INTRODUCTION
Wood is present in many natural and anthropogenic settings and is one of the most common materials analyzed by the radiocarbon dating method. Moreover, wood is also the key material used for the construction of the 14C calibration curve. In fact, the very first set of 14C samples included wood samples (Arnold and Libby Reference Arnold and Libby1949), and wood continues to be one of the largest classes of materials dated by 14C. It is not always easy to track down the treatment applied to samples that were measured decades ago. Even among laboratories involved in measurements of wood for 14C calibration, different protocols were applied (McCormac et al. Reference McCormac, Hogg, Higham, Baillie, Palmer, Xiong, Pilcher, Brown and Hoper1998 and references therein). The discussion of the proper treatment has been revitalized due to the involvement of accelerator mass spectrometry (AMS) laboratories in work on the calibration curve (Hogg et al. Reference Hogg, Turney, Palmer, Southon, Kromer, Bronk Ramsey, Boswijk, Fenwick, Noronha, Staff, Friedrich, Reynard, Guetter, Wacker and Jones2013). The wide range of treatment from acid-base-acid (ABA) application to whole wood through separation of hemicellulose and α-cellulose demonstrates the search for the optimal pretreatment protocol.
Cellulose (α-cellulose), which is an important component of cell walls in green plants, is considered to be the ultimate material for isotopic studies of wood, as it is the most abundant and is a moderately inert organic compound (C6H10O5)n in wood. The second group of carbohydrates in wood is hemicelluloses. These compounds form a matrix, which surrounds the cellulose fibers in cell walls; they are therefore considered to be a secondary cell wall material. New developments in separation of α-cellulose have focused on reducing contamination sources in 14C analysis because the established protocols, which were originally designed for stable isotope research, employed carbon-bearing chemicals such as acetic acid (for overview see Boettger et al. Reference Boettger, Haupt, Knoller, Weise, Waterhouse, Rinne, Loader, Sonninen, Jungner, Masson-Delmotte, Stievenard, Guillemin, Pierre, Pazdur, Leuenberger, Filot, Saurer, Reynolds, Helle and Schleser2007). Another factor, which must also be taken into account, is the required amount of material necessary for sample treatment considering that no treatment affords 100% yield. Many applications of 14C dating are pushing for the smallest amount of material used for analysis, as in the case of paintings and art masterpieces (Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Küffner, Scherrer and Ferreira2016), while at the same time requiring the highest precision. When considering cellulose separation from wood, the required amount of starting material may vary considerably depending on the type of wood. Consequently, limits on the necessary amount of wood must also be set. Therefore, evaluating the effectiveness of alternative methods is important when an informed decision must be made on sampling and treatment. Moreover, this evaluation must take into account that wood can have two different conditions of storage: the natural preservation in the field environment and preservation in human archives where chemicals may be purposely applied in conservation. In the case of the latter, separation of cellulose provides an opportunity to analyze samples that might be contaminated with conservatives and/or infected by remains of woodworms that can potentially go undetected when wood is sampled by drilling. The issue of the chemical reagents used for conservation of wood and their effect on 14C ages is complex and requires extensive tests and research. Even cellulose separation might be ineffective in some cases of conservations (Isler and Hajdas, unpublished data). On the other hand, separation of cellulose might be only needed and applied in very specific cases. This is because the whole cellulose separation procedure, which is much more aggressive than ABA, requires a large amount of wood as a starting material as well as well-ventilated laboratories in order to dissipate aggressive and toxic fumes of the bleach. In contrast, when dealing with wood found in natural deposits, a thorough ABA treatment is usually sufficient. Moreover, in the case of wood preserved in very wet environments, cellulose may be decomposed to varying degrees, resulting in very low yields during pretreatment. In this study, we perform analysis on fossil trees found in ~30,000-yr-old sediments. Results of 14C dating of whole wood treated by ABA and acid-base oxidation (ABOX) treatments are compared to those obtained after cellulose separation.
SITES – ORIGIN OF THE WOOD SAMPLES
The trunks analyzed in this research were collected in the Friuli Venezia Giulia Region (NE Italy), at the sites of Illegio and Strassoldo (Figure 1). In the area of Illegio, near Tolmezzo in the Carnic Alps, two trunks were found in 2005 at an elevation of 585 m above sea level (asl) (46°26′08″N, 13°04′0.0″E) in the valley of Frondizzon Creek, a tributary of the But River (Monegato and Fontana Reference Monegato and Fontana2006). The tree trunks had been exposed by recent erosive processes, but they were inside a slope deposit, covered by fluvioglacial gravels related to the LGM glacial advance. For our study, we analyzed only one of the fossil trees that is part of a trunk with a length of 1.5 m and 37 cm in diameter. The bark and parts of the tree trunk were not preserved, and probably were removed by transport within the landslide. Samples of this trunk have been previously dated with conventional AMS methods (Monegato and Fontana Reference Monegato and Fontana2006; Kromer et al. Reference Kromer, Lindauer, Synal and Wacker2013).

Figure 1 Location of Illegio and Strassoldo sites
The other wood sample was collected near Strassoldo, in the distal sector of the Venetian–Friulian Plain (45°52′04″N, 13°18′47″E), at an elevation of 7 m asl. In the site, a mechanical core recovered a piece of wood at a depth of 25.55–25.75 m below the surface. The sample was at the base of a layer with a thickness of 1.4 m and characterized by the alternation of decimetric intervals of silt and organic-peaty silt. Above this layer, the stratigraphic sequence consists of sandy gravels that, according to the regional stratigraphic framework, were deposited during the Last Glacial Maximum (LGM) (Fontana et al. Reference Fontana, Mozzi and Marchetti2014).
SAMPLES AND PREPARATION PROCEDURE
Eight samples of Illegio fossil tree (IL1) were taken across the trunk slab, starting from the inner rings (oldest) of the trunk through to the outermost rings (younger) (see subsample location in Figure 2a). The samples were then weighed and placed in centrifuge tubes. The study was conducted using the two innermost and two outermost samples.

Figure 2 (a) Sampling of Illegio tree: IL1_1 (rings 1–5), IL1_2 (rings 6–10), IL1_7 (ring_128), IL1_8 (ring 138); (b) Strassoldo: STRA_1 and STRA_2
In the case of the first two samples, IL1_1 and IL1_2, four treatments were investigated, namely ABA, ABA and bleach, ABOX, and base-acid-base-acid-bleaching (BABAB). For the last two samples, IL1_7 and IL1_8 (Table 1), a modified version of the procedure from Loader et al. (Reference Loader, Robertson, Barker, Switsur and Waterhouse1997) was additionally applied to separate α-cellulose. Thus, each sample was split into subsamples and weighed before treatment. Because only a fragment of the Strassoldo trunk was extracted from the core, the inner rings could not be distinguished from the outer ones. Moreover, the center of the trunk was missing, thereby limiting the possibility of dendrochronology analysis. Hence, two samples were taken, as illustrated in Figure 2b. As the wood was still wet at the time of sampling, the amount taken was generously overestimated as all five methods were to be tested.
Table 1 Results of 14C ages (BP) for different treatment methods, yield, as well as amount of C pressed in the target. Calibrated ages are given for mean values obtained using the Combine function of OxCal v 4.02 (Bronk Ramsey Reference Bronk Ramsey2009) and IntCal13 data set (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013).
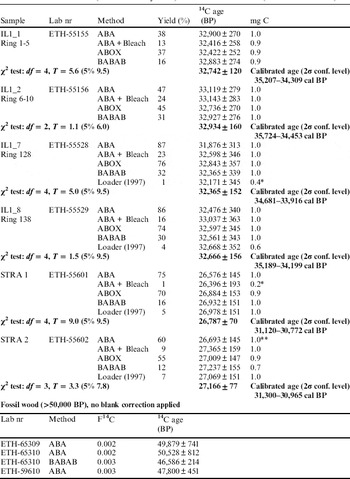
Note: * very small amount of C. ** Stra2/ABA was excluded from combined mean value.
Treatment Methods
Despite the fact that Illegio and Strassoldo wood were expected to be older than 20 kyr and such old wood is assumed to contain no resins, both were treated by Soxhlet extraction in order to assure the compatibility of the methods applied to various samples in the laboratory. All samples were placed in the Soxhlet system described by Hajdas et al. (Reference Hajdas, Bonani, Thut, Leone, Pfenninger and Maden2004), where the samples are successively immersed in warm hexane, acetone, and ethanol for 1 hr each. The Soxhlet is designed to soak the sample in the warm solvent, which is circulated through the Soxhlet apparatus by heating the solvent to its boiling temperature. Contaminations are thereby removed as the distilled solvent’s vapors cool against the condenser and drip back down into the sample. Contaminants such as oils or resins are thus dissolved and removed from the sample through the multiple washes. The solvent order (hexane first, followed by acetone and ethanol bath) is designed to wash out any remains of previous solvent with the next bath, with a final step using ethanol, which dissolves in water and is easily washed out by the ABA treatment (Hajdas Reference Hajdas2008). All samples were subjected to ABA treatment, before being dried and divided into subsamples prior to application of the different procedures: bleaching, oxidation with dichromate solution (ABOX), or bleaching followed by strong base (Loader et al. Reference Loader, Robertson, Barker, Switsur and Waterhouse1997).
ABA or AAA
The standard acid-base-acid (ABA) or acid-alkali-acid (AAA) procedure involves a sequence of washes that remove contamination incorporated into the sample during the deposition and burial. This treatment consists of the following steps: acid 0.5M HCl at 60°C, to remove carbonates, followed by a weak base of 0.1M NaOH at 60°C, which dissolves humic acids. The final step is again a hot acid wash of 0.5M HCl at 60°C, removing any carbonates that precipitated from modern atmospheric carbon dioxide, which dissolved in the alkali solution. Following each step, the sample is rinsed with Milli-Q® water until a neutral pH is obtained. Each step is generally conducted for at least 6 hr.
ABA and Bleach
Similar to the study of Southon and Magana (Reference Southon and Magana2010), samples pretreated with ABA were additionally treated with a bleaching step. After addition of 5% NaClO2 and five drops of 4% HCl, the samples were agitated for 2 hr at 80°C, followed by 15 min in the ultrasonic bath. After washing with Milli-Q water to pH neutral, the sample was dried in the oven.
ABOX or Wet Oxidation
The ABOX procedure developed by Bird et al. (Reference Bird, Ayliffe, Fifield, Turney, Cresswell, Barrows and David1999) is an alternative to the conventional ABA treatment when pretreating old material. This procedure follows the conventional ABA scheme; however, the final acid step with an oxidizing step applying 0.1M K2Cr2O7 in a 2M solution of H2SO4. This aggressive acid-dichromate solution leads to excessive destruction of material and poor yields when applied for long time periods. Therefore, a compromise between reaction time and applied temperature was suggested by Santos et al. (Reference Santos, Bird, Pillans, Fifield, Alloway, Chappell, Hausladen and Arneth2001), of treating the samples with potassium dichromate for 30 min at 60°C. After this rather short oxidizing step, the woody components were washed with Milli-Q water until a neutral pH was reached.
BABAB
The BABAB method (Nemec et al. Reference Nemec, Wacker, Hajdas and Gaggeler2010) is another modification of the commonly applied ABA method (Gaudinski et al. Reference Gaudinski, Dawson, Quideau, Schuur, Roden, Trumbore, Sandquist, Oh and Wasylishen2005; Anchukaitis et al. Reference Anchukaitis, Evans, Lange, Smith, Leavitt and Schrag2008). Two additional steps extend the ABA procedure and result in the separation of hemicellulose. An alkaline step at the very beginning enables a higher decomposition of the hemicellulose woody components, which allows cellulose to be more accessible in the following steps. After overnight treatment with 4% NaOH at 80°C, samples underwent conventional ABA at 80°C, using 4% HCl and 4% NaOH solutions, each step for an hour while placed in a shaker as described by Nemec et al. (Reference Nemec, Wacker, Hajdas and Gaggeler2010). In the final bleaching step, the samples were agitated for 2 hr at 80°C after addition of 5% NaClO2 and five drops of 4% HCl, followed by 15 min in the ultrasonic bath. The resulting suspension was washed with Milli-Q water until pH neutral was reached. At this point, the cellulose should be quite white. In the case of the remaining material, if a yellow or orange tint exists, the sample should be further oxidized and rinsed again. The final product was dried in the oven at 60°C overnight.
Loader α-Cellulose (Modified)
Separation of α-cellulose is of primary interest as it is chemically inert with respect to carbon exchange. The method of Loader et al. (Reference Loader, Robertson, Barker, Switsur and Waterhouse1997) was applied using two modifications. The first was the additional ABA treatment prior to application of the Loader protocol. Secondly, as in the BABAB method (Nemec et al. Reference Nemec, Wacker, Hajdas and Gaggeler2010), in order to avoid any potential contamination, hydrochloric acid (4% HCl) was chosen instead of acetic acid for the bleaching step. Moreover, centrifuge tubes were used instead of Soxhlet thimbles. Also, similar to the ABA protocol, a final acid wash was added to remove the possible contamination with modern CO2 dissolved during the NaOH step. The samples were immersed in the alternate bleaching reagents (5 mL of 5% NaClO2 and five drops of 4% HCl) and set in an ultrasonic bath at 80°C for 4 hr, where the bleaching reagents were replaced each hour in order to oxidize lignin. The bleaching reagents were added as long as the remaining cellulose remained yellow. The final product, which consisted of white cellulose, was washed with Milli-Q water to pH neutral, 10% NaOH was added, and the sample was subjected to ultrasound for 45 min at 80°C. The sample was again washed to pH neutral and 17% NaOH was added. It was agitated for another 45 min at room temperature to decompose the hemicelluloses. After washing with Milli-Q water, the sample was treated with 4% HCl for 30 min in the ultrasonic bath. After a final wash to pH neutral, the sample was dried in the oven at 60°C.
Graphitization and AMS Analysis
The last preparation step before AMS measurement is the graphitization. This was achieved using the fully automated graphitization system AGE (Wacker et al. Reference Wacker, Nemec and Bourquin2010b). The cleaned material was weighed and wrapped in tin cups before being combusted in an elemental analyzer and transferred to the graphitization unit. In order to obtain the equivalent of 1 mg carbon after combustion, an excess of material was initially weighed (for wood/cellulose between 2.5 and 3 mg). This was not always possible as some of the treatments resulted in large loss of material (Table 1). Once combusted and purified, the resulting CO2 was transferred into the reaction tube containing 3.5 mg of catalyzer (Fe powder), H2 was added and the reactor was heated to 580°C. The reduction reaction was completed after 2.5 hr and the resulting graphite was then pressed into the aluminum targets for AMS analysis. Samples of phthalic anhydride were also graphitized along with wood and cellulose and were measured together with Illegio and Strassoldo samples in three independent cassettes. Oxalic acid II was also prepared and measured with the samples. Isotopic ratios of carbon 14C/12C and 13C/12C were determined through the dedicated carbon dating system MICADAS at the ETH AMS facility in Zurich (Wacker et al. Reference Wacker, Bonani, Friedrich, Hajdas, Kromer, Nemec, Ruff, Suter, Synal and Vockenhuber2010a). After correction for blank and fractionation (δ13C measured on graphite), 14C ages were calculated following the convention of Stuiver and Polach (Reference Stuiver and Polach1977).
IR for Cellulose Purity
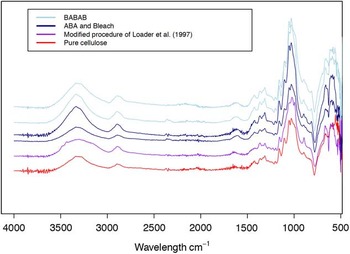
The purity of extracted cellulose was investigated using Fourier transformed infrared (FTIR) spectroscopy (Figure 3). Figure 4 shows a comparison between the different absorption spectra obtained for samples prepared by the different methods as well as pure cellulose (purchased from Sigma-Aldrich). All the measured spectra show the main peaks of cellulose (Figure 3) and little contamination compared to spectra of pure cellulose. A band at 1690 cm–1 could arise due to C=O bonds, which would indicate the presence of resins (Browning Reference Browning1967), but this band was absent in all samples. Other complex features of IR spectra of whole wood such as lignin and hemicellulose absorption peaks at 1649 cm–1 and at 1500 cm–1 are also not present. The small peak observed at 1619 cm–1, mainly for ABA and BABAB products, was also observed in previous studies (Rinne et al. Reference Rinne, Boettger, Loader, Robertson, Switsur and Waterhouse2005) and was linked to water in cellulose-OH (Harrington et al. Reference Harrington, Higgins and Michell1964). Similar to the study conducted by Rinne et al. (Reference Rinne, Boettger, Loader, Robertson, Switsur and Waterhouse2005) on the comparison of various treatments, in our study the IR spectra of all end products separated using different preparation methods closely reproduce the spectrum of pure α-cellulose.

Figure 3 IR spectra of samples treated using various methods: ABA+bleach (dark blue; two samples) and BABAB (light blue; two samples) and for Loader cellulose (purple; one sample) compared to pure cellulose (red line; Sigma-Aldrich).
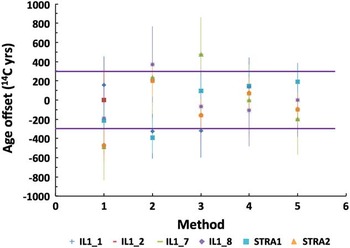
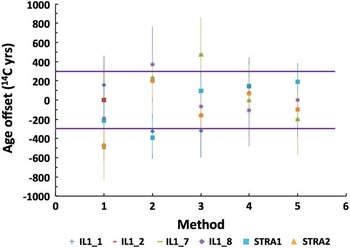
Figure 4 Offset of 14C ages of individual fractions from the mean value with 2σ range marked by horizontal lines: (1) ABA, (2) ABA+Bleach, (3) ABOX, (4) BABAB, (5) modified Loader.
RESULTS AND DISCUSSION
The results of 14C analyses on Illegio and Strassoldo samples are summarized in Table 1 and Figure 4. The combustion and graphitization blank (phthalic anhydride) values, measured along with the samples, were at the regular level of blank dating to 47,000–50,000 BP (0.003–0.002 F14C) as reported for MICADAS (Wacker et al. Reference Wacker, Bonani, Friedrich, Hajdas, Kromer, Nemec, Ruff, Suter, Synal and Vockenhuber2010a). This level of F14C is typically measured in old fossil wood prepared at ETH laboratory by either ABA or cellulose (Table 1). 14C ages were calculated using the Combine function of OxCal v 4.02 (Bronk Ramsey Reference Bronk Ramsey2009) and IntCal13 data set (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). All but one ABA-treated sample of STRA1 gave coherent 14C ages and a weighted mean was calculated for each sample. We counted 138 tree rings between sample 1 and 8. The ages do not differ significantly as would be expected for such an old piece with a short timespan. The outer ring is dated to 32,666±156 BP, while Kromer et al. (Reference Kromer, Lindauer, Synal and Wacker2013) report 32,940±180 BP by gas counting for Illegio 152, which is the same tree trunk fragment used in our study (IL1). The same sample analyzed by AMS resulted in an older age of 33,440±190 BP and 33,490±190 BP (MAMS laboratory).
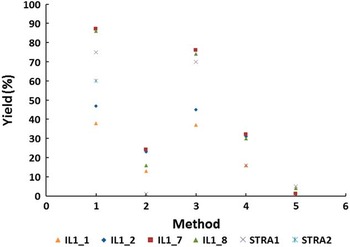
The overall agreement of 14C ages measured on differently prepared cellulose or whole wood is illustrated in Figure 4 where the difference between 14C ages of individual fractions and the mean value is plotted. Although the difference remains in the 2σ range and no trend can be observed, the data obtained on cellulose (BABAB method #4 and modified Loader method #5) appear most consistent (Figure 4). On the other hand, the yield of both cellulose separation methods is very low (see Table 1 and Figure 5), which sets a limit on the choice of treatment when dealing with small samples.
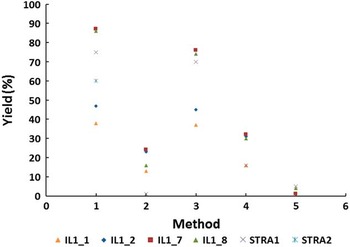
Figure 5 Yield of methods applied to treatment methods of wood: (1) ABA, (2) ABA+Bleach, (3) ABOX, (4) BABAB, (5) modified Loader.
Our results are in agreement with the previously published studies, which showed that ABA and ABOX might be equally efficient in removing contamination from the wood samples, as is the separation of cellulose (Southon and Magana Reference Southon and Magana2010). Nevertheless, we recognize the fact that, as in the case of the Wk5383 kauri wood in the study of Southon and Magana (Reference Southon and Magana2010), some wood requires separation of cellulose. This might be related to the wood type and the preservation at different sites. The sample-specific choice of treatment can be optimized by implementation of FTIR spectroscopy to prescreening the samples as well as controlling the quality of the end product (Rinne et al. Reference Rinne, Boettger, Loader, Robertson, Switsur and Waterhouse2005). Moreover, the sampling site gives an indication on which treatment should be applied. Similar to the presented study of Illegio and Strassoldo wood, numerous double (ABA and BABAB) preparations and cross-check 14C analyses of wood from natural archives submitted to the laboratory result in coherent 14C ages. This, however, cannot always be extended to samples of archived wood, which is a subject of an ongoing study.
Finally, our methodological study resulted in 14C ages that provide chronological framework for archives of past environment and will be discussed in detail elsewhere. Both pieces of wood predate LGM and their 14C ages are in a broad agreement with chronologies of climatic events of the MIS 3 in the study area (Monegato et al. Reference Monegato, Ravazzi, Donegana, Pini, Calderoni and Wick2007; Fontana et al. Reference Fontana, Mozzi and Bondesan2010, Reference Fontana, Mozzi and Marchetti2014). Such unique finds provide an excellent opportunity for more precise chronological reconstructions.
CONCLUSIONS
Multiple methods were used to prepare Illegio and Strassoldo wood for 14C analysis and resulted in coherent 14C ages of the two fossil trees dated to 32,900 and ~27,000 BP, respectively. Also, FTIR spectra of all end products validated the effectiveness of the purification methods. The dramatically low yields, which result after cellulose separation, demonstrate the need for alternative treatment in cases where only a few milligrams of wood are available. Furthermore, this study shows that for most wood found in natural archives, a simple ABA treatment may be all that is required.
ACKNOWLEDGMENTS
We thank Mantana Maurer and Maria Belen Röttig for their help in laboratory. Lukas Wacker and Cameron McIntyre are thanked for their support in performing AMS analysis. The authors are grateful to the Comitato San Floriano (responsible Aurora Cagnana and Alessio Geretti) for supporting the fieldwork in Illegio and to Umberto Stefanel for providing the core from Strassoldo.