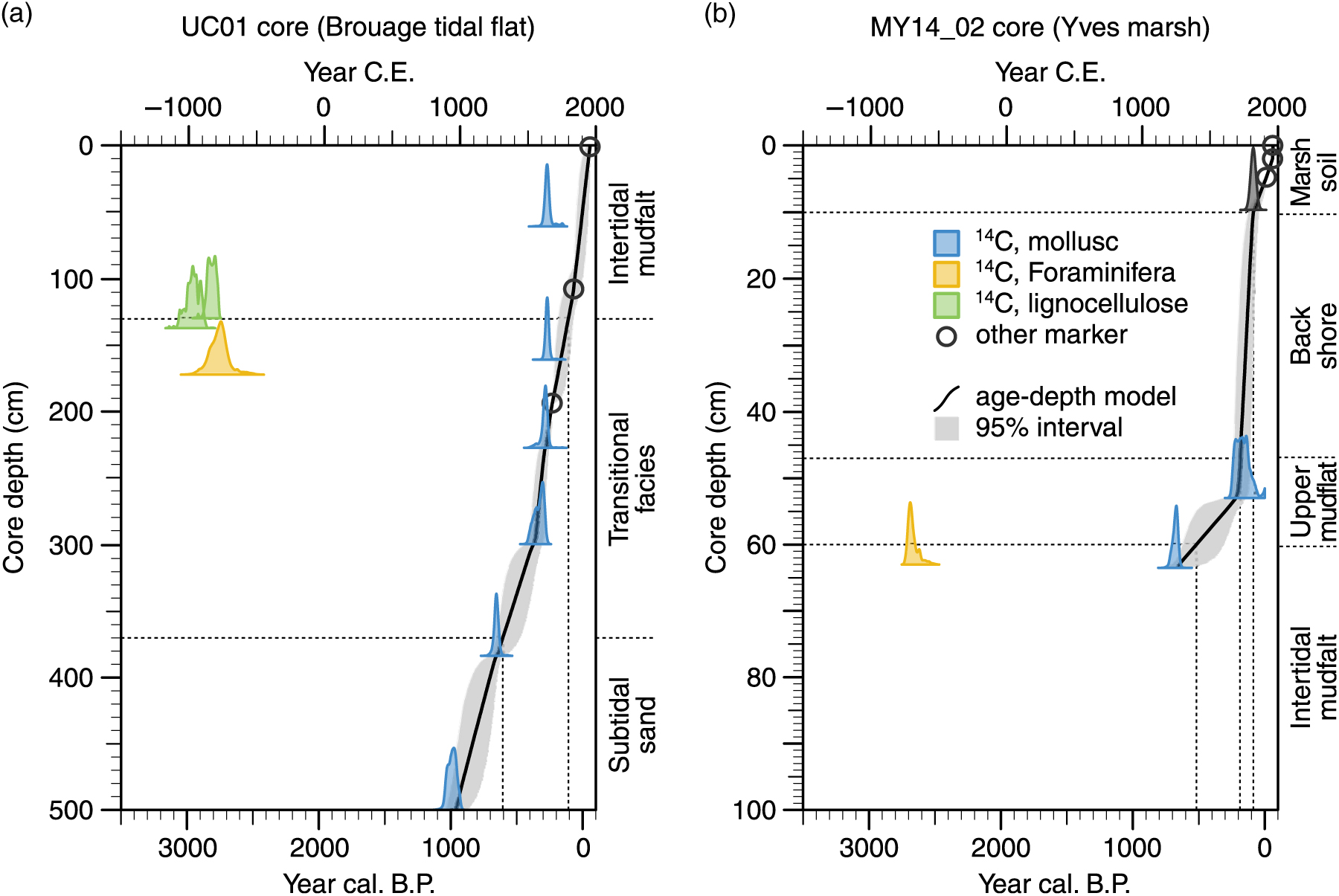
Figure 2 Age-depth models. (a) M7UC01 core, Brouage tidal flat. (b) MY14 02 core, Yves marsh. Chronological markers in green and orange significantly deviate from the grey-shaded 95% confidence interval of modeled age-depth relationship. Black solid line corresponds to the most probable age-depth relationship. Thin dashed horizontal lines delineate the sedimentary units as interpreted in the original core descriptions (Poirier et al. Reference Poirier, Chaumillon and Arnaud2011; Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017).
M7UC01 Core
The M7UC01 age-depth model relies (1) on the two independent chronological markers described above, including the AD 1720 ± 2 yr silt deposit at 194 ± 2 cm and the AD 1882 seafloor depth record at 108 ± 20 cm, and (2) on four mollusk shells out of six, which dated from 1420 ± 30 BP Poz-31120 or cal AD 865–1138 (2σ) at 502.5 cm, to 615 ± 30 BP Poz-32937 or cal AD 1539–1724 at 227.5 cm. Two 14C ages appear slightly older than the predicted age-depth relationship. At 160.5 cm, mollusk shell dated to 590 ± 25 BP Poz-31317 or cal AD 1615–1814, which is about 70 years older than the predicted age (95% confidence interval AD 1743–1839). At 61 cm, mollusk shell dated to 590 ± 30 BP Poz-31123 or cal AD 1587–1815, which is about 90 years older than the predicted age (95% confidence interval AD 1903–1923).
Three 14C ages deviate strongly from the predicted age-depth relationship, including the one determined on Haynesina germanica individuals picked from the total foraminifera assemblage at 172.5 cm (2950 ± 70 BP Poz- 32935), and the two determined on terrestrial lignocellulose debris at 137.5 and 130 cm (2845 ± 35 BP Poz-31315, 2715 ± 35 BP Poz-31316). These three samples dated to cal BC 987–610, cal BC 1112–916 and cal BC 921–806 respectively (Table 1), while the Bchron age-depth model predicts that sediments should not be older than cal AD 1690–1790 above 172.5 cm.
MY14_02 Core
The MY14_02 age-model relies on (1) short-lived isotope dating of sub-surface sediments, and (2) two 14C ages determined on mollusk shells. The first 2 cm of sub-surface marsh soil contained low levels of 210Pbxs indicative of reworked sediments. Based on a well-resolved peak of 137Cs at 4.8 cm (AD 1963), extrapolation of the sub-surface sedimentation rate gave an age of AD 2010 ± 1 year at 2 cm (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). Between 2 and 6.5 cm, 210Pbxs age-depth model provided an average sedimentation rate of 0.053 ± 0.005 cm.yr−1 (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). Zero-intercept of the log-linear 210Pbxs age-depth curve infers a date of AD 1863 ± 20 years at 9.7 cm. Mollusk shells dated to 1100 ± 30 BP Beta-450672 or cal AD 1199–1324 at 63.5 cm, and to 500 ± 30 BP Beta-450673 or cal AD 1689–1899 at 53 cm.
A similar discrepancy between mollusk and foraminifera 14C ages is observed in the MY14_02 core. Total foraminifera assemblage from the same level (63 cm) than Beta-450672 (cal AD 1199–1324) was dated to 2850 ± 30 BP Beta-44311 or cal BC 786–567.
DISCUSSION
In both the M7UC01 and MY14_02 cores, 14C ages determined on lignocellulose debris and foraminifera therefore appear older by about 2500–2000 years than the expected timing of sediment deposition predicted by the Bchron age models, mostly on the basis of 14C-dated mollusk shells. The validity of the age-depth models is first discussed, before identifying the factors that could explain the 14C age anomalies obtained in the study area.
Validity of Age-Depth Models
M7UC01 Core
The Holocene sediment infill of the Marennes-Oléron bay has been extensively studied with very high resolution seismic reflexion profiles, ground-truthed by sediment cores and 14C-dated mollusk shells (Chaumillon et al. Reference Chaumillon, Tessier, Weber, Tesson and Bertin2004; Billeaud et al. Reference Billeaud, Chaumillon and Weber2005; Chaumillon and Weber Reference Chaumillon, Weber, Dalrymple, Leckie and Tillman2006; Allard et al. Reference Allard, Chaumillon, Poirier, Sauriau and Weber2008, Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010; Poirier et al. Reference Poirier, Chaumillon and Arnaud2011). A distinctive first-order seismic boundary, named EU1 (erosional unconformity 1), was recognized on seismic profiles of the area as a high amplitude reflector correlated with a 0.2–0.5-m-thick shell bed. EU1 is a bay-scale channelized surface with a depth range of about 16 m, interpreted as the consequence of intense tidal ravinement induced by major paleogeographical changes in the area (Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010). The sequence described in the M7UC01 core corresponds to sediments deposited above the EU1 boundary (seismic units UT3 to UT4.2 in Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010 and Poirier et al. Reference Poirier, Chaumillon and Arnaud2011). The mollusk recovered at the base of the M7UC01 sequence, just above the EU1 boundary (Poz-31120, 502.5 cm), is dated to cal AD 865–1138. It is consistently younger than the most recent 14C sample recovered below EU1, which was dated to cal AD 598–762 (Poz-20106; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010). It is therefore very likely that the sample Poz-31120 at the base of the M7UC01 sequence was not reworked, which implies that the age-depth model is properly anchored in time. The two 14C dates bounding the EU1 erosional surface suggest that tidal ravinement occurred between about cal AD 680–950 (median calibrated ages), which shifts and narrows the chronological range previously estimated from historical testimonies and maps between 850 and 1450 cal yr BP (Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010).
Internal architecture of the Marennes-Oléron bay sediment fill varies from west to east, but seismic units above EU1 erosional surface are identical (Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010; Poirier et al. Reference Poirier, Chaumillon and Arnaud2011). In the western part of the bay, mollusk shells were recovered on top of the sand-rich unit UT3 (Chaumillon et al. Reference Chaumillon, Tessier, Weber, Tesson and Bertin2004; Billeaud et al. Reference Billeaud, Chaumillon and Weber2005), which was sampled with three short cores (M5VC33, 34, 35; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010). Shells dated to 1165 ± 30 BP Poz-26302, to 1245 ± 30 BP Poz-26303 and to 1235 ± 30 BP Poz-26304. Ages are consistent with those obtained in M7UC01 sandy sediments between depths of 502.5 cm (1420 ± 30 BP Poz-31120) and 383 cm (1065 ± 30 BP Poz-31121). Likewise, the silt-rich unit UT4.2 dated to 630 ± 30 BP Poz-20250 (Allard et al. Reference Allard, Chaumillon, Poirier, Sauriau and Weber2008) and 570 ± 30 BP Poz-20120 (Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010). Ages are again consistent with those obtained in M7UC01 in silty sediments at depths of 160.5 cm (590 ± 25 BP Poz-31317) and 61 cm (590 ± 30 BP Poz-31123).
These two latter samples appear slightly older than the predicted age-depth relationship (Figure 2a). 14C dating of marine samples younger than 600 14C yr BP is challenging. Owing to a plateau in the Marine13 curve, calibration of such samples may yield relatively large uncertainties (up to 230 years for Poz-31123) that are poorly compatible with the rapid sedimentation predicted in the uppermost section of the M7UC01 core. The two 14C samples are therefore considered as less robust chronological markers than the AD 1720 ± 2 yr silt deposit and the AD 1882 lead line bathymetry, which are both precisely dated. Vertical position of these two independent markers is also consistent with the average sedimentation rates predicted in underlying deposits (Figure 2a).
From these observations, it is therefore expected that the M7UC01 age-depth model is valid and reflects the true timing of sediment deposition along the core.
MY14_02 Core
The seaward margin of the Yves marsh, in which the MY14_02 core was sampled (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017), is a small beach ridge system composed of meter thick wave-built sandy barriers and intervening muddy swales. The most inland ridge, which is located about 1 km northeastward of the core (Figure 1), was dated to 1280 ± 90 BP Gif-3857 or cal AD 909–1280 (Regrain Reference Regrain1980). In MY14_02 core, intertidal mudflat sediments at 63.5 cm have been dated to cal AD 1199–1324 (Beta-450672). These two ages are consistent with a westward progradation of the marsh and beach ridge system, in agreement with the global regression trend evidenced in the Pertuis Charentais area (Chaumillon et al. Reference Chaumillon, Tessier, Weber, Tesson and Bertin2004; Chaumillon and Weber Reference Chaumillon, Weber, Dalrymple, Leckie and Tillman2006).
Age-depth model in MY14_02 predicts a date of cal AD 1763 ± 40 yr for the mudflat to back barrier transition identified from micropaleontological content at 47 cm (Figure 2b). This age estimate is consistent with the expected timing of marsh isolation, which is thought from historical maps to have occurred between AD 1708 and 1886 (Baumann et al. Reference Baumann, Chaumillon, Schneider, Jorissen, Sauriau, Richard, Bonnin and Schmidt2017). Sedimentation rates predicted by the MY14_02 age-depth model range between 0.5 mm.yr−1 (intertidal mudflat) to 3.8 mm.yr−1 (back barrier). These values are comparable to those observed in similar depositional environments.
From these observations, it is therefore expected that the MY14_02 age-depth model is valid and reflects the true timing of sediment deposition along the core.
Factors Explaining Anomalous 14C Ages
Foraminifera appear older by about 2500–2000 years than the modeled age-depth relationships in M7UC01 and MY14_02 cores. The most probable factors that may explain this discrepancy are (1) reworking from older strata, which has been frequently invoked as a cause of anomalous ages obtained from 14C-dated foraminifera (Heier-Nielsen et al. Reference Heier-Nielsen, Conradsen, Heinemeier, Knudsen, Nielsen, Rud and Sveinbjörnsdóttir1995; Cearreta and Murray Reference Cearreta and Murray2000) and (2) incorporation of pore water 14C. Both hypotheses are discussed in the following sections.
Reworking from Older Strata
Micropalentological content of the M7UC01 and MY14_02 sedimentary cores is dominated by three species, including Haynesina germanica, Ammonia tepida and Elphidium excavatum. This peculiar assemblage is typically encountered on upper intertidal mudflats, where important fluctuations in salinity and exposure to abundant organic matter induce strong environmental stress favoring the development of such stress-tolerant species (Armynot du Châtelet et al. Reference Armynot du Châtelet, Debenay, Degré and Sauriau2005; Debenay et al. Reference Debenay, Bicchi, Goubert and Armynot du Châtelet2006; Armynot du Châtelet et al. Reference Armynot du Châtelet, Degré, Sauriau and Debenay2009). In addition to the excellent preservation of fossil foraminifera in the cores, the low abundance (< 10% in MY14_02) or absence (in M7UC01) of open marine calcareous species (Quinqueloculina seminula, Cibicidoides lobatulus; Pujos-Lamy Reference Pujos-Lamy1984), as well as the absence of salt marsh agglutinated species (e.g. Leorri et al. Reference Leorri, Gehrels, Horton, Fatela and Cearreta2010), rules out the risk of incorporation of far-transported tests within the sediments (allochthonous fossil assemblages; Kidwell and Bosence Reference Kidwell, Bosence, Allison and Briggs1991). Good preservation of calcareous foraminifera is usually encountered in unvegetated mudflat deposits, where aerobic degradation processes producing acid conditions are limited (Berkeley et al. Reference Berkeley, Perry, Smithers, Horton and Taylor2007).
Most of the Marennes-Oléron bay sediment infill corresponds to seismic unit UT2 (Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010). UT2 is composed of fine sands and silts, dated from mollusk shells between cal BC 1076–827 (3095 ± 35 BP Poz-23454) and cal AD 598–762 (1695 ± 30 BP Poz-20106; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010). The anomalous 14C ages found in the M7UC01 and MY14_02 cores, around cal BC 1000–700, are therefore contemporary with the period of deposition of the UT2 unit. Mollusk fossil assemblages indicate that UT2 sediments were deposited in a shallow subtidal, but not intertidal, context (Poirier et al. Reference Poirier, Sauriau, Chaumillon and Allard2009). Foraminifera fossil assemblages in the UT2 unit are dominated by miliolids (e.g. Quinqueloculina sp.) and discorbids (e.g. Gavelinopis sp.), with a high proportion of abraded, broken and/or pyrite-filled tests. H. germanica, A. tepida and E. excavatum are less abundant, reflecting a greater exposure to open marine conditions than in the M7UC01 and MY14_02 cores (unpublished data; Poirier Reference Poirier2010). Such poorly preserved, high-energy assemblages were not found in the M7UC01 and MY14_02 cores, which rules out the risk of incorporation of old tests within the sediments (leaked assemblages; Kidwell and Bosence Reference Kidwell, Bosence, Allison and Briggs1991). Furthermore, stratigraphical and paleontological data from the Marennes-Oléron bay (Poirier et al. Reference Poirier, Sauriau, Chaumillon and Allard2009; Allard et al. Reference Allard, Chaumillon, Bertin, Poirier and Ganthy2010) do not indicate the existence of intertidal deposits dated to around cal BC 1000–700 that would have been reworked, and would have mimicked the Haynesina germanica dominated assemblage found in the cores.
The 14C-dated foraminiferal assemblages picked from the M7UC01 and MY14_02 cores are therefore compatible with their depositional environment, and they were not contaminated by out-of-habitat species, nor by material reworked from older strata. They thus correspond to autochthonous or para-autochthonous fossil assemblages (Kidwell and Bosence Reference Kidwell, Bosence, Allison and Briggs1991) that reflect in-situ foraminiferal communities living in the area prior to definitive burial. It is therefore unlikely that reworking from older strata explains the discrepancy in 14C age of foraminifera samples.
Incorporation of Pore Water 14C
Calcification in marine organisms requires calcium ions Ca2+ and dissolved inorganic carbon (DIC). DIC occurs in seawater as carbon dioxide CO2, carbonate ions CO32– and bicarbonate ions HCO3−, the latter contributing to 90% of the total DIC pool at seawater pH (Bach Reference Bach2015). In hyaline foraminifera like H. germanica, calcification is performed by exocytosis of intracellular vesicles separately containing DIC and Ca2+, which are both primarily obtained from the surrounding seawater (de Nooijer et al. Reference de Nooijer, Spero, Erez, Bijma and Reichart2014). Dietary carbon is not incorporated in the tests of adult H. germanica specimens, but laboratory experiments could not rule out the existence of a specific metabolic transfer pathway in growing juveniles (Mojtahid et al. Reference Mojtahid, Zubkov, Hartmann and Gooday2011). H. germanica is an infaunal species, which actively avoids the sediment/water interface and migrates vertically within the sub-surface sediment (Seuront and Bouchet Reference Seuront and Bouchet2015). The basis assumption of the present study is therefore that DIC and Ca2+ in H. germanica, which is the dominant foraminifera species in M7UC01 and MY14_02 cores, is derived from the sediment interstitial water rather than from the seawater column.
Chemical composition of the sediment interstitial water of the eastern Marennes-Oléron bay mudflat was studied with a 1 m long sediment core, sampled about 3 km northeastward of the M7UC01 core (El Ghobary and Dumon Reference El Ghobary and Dumon1984). Analyses have shown decreased SO42– and increased HCO3− concentrations in the top 5 cm and downward 20 cm below the surface, which were interpreted as the consequence of organic matter degradation under anoxic conditions. Sulfate reduction and bicarbonate production evidenced by El Ghobary and Dumon (Reference El Ghobary and Dumon1984) point to anaerobic oxidation of methane (AOM; Hinrichs et al. Reference Hinrichs, Hayes, Sylva, Brewer and DeLong1999; Boetius et al. Reference Boetius, Ravenschlag, Schubert, Rickert, Widdel, Gieseke, Amann, Jørgensen, Witte and Pfannkuche2000), which is mediated by methanotrophic Archaea (ANME) according to the reaction CH4 + SO42– → HCO3− + HS− + H2O.
The Marennes-Oléron bay depocentre harbors rich communities of active methane cycling bacteria, which thrive on high concentrations of organic matter in the sediment deposited under rapid sedimentation rates (Bertin and Chaumillon Reference Bertin and Chaumillon2005; Roussel et al. Reference Roussel, Sauvadet, Allard, Chaduteau, Richard, Bonavita and Chaumillon2009). As described above, lignocellulose derived from terrestrial vascular plants and supplied by coastal rivers is likely the dominant source of suspended particulate organic matter in the area. Degradation of lignocellulose in marine environments predominantly involves bacteria, and fungi to a lesser extent (Benner et al. Reference Benner, Moran and Hodson1986). Ji et al. (Reference Ji, Wang, Tan, Chen, Schwarz and Li2012) have cultured in anaerobic and thermophilic conditions a bacterial community living in nearshore sediments of the Yellow Sea (Qingdao, China), which showed strong abilities for cellulose degradation. The community was dominated by the species Clostridium thermocellum. Gene sequences showed little similarities with reference databases, suggesting the existence of unknown taxonomic diversity and metabolic pathways. Degradation end-products included ethanol CH3—CH2—OH, acetic acid CH3—COOH and butanoic acid CH3— (CH2)2—COOH.
Along with methanotrophic Archaea mediating AOM, bacterial communities of the Marennes-Oléron bay include methanogenic Archaea belonging to the Methanococcoides, Methanosarcina, Methanosaeta and Methanomicrobiales genera (Roussel et al. Reference Roussel, Sauvadet, Allard, Chaduteau, Richard, Bonavita and Chaumillon2009). Among these, Methanosaeta sp. is a genus of obligate acetotrophic methanogens that degrade acetic acid according to the reaction CH3COO− + H2O → CH4 + HCO3− (Ozuolmez et al. Reference Ozuolmez, Na, Lever, Kjeldsen, Jørgensen and Plugge2015), while butanoic acid does not seem to be a proper metabolic substrate (Patel and Sprott Reference Patel and Sprott1990). Ozuolmez et al. (Reference Ozuolmez, Na, Lever, Kjeldsen, Jørgensen and Plugge2015) emphasized that “aceticlastic methanogens, specifically Methanosaeta species, may be important in contributing to acetate degradation in marine sediments, in particular the tidal flat sediments, which have an abundant supply of organic matter.” Alternative metabolic pathways that involve other Archaea interacting with sulfate reducing bacteria may also occur. The corresponding chemical reactions contribute to acetate and SO42– reduction, and to HCO3− production (Ozuolmez et al. Reference Ozuolmez, Na, Lever, Kjeldsen, Jørgensen and Plugge2015), consistent with in-situ analysis of the sediment pore water chemistry (El Ghobary and Dumon Reference El Ghobary and Dumon1984).
Given that terrestrial lignocellulose debris and in situ Haynesina germanica have similar, older than expected 14C ages, we therefore suggest the following pathway to explain the transfer of old carbon from the former to the latter compartment (Figure 3). Lignocellulose debris that originate from the Charente and Gironde estuary, after transport, settling and burial, could have been degraded in anoxic sediments of the Pertuis d’Antioche and Marennes-Oléron by a Clostridium-dominated bacterial community (Ji et al. Reference Ji, Wang, Tan, Chen, Schwarz and Li2012). Production of acetic acid could then have served as the basis substrate for active methane cycling methanogenic Archaea that are known to live in the study area (Roussel et al. Reference Roussel, Sauvadet, Allard, Chaduteau, Richard, Bonavita and Chaumillon2009). Bacterial activity could have contributed to the enrichment of 14C-depleted HCO3− ions in the sediment pore water (El Ghobary and Dumon Reference El Ghobary and Dumon1984), which would have been used by the foraminifer Haynesina germanica as its primary source of DIC for calcification (de Nooijer et al. Reference de Nooijer, Spero, Erez, Bijma and Reichart2014).
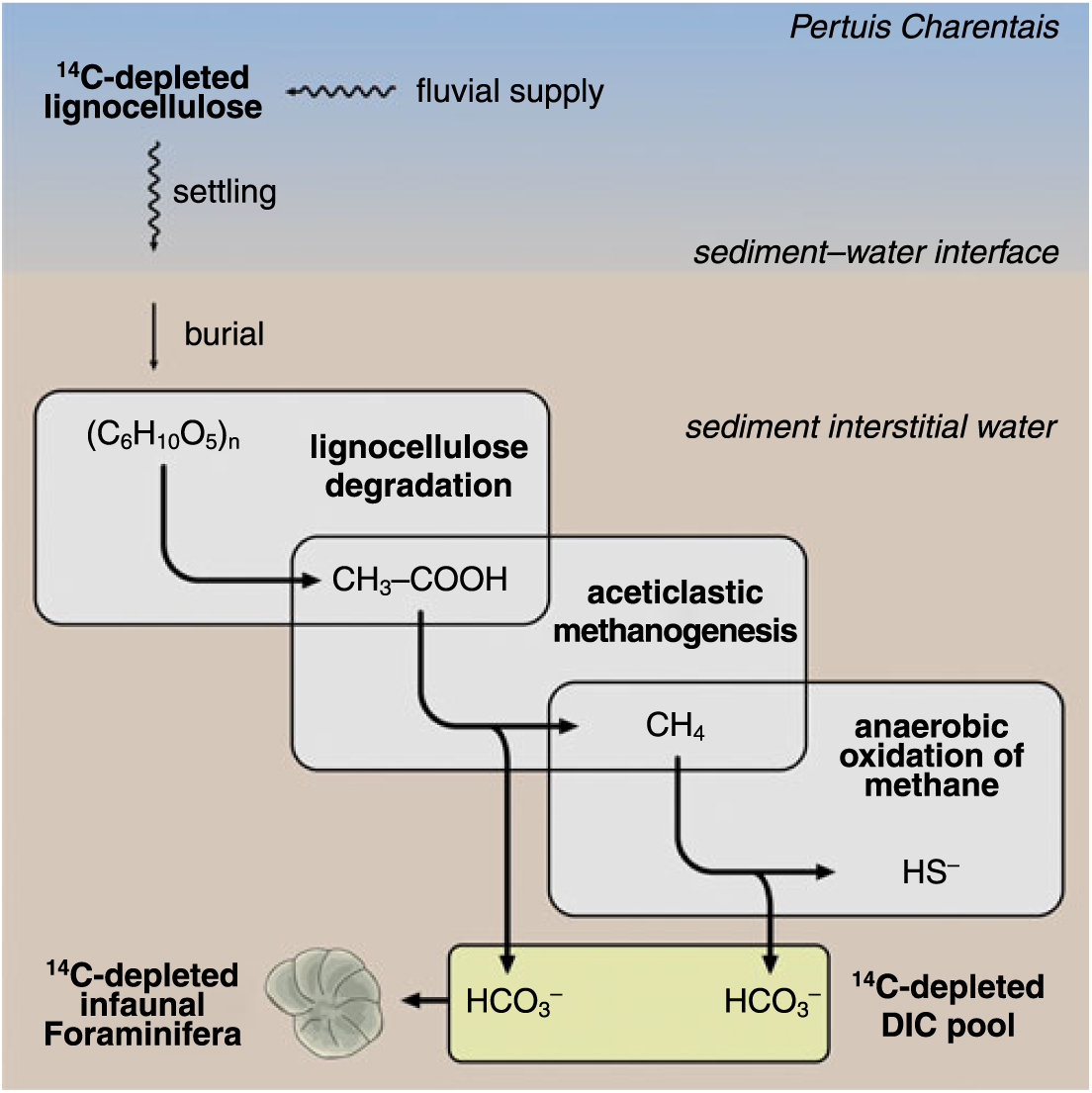
Figure 3 Probable pathway of transfer of 14C-depleted carbon from lignocellulose debris to the foraminifer Haynesina germanica in the Pertuis d’Antioche and Marennes-Oléron Bay. Grey boxes symbolize metabolic pathways.
H. germanica is known to migrate vertically within the sub-surface sediment (Seuront and Bouchet Reference Seuront and Bouchet2015), but the species mostly lives in the first 2 cm below the sediment/water interface (Cesbron et al. Reference Cesbron, Geslin, Jorissen, Delgard, Charrieau, Deflandre, Jézéquel, Anschutz and Metzger2016). Although El Ghobary and Dumon (Reference El Ghobary and Dumon1984) reported anoxic conditions in the first 5 cm of sediments, such a thin layer would be easily resuspended during stormy conditions, contributing to downward dilution of the 14C-depleted DIC pool with contemporary seawater into the sediments. It is also unclear why mollusks appear to be unaffected by this hypothetical contamination, although most of the carbon they use for calcification is also derived from ambient DIC (>90% for Mytilus edulis; Gillikin et al. Reference Gillikin, Lorrain, Bouillon, Willenz and Dehairs2006). Resuspension would inversely contribute to upward release of the 14C-depleted DIC pool into the seawater.
Further work is therefore needed to elucidate these issues. Comprehensive studies involving biogeochemical, sedimentological and geochronological methods would help to better understand the external factors controlling organic matter transport, settling and burial, bacterial activity and isotopic fractionation in the Pertuis Charentais (Lavergne et al. Reference Lavergne, Agogué, Leynaert, Raimonet, De Wit, Pineau, Bréret, Lachaussée and Dupuy2017) and in other coastal environments.
Implications for Paleoenvironmental Reconstructions
Suspended particulate organic matter (SPOM) delivered to the Pertuis d’Antioche and Marennes-Oléron bay involves both autochthonous and allochthonous sources (Malet et al. Reference Malet, Sauriau, Ryckaert, Malestroit and Guillou2008). The relative contribution of each source varies seasonally. In winter (high river discharge), the SPOM δ13C content increases, reflecting the greater contribution of allochthonous, terrestrial inputs relative to autochthonous and marine ones. The ratio of particulate organic carbon to chlorophyll a becomes more variable, which has been interpreted as the signature of pulsed supplies of decomposed terrigenous organic matter to the coast (Malet et al. Reference Malet, Sauriau, Ryckaert, Malestroit and Guillou2008). Soil erosion is the main process of surface transfers across the Charente and Gironde catchments, on which agricultural activities are dominant (Le Bissonnais et al. Reference Le Bissonnais, Thorette, Bardet and Daroussin2002; Poirier et al. Reference Poirier, Chaumillon and Poitevin2016). Sub-surface organic soil samples at the northern boundary of the lower Charente basin (30 km northeastward of Marennes-Oléron bay) were dated to 7370 ± 80 BP HAM-3035 (Becker-Heidmann et al. Reference Becker-Heidmann, Scharpenseel and Wiechmann1996). Similar organic soil samples from the Vienne basin, a tributary of the Loire River (120 km northeastward of Marennes-Oléron bay) ranged from modern ages to 5805 (uncal.?) BP (Chabbi et al. Reference Chabbi, Kögel-Knabner and Rumpel2009). Values are consistent with those found on terrestrial organic matter deposited in the temperate Saanich Inlet basin, around 7.9 ± 5.0 ka BP (Vonk et al. Reference Vonk, Drenzek, Hughen, Stanley, McIntyre, Montluçon, Giosan, Southon, Santos, Druffel, Andersson, Sköld and Eglinton2019).
These results are in agreement with our 14C age estimates of the lignocellulose debris, which overall suggest a long retention time of decomposed organic matter on the catchments, on the order of several thousands of years. Similar age offsets have been reported in the Chesapeake Bay, USA (Colman et al. Reference Colman, Baucom, Bratton, Cronin, McGeehin, Willard, Zimmerman and Vogt2002), in the Loire estuary, France (Durand et al. Reference Durand, Mojtahid, Maillet, Proust, Lehay, Ehrhold, Barré and Howa2016), and on the Paraná coast, Brazil (Angulo et al. Reference Angulo, de Souza, Assine, Pessenda and Disaró2008), where 14C-dated plant debris and/or bulk organic sediments were consistently older than bivalve mollusk shells by several centuries to millennia. The late Holocene evolution of the Vilaine bay, nearby the Loire estuary, has been reconstructed from 21 14C dates, out of which 17 were determined on plant debris or bulk organic sediment (Traini et al. Reference Traini, Menier, Proust and Sorrel2013). Based on their age model, the authors proposed a local correction of the relative sea level curve for the early Holocene period (ca. 11–8 ka BP). The locally revised curve is in disagreement with the overall well-constrained chronological framework of sea level changes along French Atlantic coasts (Lambeck Reference Lambeck1997; Allard et al. Reference Allard, Chaumillon, Poirier, Sauriau and Weber2008; Stéphan and Goslin Reference Stéphan and Goslin2014), showing an offset of about 2000 years toward older ages. In the light of our results, and those obtained on the Loire estuary (Durand et al. Reference Durand, Mojtahid, Maillet, Proust, Lehay, Ehrhold, Barré and Howa2016), we therefore question the validity of this local revision of the relative sea level curve, and carefully suggest that the age model built upon terrestrial organic matter might be biased (Traini et al. Reference Traini, Menier, Proust and Sorrel2013).
The present study suggests that dating of in-situ coastal foraminifera could be problematic. Old organic matter supplied by rivers may have the potential to be transformed into dissolved inorganic carbon within the sediment, following complex pathways possibly involving bacterial communities. In the absence of datable mollusk shells or other macroscopic in-situ material, 14C age determination of indeterminate organic matter and foraminifera might be compulsory, but precautions should be then taken to avoid incorrect interpretation of age models, as suggested by Durand et al. (Reference Durand, Mojtahid, Maillet, Proust, Lehay, Ehrhold, Barré and Howa2016). A significant proportion of carbon produced by terrestrial vascular plants is subjected to “pre-aging” on catchments prior to their release in coastal environments (Vonk et al. Reference Vonk, Drenzek, Hughen, Stanley, McIntyre, Montluçon, Giosan, Southon, Santos, Druffel, Andersson, Sköld and Eglinton2019). The hypothetical contamination process described in the present study may thus be a widespread phenomenon that is not unique to the Pertuis Charentais area, and would therefore deserve further consideration.
CONCLUSION
In the Pertuis Charentais, 14C-dated Haynesina germanica picked from two late Holocene sediment cores appeared about 2500–2000 years older than their expected age of deposition, which was estimated from robust age-depth models. Stratigraphical and micropaleontogical data have ruled out the possible effect of reworking from previous strata. The Charente and Gironde Rivers supply significant amounts of suspended particulate organic matter (SPOM) derived from their catchments to the Pertuis Charentais. SPOM, occurring as lignocellulose debris, was likely released by erosion after centuries to millennia of retention within soils. H. germanica acquired the same 14C-depleted signature than that of organic debris, suggesting the existence of an unexplored pathway of carbon transfer between the two compartments. We have collated evidence from the literature to address this issue. Assuming that H. germanica exploited dissolved inorganic carbon for calcification from tidal flat interstitial waters, we have proposed that degradation of lignocellulose debris into 14C-depleted HCO3− ions involved bacterial communities, following three main steps. These include (1) lignocellulose degradation into acetic acid possibly by a Clostridium-dominated community, (2) conversion to methane through aceticlastic methanogenesis by Methanosaeta sp., and (3) anaerobic oxidation of methane by ANME Archaea. In the two latter metabolic pathways, HCO3− ions are by-products that could have contributed to local enrichment in 14C-depleted carbon. Further work is now needed to elucidate this transfer pathway with more robust, direct evidence, which would require specific studies in the Pertuis Charentais area and in other coastal environments.
ACKNOWLEDGMENTS
The authors wish to thank Associate Editor Dr. John Southon and two anonymous reviewers for their useful comments and constructive criticism. We also thank Dr. Irka Hajdas (ETH Zürich) for her useful advice prior to manuscript preparation, as well as Dr. Meryem Mojtahid (University of Angers) who kindly guided us in the early discussion process. J. Baumann Ph.D. was funded by a grant from the Région Poitou-Charentes, and the Parc Naturel Régional du Marais Poitevin supported operating costs.






